Clinical Pharmacy Activities in Swiss Hospitals: How Have They Evolved from 2013 to 2017?
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey
2.2. Data Analysis
3. Results
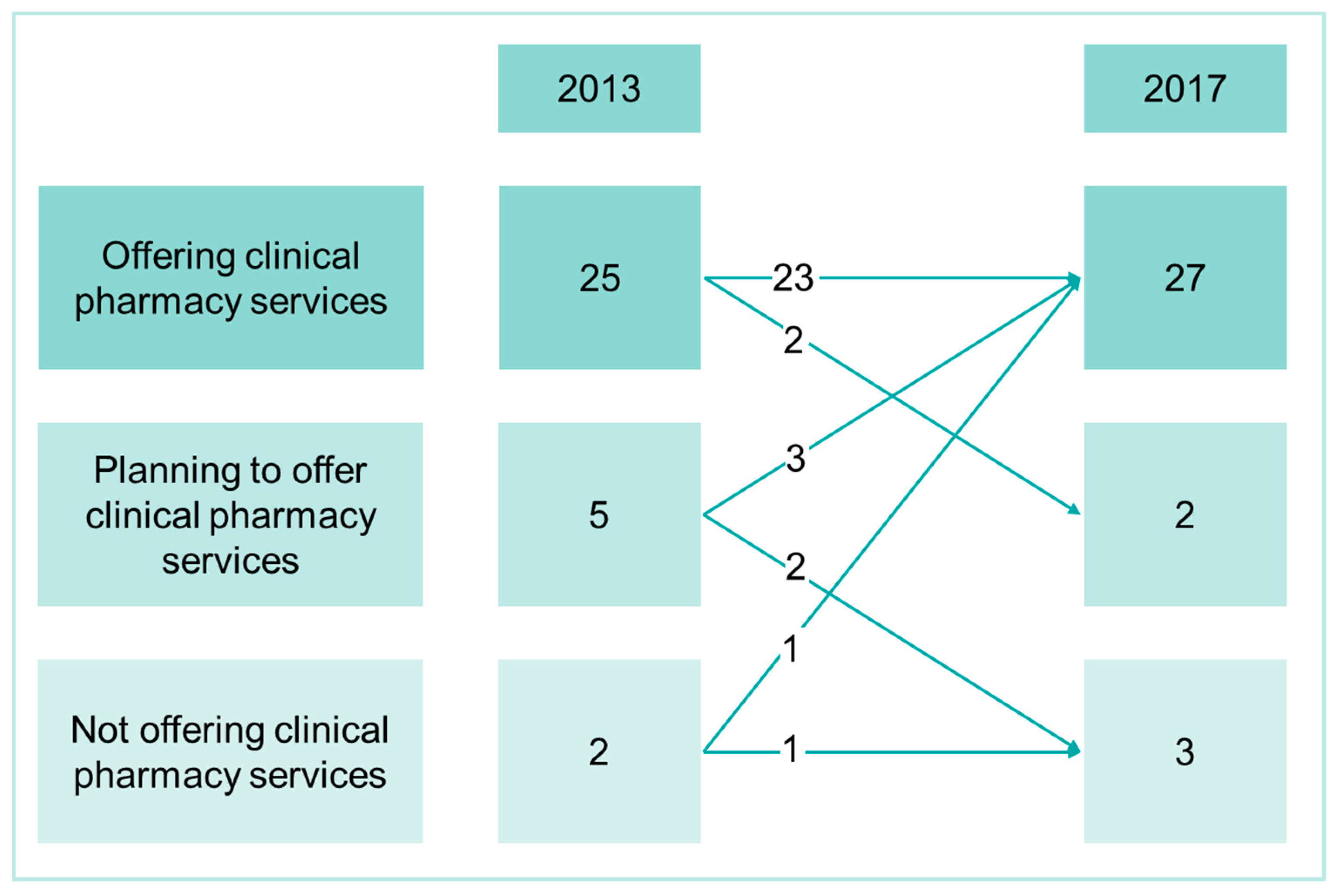
3.1. Extent of Clinical Pharmacy Activities and Human Resources
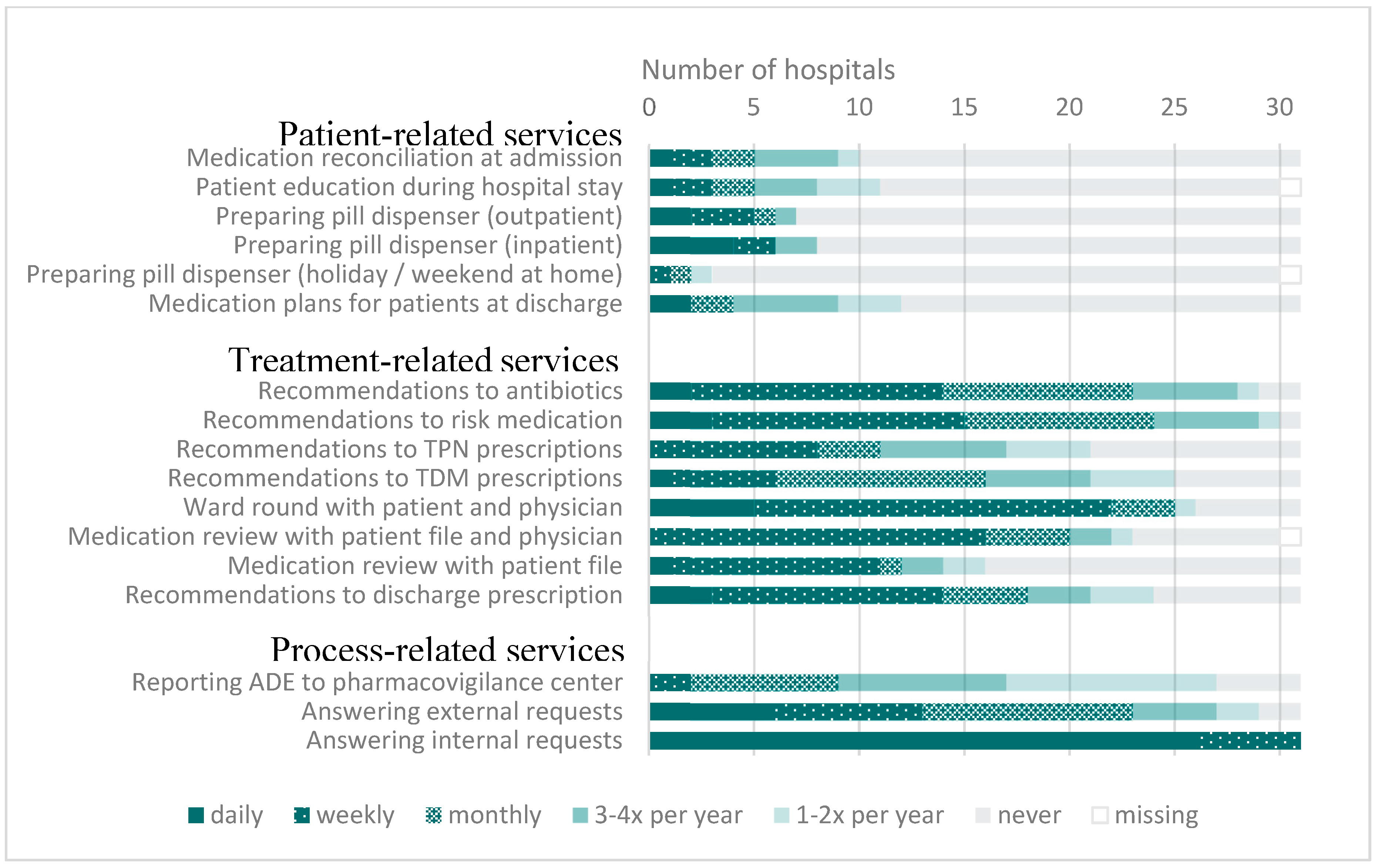
