Validation of a Novel Electronic Device for Medication Adherence Monitoring of Ambulatory Patients
Abstract
:1. Introduction
2. Materials and Methods
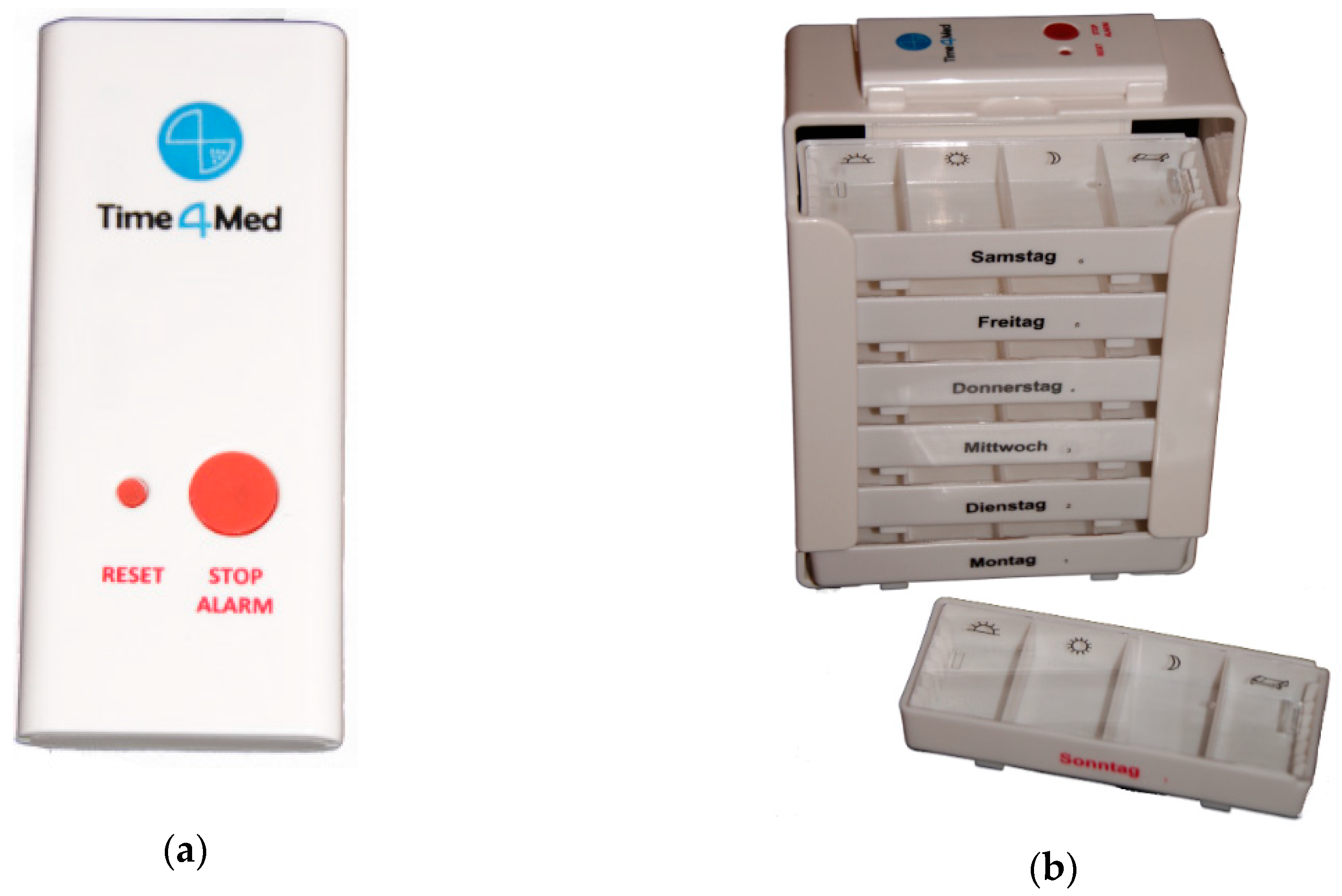
2.1. Time4MedTM Design
2.2. Validation Study
- Field: 20 smart cards were used at home by non-medicating volunteers.
- Purse: 2 smart cards were carried in women personal purses, without further protection.
- Dropped: 20 smart cards were used, then dropped from a height of 1.2 m once, and then used again once.
