Substandard and Falsified Medicines in Myanmar
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Areas
2.2. Sample Collection
2.3. Observation Testing
2.4. Authenticity Investigation
2.5. Quality Analysis
2.6. Statistical Analysis
2.7. Ethics
3. Results
3.1. Sampling Collection
3.1.1. Sampling Sites
3.1.2. Samples
3.2. Observation Testing
3.2.1. Observation of Shops
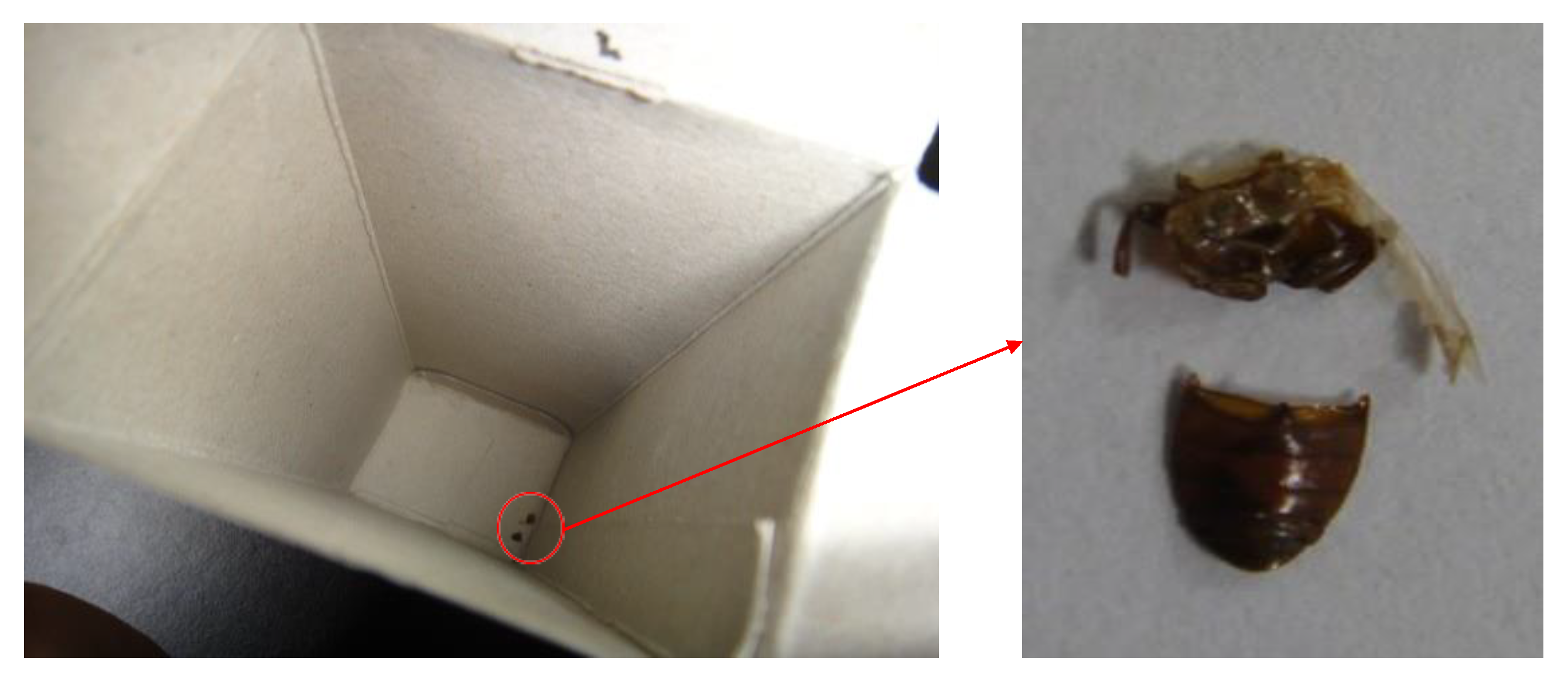
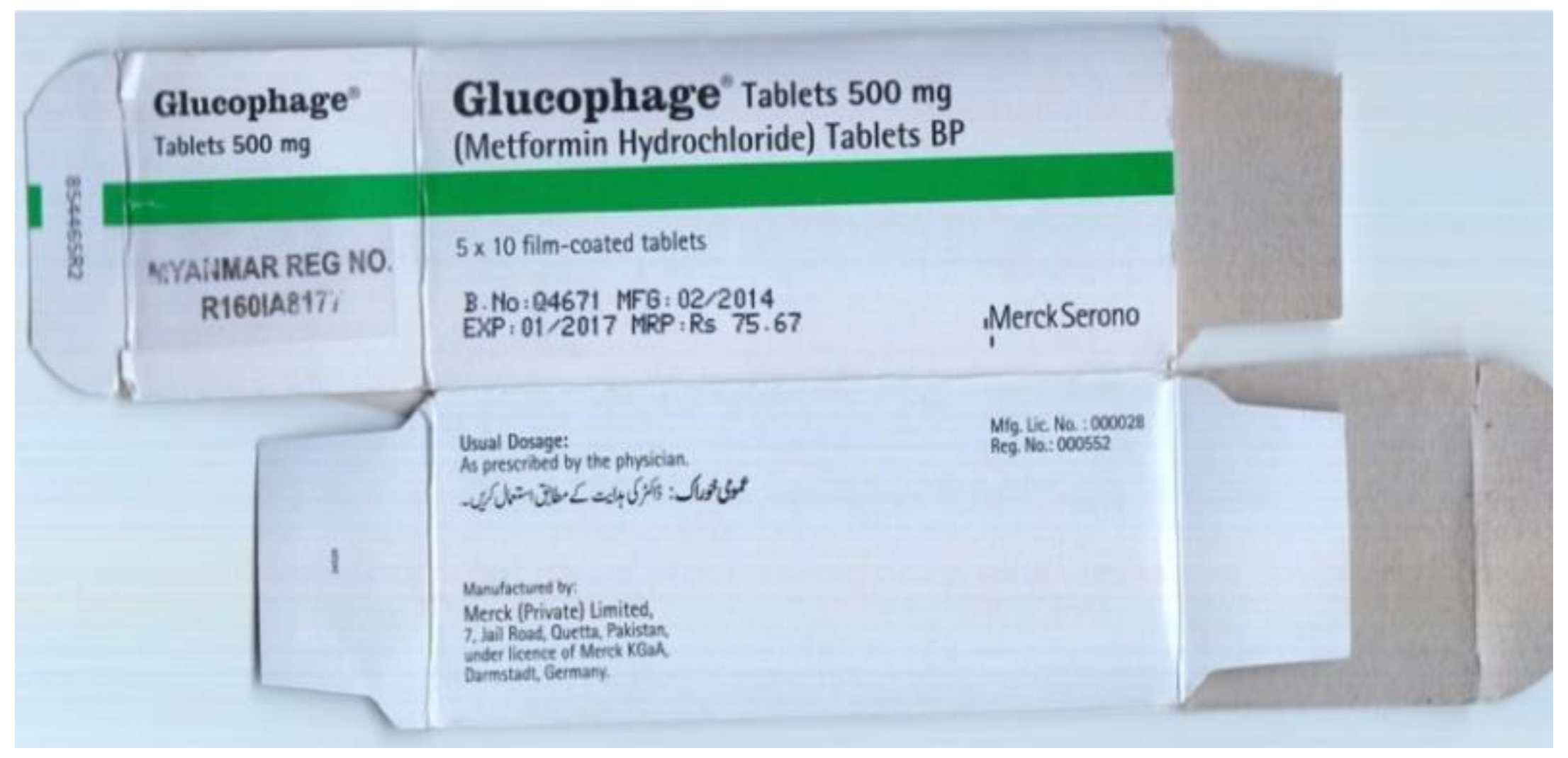
3.2.2. Observation of Samples
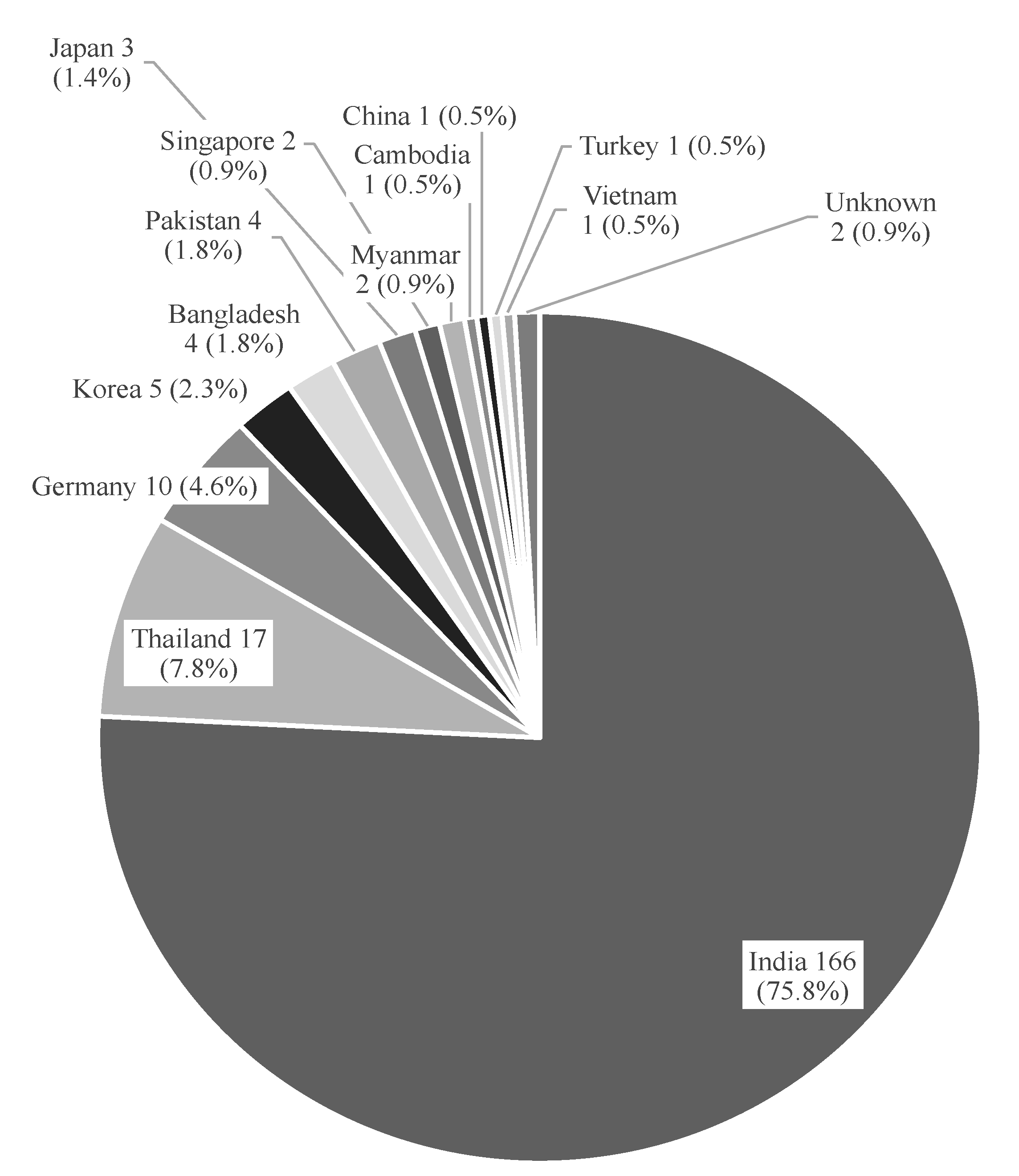
3.3. Authenticity Investigation
3.4. Quality Analysis
3.5. Factors Influencing the Outcome of the Quality Testing
4. Discussion
4.1. Observations
4.2. Authenticity
4.3. Quality Analysis
4.4. Price and Falsification
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO Seventieth World Health Assembly Update, 29 May 2017. Available online: https://www.who.int/news-room/detail/29-05-2017-seventieth-world-health-assembly-update-29-may-2017 (accessed on 27 January 2020).