3.2. Time and Organization of Patient-Oriented Activities
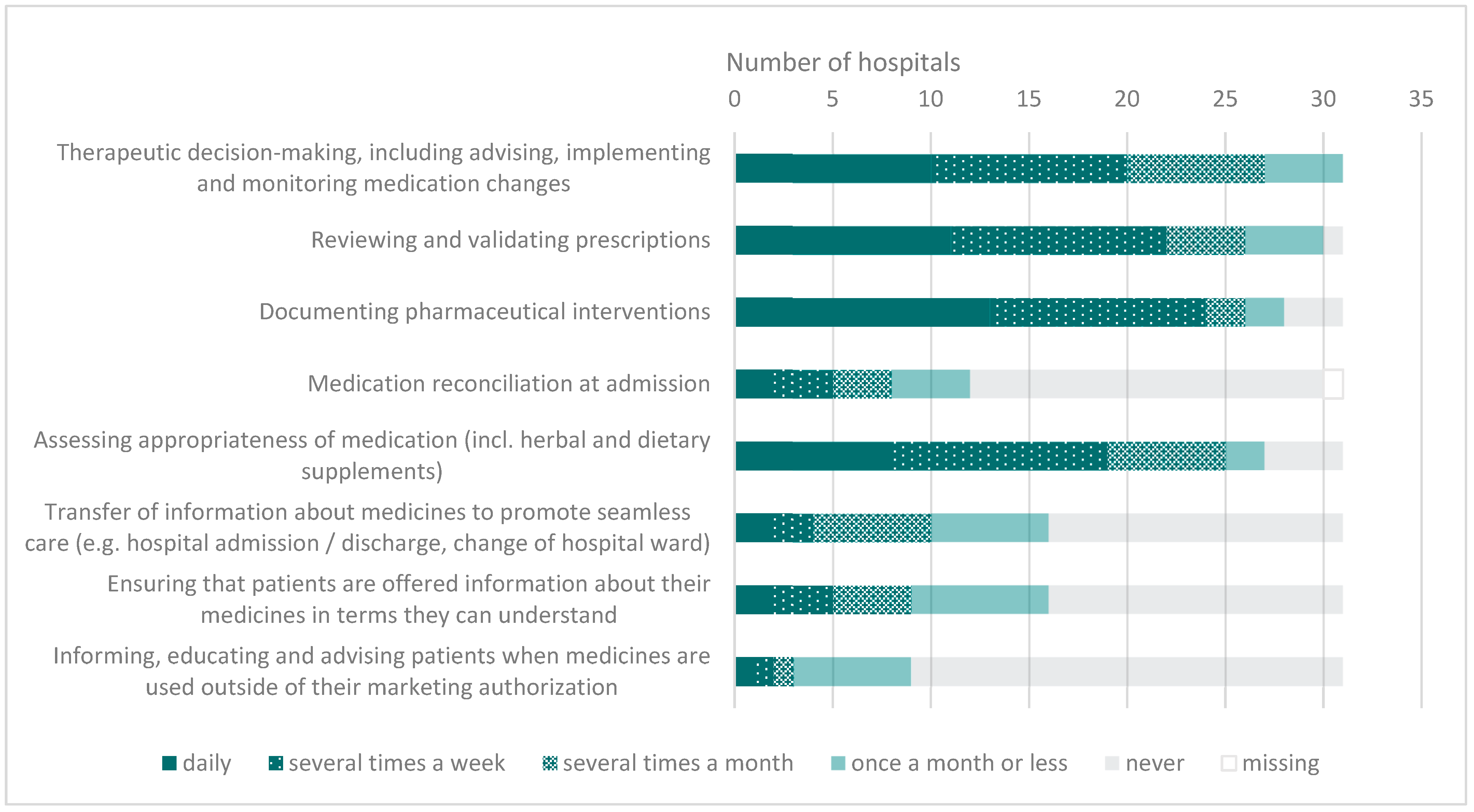
3.3. Implementation of Activities Related to the EAHP Statements in Clinical Pharmacy
4. Discussion
4.1. Development of Clinical Pharmacy in Swiss Hospitals
4.2. Comparison to International Community
4.3. Activities Related to EAHP Statements
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Joint FIP/WHO Guidelines on Good Pharmacy Practice: Standards for Quality of Pharmacy Services. Available online: https://apps.who.int/medicinedocs/documents/s18676en/s18676en.pdf (accessed on 20 January 2020).