- Post mail: 2 smart cards were packed in a plastic protective case (135 × 99 ×18 mm; Renfer GmbH, Lengnau, Switzerland; see Figure 3), put into a common A5 envelope and post mailed.
- Refrigerator: 20 smart cards were stored in a common refrigerator at 2–12 °C.
3. Results
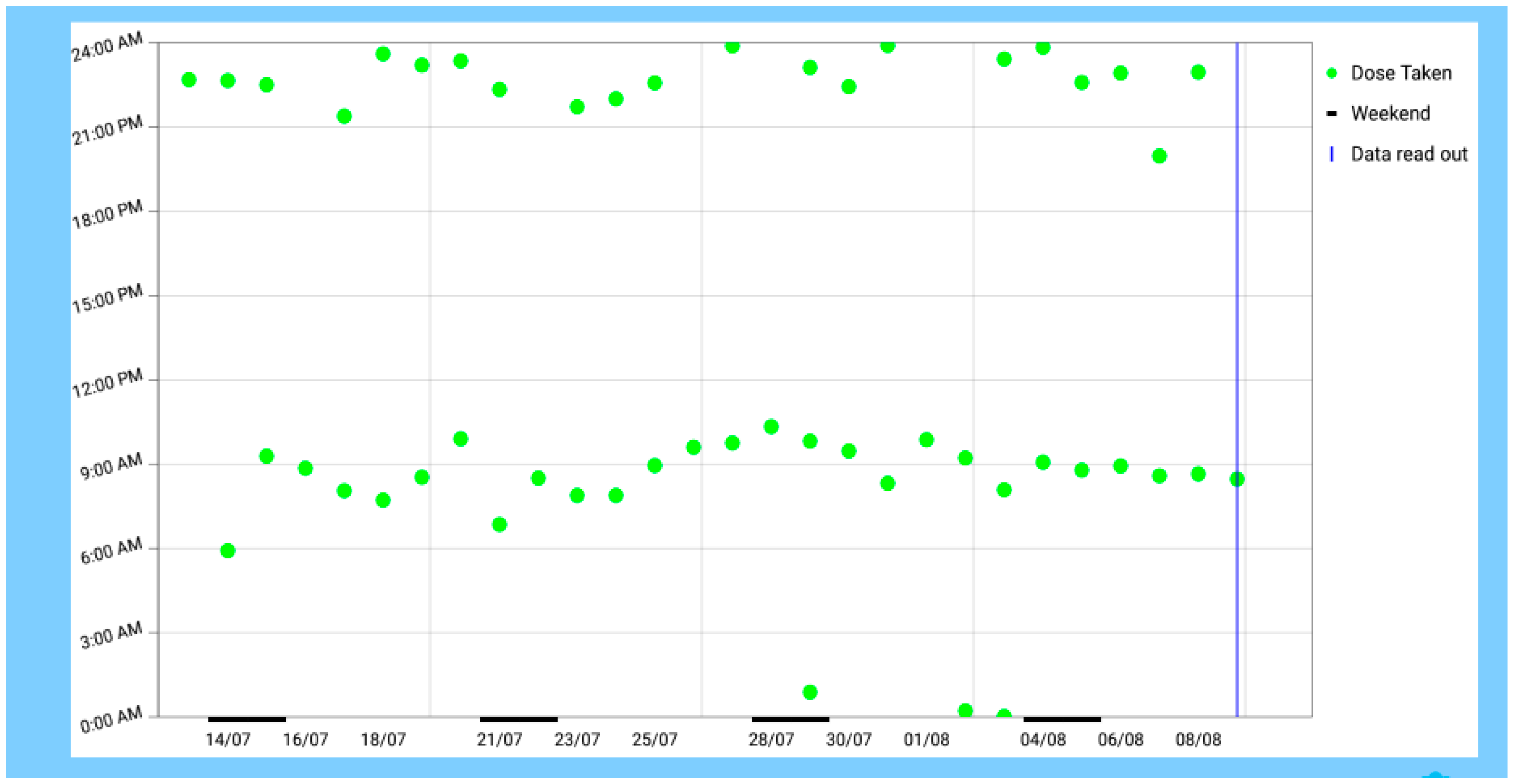
3.1. Field Condition
3.2. Purse
3.3. Dropped
3.4. Post Mail
3.5. Refrigerator
3.6. Summertime Conditions
3.7. Acceptability
3.8. Usability
3.9. Metrics
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| System Usability Scale | Fragebogen zur System-Gebrauchstauglichkeit |
|---|---|
| I think that I would like to use this system frequently. | Ich denke, dass ich das System gerne häufig benutzen würde. |
| I found the system unnecessarily complex. | Ich fand das System unnötig komplex. |
| I thought the system was easy to use. | Ich fand das System einfach zu benutzen. |
| I think that I would need the support of a technical person to be able to use this system. | Ich glaube, ich würde die Hilfe einer technisch versierten Person benötigen, um das System benutzen zu können. |
| I found the various functions in this system were well integrated. | Ich fand, die verschiedenen Funktionen in diesem System waren gut integriert. |
| I thought there was too much inconsistency in this system. | Ich denke, das System enthielt zu viele Inkonsistenzen. |
| I would imagine that most people would learn to use this system very quickly. | Ich kann mir vorstellen, dass die meisten Menschen den Umgang mit diesem System sehr schnell lernen. |
| I found the system very cumbersome to use. | Ich fand das System sehr umständlich zu nutzen. |
| I felt very confident using the system. | Ich fühlte mich bei der Benutzung des Systems sehr sicher. |
| I needed to learn a lot of things before I could get going with this system. | Ich musste eine Menge lernen, bevor ich anfangen konnte das System zu verwenden. |
References
- Osterberg, L.; Blaschke, T. Adherence to medication. N. Engl. J. Med. 2005, 353, 487–497. [Google Scholar] [CrossRef] [PubMed]