- Khan, M.H.; Hatanaka, K.; Sovannarith, T.; Nivanna, N.; Casas, L.C.; Yoshida, N.; Tsuboi, H.; Tanimoto, T.; Kimura, K. Effects of packaging and storage conditions on the quality of amoxicillin-clavulanic acid an analysis of Cambodian samples. BMC Pharmacol. Toxicol. 2013, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Kosugi, K.; Sanami, Y.; Hanada, M.; Yoshida, N.; Tsuboi, H. Sample Collection and Testing of Target Essential Medicines from the Private Sector Markets in Four Areas in Cambodia, to Ascertain Their Quality Based on Selected Criteria; WHO Registration 2013/346475-0; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Yoshida, N.; Yuasa, M.; Sovannarith, T.; Dararth, E.; Keila, T.; Kiet, H.B.; Tsuboi, H.; Tanimoto, T.; Kimura, K. A Cross-Sectional Investigation for Verification of Globalization of Falsified Medicines in Cambodia, Indicated by Tablets of Sildenafil Citrate. Pharmacy (Basel) 2019, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Wikipedia. Available online: https://en.wikipedia.org/wiki/Myanmar#Democratic_reforms (accessed on 27 January 2020).
- Wondemagegnehu, E. Counterfeit and Substandard Drugs in Myanmar and Viet Nam, p13 & Annex 3, WHO/EDM/QSM/99.3, Essential Drugs and Other Medicines; World Health Organization: Geneva, Switzerland, 1999; Available online: https://apps.who.int/medicinedocs/pdf/s2276e/s2276e.pdf#search=%276.+Wondemagegnehu+E.%2C+Counterfeit+and+substandard+drugs+in+Myanmar+and+Viet+Nam%2C+p13+%26+Annex+3%2C+WHO%2FEDM%2FQSM%2F99.3%27 (accessed on 27 January 2020).
- Newton, P.; Proux, S.; Green, M.; Smithuis, F.; Rozendaal, J.; Prakongpan, S.; Chotivanich, K.; Mayxay, M.; Looareesuwan, S.; Farrar, J.; et al. Fake artesunate in southeast Asia. Lancet 2001, 357, 1948–1950. [Google Scholar] [CrossRef]
- Newton, P.N.; Fernández, F.M.; Plançon, A.; Mildenhall, D.C.; Green, M.D.; Ziyong, L.; Christophel, E.M.; Phanouvong, S.; Howells, S.; McIntosh, E.; et al. A collaborative epidemiological investigation into the criminal fake artesunate trade in South East Asia. PLoS Med. 2008, 5, e32. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Yoshida, N.; Kimura, K.; Uwatoko, C.; Rahman, M.S.; Kumada, S.; Endo, J.; Ito, K.; Tanimoto, T.; Zin, T.; et al. An Investigation into the Quality of Medicines in Yangon, Myanmar. Pharmacy 2018, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Kakio, T.; Nagase, H.; Takaoka, T.; Yoshida, N.; Hirakawa, J.; Macha, S.; Hiroshima, T.; Ikeda, Y.; Tsuboi, H.; Kimura, K. Survey to Identify Substandard and Falsified Tablets in Several Asian Countries with Pharmacopeial Quality Control Tests and Principal Component Analysis of Handheld Raman Spectroscopy. Am. J. Trop Med. Hyg. 2018, 98, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Global Report on Diabetes. Available online: http://www.who.int/diabetes/global-report/en/ (accessed on 27 January 2020).
- WHO Essential Medicines Selection Myanmar. Available online: http://www.who.int/selection_medicines/country_lists/mmr/en/ (accessed on 14 September 2017).
- Newton, P.N.; Lee, S.J.; Goodman, C.; Fernández, F.M.; Yeung, S.; Phanouvong, S.; Kaur, H.; Amin, A.A.; Whitty, C.J.; Kokwaro, G.O.; et al. Guidelines for Field Surveys of the Quality of Medicines. PLoS Med. 2009, 6, e52. [Google Scholar] [CrossRef] [PubMed]
- FIP Tool for Visual Inspection of Medicines. Available online: http://www.fip.org/files/fip/counterfeit/VisualInspection/A%20tool%20for%20visual%20inspection%20of%20medicines%20EN.pdf (accessed on 27 January 2020).