- International Pharmaceutical Federation. Revised FIP Basel Statements on the Future of Hospital Pharmacy. Available online: https://www.fip.org/files/content/pharmacy-practice/hospital-pharmacy/hospital-activities/basel-statements/fip-basel-statements-on-the-future-of-hospital-pharmacy-2015.pdf (accessed on 20 January 2020).
- European Association of Hospital Pharmacists. European Statements of Hospital Pharmacy. Available online: https://statements.eahp.eu/ (accessed on 7 February 2020).
- European Association of Hospital Pharmacists. The European Statements of Hospital Pharmacy. Eur. J. Hosp. Pharm. 2014, 21, 256–258. [Google Scholar] [CrossRef]
- Schweizerischer Verein der Amts- und Spitalapotheker. Definition of Clinical Pharmacy in a Hospital Setting according to the GSASA. Available online: https://www.gsasa.ch/de/aktivitaeten/pharmazeutische-dienstleistungen/klinische-aktivitaeten/?oid=10135&lang=de (accessed on 1 November 2019).
- Fishman, L.; Bruhwiler, L.; Schwappach, D. Medication safety in Switzerland: Where are we today? Medikationssicherheit: Wo steht die Schweiz? Bundesgesundheitsblatt Gesundh. Gesundh. 2018, 61, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Vincent, C.; Staines, A. Enhancing the Quality and Safety of Swiss Healthcare. Available online: https://www.bag.admin.ch/bag/en/home/versicherungen/krankenversicherung/krankenversicherung-qualitaetssicherung.html (accessed on 30 January 2020).
- Messerli, M.; Maes, K.A.; Hersberger, K.E.; Lampert, M.L. Mapping clinical pharmacy practice in Swiss hospitals: A cross-sectional study. Eur J Hosp Pharm 2016, 23, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Maes, K.A.; Tremp, R.M.; GSASA Working Group on Clinical Pharmacy; Hersberger, K.E.; Lampert, M.L. Demonstrating the clinical pharmacist’s activity: Validation of an intervention oriented classification system. Int. J. Clin. Pharm. 2015, 37, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, A.B.; McLachlan, A.J.; Brien, J.A. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: A systematic review and meta-analysis. BMJ Open 2016, 6, e010003. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, U.; Alassaad, A.; Henrohn, D.; Garmo, H.; Hammarlund-Udenaes, M.; Toss, H.; Kettis-Lindblad, A.; Melhus, H.; Morlin, C. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: A randomized controlled trial. Arch. Int. Med. 2009, 169, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Horak, P.; Underhill, J.; Batista, A.; Amann, S.; Gibbons, N. EAHP European Statements Survey 2017, focusing on sections 2 (Selection, Procurement and Distribution), 5 (Patient Safety and Quality Assurance) and 6 (Education and Research). Eur. J. Hosp. Pharm. 2018, 25, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Horák, P.; Gibbons, N.; Sýkora, J.; Batista, A.; Underhill, J. EAHP statements survey 2016: Sections 1, 3 and 4 of the European Statements of Hospital Pharmacy. Eur. J. Hosp. Pharm. 2017, 24, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Fischer, A.; Vogt, W.; Leichenberg, K.; Warnke, U.; Liekweg, A.; Georgi, U.; Langebrake, C.; Hoppe-Tichy, T.; Dörje, F.; et al. Clinical pharmacy services in Germany: A national survey. Eur. J. Hosp. Pharm. 2019. [Google Scholar] [CrossRef]
- Somers, A.; Claus, B.; Vandewoude, K.; Petrovic, M. Experience with the Implementation of Clinical Pharmacy Services and Processes in a University Hospital in Belgium. Drugs Aging. 2016, 33, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, A.B.; McLachlan, A.J.; Brien, J.A. Pharmacy-led medication reconciliation programmes at hospital transitions: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2016, 41, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Cornu, P.; Steurbaut, S.; Leysen, T.; De Baere, E.; Ligneel, C.; Mets, T.; Dupont, A.G. Effect of medication reconciliation at hospital admission on medication discrepancies during hospitalization and at discharge for geriatric patients. Ann Pharm. 2012, 46, 484–494. [Google Scholar] [CrossRef] [PubMed]
| EAHP Statements on Clinical Pharmacy Services |
|---|
| 4.1 Hospital pharmacists should be involved in all patient care settings to prospectively influence collaborative, multidisciplinary therapeutic decision-making; they should play a full part in decision making, including advising, implementing and monitoring medication changes in full partnership with patients, carers and other health care professionals. |
| 4.2 All prescriptions should be reviewed and validated as soon as possible by a hospital pharmacist. Whenever the clinical situation allows, this review should take place prior to the supply and administration of medicines. |
| 4.3 Hospital pharmacists should have access to the patients’ health record. Their clinical interventions should be documented in the patients’ health record and analysed to inform quality improvement interventions. |
| 4.4 All the medicines used by patients should be entered on the patient’s medical record and reconciled by the hospital pharmacist on admission. Hospital pharmacists should assess the appropriateness of all patients’ medicines, including herbal and dietary supplements. |
| 4.5 Hospital pharmacists should promote seamless care by contributing to the transfer of information about medicines whenever patients move between and within healthcare settings. |
| 4.6 Hospital pharmacists, as an integral part of all patient care teams, should ensure that patients and carers are offered information about their clinical management options, and especially about the use of their medicines, in terms they can understand. |
| 4.7 Hospital pharmacists should inform, educate and advise patients, carers and other health care professionals when medicines are used outside of their marketing authorisation. |
| 4.8 Clinical pharmacy services should continuously evolve to optimise patients’ outcomes. |
| Hospitals | FTETotPharm * | Median Number of Beds (IQR) ** | Frequency by Hospital Type | |
|---|---|---|---|---|
| Offering clinical pharmacy services (n = 31) | 230.1 | 460.0 (950) | University hospital | 5 |
| Cantonal or regional hospital | 10 | |||
| Private hospital or specialized clinic | 7 | |||
| Networks | 9 | |||
| Planning to offer clinical pharmacy services (n = 4) | 18.7 | 370.0 (275.0) | University hospital | 0 |
| Cantonal or regional hospital | 3 | |||
| Private hospital or specialized clinic | 1 | |||
| Networks | 0 | |||
| Not offering clinical pharmacy services (n = 9) | 17.0 | 180.0 (138.5) | University hospital | 0 |
| Cantonal or regional hospital | 5 | |||
| Private hospital or specialized clinic | 3 | |||
| Networks | 1 | |||
| All Participating Hospitals 2017, n = 44 | ||||