- The United States Pharmacopeial Convention. United States Pharmacopeia 37; The United States Pharmacopeial Convention 12601 Twinbrook Parkway: Rockville, MD, USA, 2014; pp. 2103–2105, 2352–2354, 3536–3538, 3729–3741, 4308–4311. [Google Scholar]
- British Pharmacopeia Commission. British Pharmacopoeia 2015; Stationary Office: London, UK, 2015; Volume 1, pp. 393–395, 563–565; Volume 2, pp. 585–586. [Google Scholar]
- Pharmaceutical and Medical Device Regulatory Science Society of Japan. Japanese Pharmacopoeia, 17th ed.; Jiho, Inc.: Kanda Sarugaku Chiyoda, Tokyo, Japan, 2016; pp. 663–665, 851–854, 1271–1273, 1600–1601, 1706–1710. [Google Scholar]
- Samanidou, V.F.; Demetriou, C.E.; Papadoyannis, I.N. Direct determination of four fluoroquinolones.; enoxacin.; norfloxacin.; ofloxacin.; and ciprofloxacin.; in pharmaceuticals and blood serum by HPLC. Anal. Bioanal. Chem. 2003, 375, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Japan Meteorological Agency. Available online: http://www.data.jma.go.jp (accessed on 27 January 2020).
- Fotiou, F.; Aravind, S.; Wang, P.P.; Nerapusee, O. Impact of illegal trade on the quality of epoetin alfa in Thailand. Clin. Ther. 2009, 31, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Swinden, J.; Donyai, P. Pilot study of the short-term physico-chemical stability of atenolol tablets stored in a multi-compartment compliance aid. Eur. J. Hosp. Pharm. Sci. 2007, 13, 60–66. [Google Scholar]
- Chen, Y.C.; Ho, H.O.; Liu, D.Z.; Siow, W.S.; Sheu, M.T. Swelling/floating capability and drug release characterizations of gastroretentive drug delivery system based on a combination of hydroxyethyl cellulose and sodium carboxymethyl cellulose. PLoS ONE 2015, 10, e0116914. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Yoshida, N.; Tsuboi, H.; Keila, T.; Sovannarith, T.; Kiet, H.B.; Dararth, E.; Zin, T.; Tanimoto, T.; Kimura, K. Erroneous formulation of delayed-release omeprazole capsules: Alert for importing countries. BMC Pharmacol. Toxicol. 2017, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Hisada, N.; Takano, R.; Takata, N.; Shiraki, K.; Ueto, T.; Tanida, S.; Kataoka, M.; Yamashita, S. Characterizing the dissolution profiles of supersaturable salts.; cocrystals.; and solvates to enhance in vivo oral absorption. Eur. J. Pharm. Biopharm. 2016, 103, 192–199. [Google Scholar] [CrossRef] [PubMed]

| Active Ingredient | Candesartan (8 mg) | Ciprofloxacin Infusion (200 mg/100 mL) | Levofloxacin Infusion (500 mg/100 mL) | Metformin IR (500 mg) | Metformin ER (500 mg) | Pioglitazone (15 mg) | Pioglitazone (30 mg) | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Number of samples | 10 | 45 | 42 | 49 | 13 | 59 | 1 | 219 | |
| Number of shops | 8 | 44 | 37 | 51 | 49 | 91 | |||
| Number of samples collected at sampling site | Private hospital (n = 22) | 2 | 17 | 20 | 8 | 6 | 9 | 0 | 62 |
| Clinical pharmacy (n = 7) | 0 | 2 | 3 | 9 | 0 | 1 | 0 | 15 | |
| Community pharmacy (n = 46) | 3 | 16 | 12 | 19 | 2 | 34 | 0 | 86 | |
| Wholesaler with community pharmacy (n = 12) | 4 | 6 | 5 | 9 | 1 | 11 | 1 | 37 | |
| Wholesaler (n = 5) | 1 | 4 | 2 | 4 | 4 | 4 | 0 | 19 | |