|---|---|---|---|---|
| FTETotPharm * | Average FTETotPharm per 100 beds | FTEClinPharm ** | % FTEClinPharm of FTETotPharm | |
| Total | 265.8 (n = 44) | 1.12 | 54.1 (n = 28, nmissing = 3) | 20.4 % |
| University hospital | 81.3 (n = 5) | 1.02 | 19.6 (n = 5) | 24.1 |
| Cantonal or regional hospital | 66.2 (n = 18) | 0.97 | 7.6 (n = 10) | 11.5 |
| Private hospital or specialized clinic | 27.4 (n = 11) | 1.53 | 2.4 (n = 4, nmissing = 3) | 8.8 |
| Networks | 91.1 (n = 10) | 0.99 | 24.5 (n = 9) | 26.9 |
| German-speaking | 169.5 (n = 35) | 1.12 | 31.9 (n = 20, nmissing = 2) | 18.8 % |
| French or Italian-speaking | 96.3 (n = 9) | 1.12 | 22.2 (n = 8, nmissing = 1) | 23.1 % |
| 2013 (n) | 2017 (n) | Difference (%) | p Value † | |
|---|---|---|---|---|
| Hospitals that participated in both surveys 2013 and 2017, n = 32 | ||||
| FTETotPharm * | 196.4 (n = 32) | 244.6 (n = 32) | +48.2 (+24.5%) | <0.01 |
| University hospital | 80.0 (n = 5) | 81.3 (n = 5) | +1.3 (+1.6%) | 0.50 |
| Cantonal or regional hospital | 44.1 (n = 12) | 58.4 (n = 12) | +14.3 (+32.4) | 0.02 |
| Private hospital or specialized clinic | 13.5 (n = 7) | 18.5 (n = 7) | +5.0 (+37.0) | 0.09 |
| Networks | 58.8 (n = 8) | 86.6 (n = 8) | +27.8 (+47.3) | 0.01 |
| German-speaking | 121.7 (n = 23) | 148.3 (n = 23) | +26.6 (+21.9%) | <0.01 |
| French or Italian-speaking | 74.7 (n = 9) | 96.30 (n = 9) | +21.6 (+28.9%) | 0.01 |
| FTEClinPharm ** | 31.1 (n = 32) | 50.6 (n = 29 nmissing = 3) | +19.5 (+62.7%) | 0.01 |
| University hospital | 11.4 (n = 5) | 19.6 (n = 5) | +8.2 (+71.9%) | 0.14 |
| Cantonal or regional hospital | 5.5 (n = 12) | 7.6 (n = 12) | +2.1 (+38.2) | 0.62 |
| Private hospital or specialized clinic | 1.2 (n = 7) | 2.2 (n = 4, nmissing = 3) | + 1.0 (+83.3) | 0.65 |
| Networks | 13.0 (n = 8) | 21.2 (n = 8) | + 8.2 (+63.1) | 0.09 |
| German-speaking | 13.4 (n = 23) | 28.4 (n = 21, nmissing = 2) | +15.0 (+111.9%) | 0.06 |
| French or Italian-speaking | 17.7 (n = 9) | 22.2 (n = 8, nmissing = 1) | +4.5 (25.4%) | 0.09 |
| Hospitals that offered clinical pharmacy services in both surveys 2013 and 2017, n = 23 | ||||
| FTETotPharm | 171.8 (n = 23) | 208.5 (n = 23) | +36.7 (+21.4%) | <0.01 |
| FTEClinPharm | 30.6 (n = 23) | 45.6 (n = 22, nmissing = 1) | +15.0 (+49.0%) | 0.03 |
| % FTEClinPharm of FTETotPharm | 17.8% | 21.9% | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Studer, H.; Boeni, F.; Messerli, M.; Hersberger, K.E.; Lampert, M.L. Clinical Pharmacy Activities in Swiss Hospitals: How Have They Evolved from 2013 to 2017? Pharmacy 2020, 8, 19. https://doi.org/10.3390/pharmacy8010019
Studer H, Boeni F, Messerli M, Hersberger KE, Lampert ML. Clinical Pharmacy Activities in Swiss Hospitals: How Have They Evolved from 2013 to 2017? Pharmacy. 2020; 8(1):19. https://doi.org/10.3390/pharmacy8010019
Chicago/Turabian StyleStuder, Helene, Fabienne Boeni, Markus Messerli, Kurt E. Hersberger, and Markus L. Lampert. 2020. "Clinical Pharmacy Activities in Swiss Hospitals: How Have They Evolved from 2013 to 2017?" Pharmacy 8, no. 1: 19. https://doi.org/10.3390/pharmacy8010019
APA StyleStuder, H., Boeni, F., Messerli, M., Hersberger, K. E., & Lampert, M. L. (2020). Clinical Pharmacy Activities in Swiss Hospitals: How Have They Evolved from 2013 to 2017? Pharmacy, 8(1), 19. https://doi.org/10.3390/pharmacy8010019