| Medicine | Registered | Non-Registered |
|---|---|---|
| Candesartan (n = 10) | 10 (100%) | 0 (0%) |
| Ciprofloxacin infusion (n = 45) | 43 (96%) | 2 (4%) * |
| Levofloxacin infusion (n = 42) | 42 (100%) | 0 (0%) |
| Metformin (n = 62) | 62 (100%) | 0 (0%) |
| Pioglitazone (n = 60) | 59 (98%) | 1 (2%) |
| Total (n = 219) | 219 (99%) | 3 (1%) |
| Quantity Test | Content Uniformity Test | Dissolution Test | Sterility | Endotoxin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medicine | Pass | Fail | Pass | Fail | Pending | Pass | Fail | Pending | Pass | Fail | Pass | Fail |
| Candesartan (n = 10) | 9 | 1 | 10 | 0 | 0 | 10 | 0 | 0 | n.a. | n.a. | n.a. | n.a. |
| Ciprofloxacin (n = 44) * | 38 | 6 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 44 | 0 | 44 | 0 |
| Levofloxacin (n = 42) ** | 28 | 14 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 40 | 0 | 42 | 0 |
| Metformin IR (n = 49) | 49 | 0 | 49 | 0 | 0 | 47 | 1 | 1 | n.a. | n.a. | n.a. | n.a. |
| Metformin ER (n = 13) | 8 | 5 | 10 | 0 | 3 | 4 | 0 | 9 | n.a. | n.a. | n.a. | n.a. |
| Pioglitazone (n = 60) | 59 | 1 | 59 | 0 | 1 | 52 | 8 | 0 | n.a. | n.a. | n.a. | n.a. |
| Total | 191 | 27 | 128 | 0 | 4 | 113 | 9 | 10 | 84 | 0 | 86 | 0 |
| All Pass | Any Fail | Pending | |
|---|---|---|---|
| Private hospital | 48 | 9 | 4 |
| Clinical pharmacy | 12 | 3 | 0 |
| Community pharmacy | 73 | 12 | 1 |
| Wholesaler with community pharmacy | 31 | 6 | 0 |
| Wholesaler | 13 | 5 | 1 |
| N | Mean ± SD | p Value | ||
|---|---|---|---|---|
| Candesartan (generic) | All pass | 6 | 0.125 ± 0.0186 | n.t. |
| Fail | 1 | 0.141 | ||
| Ciprofloxacin | All pass | 38 | 0.387 ± 0.177 | n.s. |
| Fail | 6 | 0.355 ± 0.134 | ||
| LVFX (generic) | All pass | 26 | 2.42 ± 0.435 | n.s. |
| Fail | 14 | 2.20 ± 0.368 | ||
| Metformin IR (generic) | All pass | 46 | 0.0383 ± 0.0254 | n.t. |
| Fail | 2 | 0.0430 ± 0.0387 | ||
| Metformin ER | All pass | 3 | 0.0324 ± 0.0116 | <0.05 |
| Any fail | 5 | 0.0559 ± 0.0128 | ||
| Pioglitazone (15 mg) | All pass | 51 | 0.0709 ± 0.0152 | <0.05 |
| Any fail | 8 | 0.0550 ± 0.0079 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakuda, M.; Yoshida, N.; Takaoka, T.; Sanada, T.; Rahman, M.S.; Tanimoto, T.; Zin, T.; Kimura, K.; Tsuboi, H. Substandard and Falsified Medicines in Myanmar. Pharmacy 2020, 8, 45. https://doi.org/10.3390/pharmacy8010045
Sakuda M, Yoshida N, Takaoka T, Sanada T, Rahman MS, Tanimoto T, Zin T, Kimura K, Tsuboi H. Substandard and Falsified Medicines in Myanmar. Pharmacy. 2020; 8(1):45. https://doi.org/10.3390/pharmacy8010045
Chicago/Turabian StyleSakuda, Mirai, Naoko Yoshida, Takashi Takaoka, Tomoko Sanada, Mohammad Sofiqur Rahman, Tsuyoshi Tanimoto, Theingi Zin, Kazuko Kimura, and Hirohito Tsuboi. 2020. "Substandard and Falsified Medicines in Myanmar" Pharmacy 8, no. 1: 45. https://doi.org/10.3390/pharmacy8010045
APA StyleSakuda, M., Yoshida, N., Takaoka, T., Sanada, T., Rahman, M. S., Tanimoto, T., Zin, T., Kimura, K., & Tsuboi, H. (2020). Substandard and Falsified Medicines in Myanmar. Pharmacy, 8(1), 45. https://doi.org/10.3390/pharmacy8010045