- van Onzenoort, H.A.; Neef, C.; Verberk, W.W.; van Iperen, H.P.; de Leeuw, P.W.; van der Kuy, P.-H.M. Determining the feasibility of objective adherence measurement with blister packaging smart technology. Am. J. Health Syst. Pharm. 2012, 69, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Gregoriano, C.; Dieterle, T.; Dürr, S.; Arnet, I.; Hersberger, K.E.; Leuppi, J.D. Impact of an electronic monitoring intervention to improve adherence to inhaled medication in patients with asthma and chronic obstructive pulmonary disease: Study protocol for a randomized controlled trial. JMIR Res. Protoc. 2017, 6, e204. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.J.; Ghosh-Dastidar, B. Electronic monitoring: Adherence assessment or intervention? HIV Clin. Trials 2002, 3, 45–51. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Heath and Care Excellence NICE. Smartinhaler for Asthma—Medtech Innovation Briefing. 2017. Available online: https://www.nice.org.uk/advice/mib90/resources/smartinhaler-for-asthma-pdf-63499461673669 (accessed on 26 March 2018).
- World Health Organization WHO. mHealth—New Horizons for Health through Mobile Technologies. WHO Library Cataloguing-in-Publication Data. 2017. Available online: http://www.who.int/goe/publications/goe_mhealth_web.pdf (accessed on 26 March 2018).
- Food and Drug Administration FDA. Mobile Medical Applications—Guidance for Industry Food Drug Administration Staff. 2015. Available online: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM263366.pdf (accessed on 26 March 2018).
- Santo, K.; Richtering, S.S.; Chalmers, J.; Thiagalingam, A.; Chow, C.K.; Redfern, J. Mobile phone apps to improve medication adherence: A systematic stepwise process to identify high-quality apps. JMIR mHealth uHealth 2016, 2, e132. [Google Scholar] [CrossRef] [PubMed]
- Dehling, T.; Gao, F.; Schneider, S.; Sunyaev, A. Exploring the far side of mobile health: Information security and privacy of mobile health apps on iOS and Android. JMIR mHealth uHealth 2015, 3, e8. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.M.; Cadogan, C.A.; Patton, D.; Ryan, C.A. Pharmaceutical strategies towards optimising polypharmacy in older people. Int. J. Pharm. 2016, 512, 360–365. [Google Scholar] [CrossRef]
- De Bleser, L.; De Geest, S.; Vincke, B.; Ruppar, T.; Vanhaecke, J.; Dobbels, F. How to test electronic adherence monitoring devices for use in daily life: A conceptual framework. Comput. Inform. Nurs. 2011, 29, 489–495. [Google Scholar] [CrossRef]
- Jekle, C.; Krämer, I. OtCM (Objective therapy Compliance Measurement): Smart blister packages for measuring patient compliance. Hosp. Pharm. Eur. 2008, 40, 47–50. [Google Scholar]
- Chu, K. An introduction to sensitivity, specificity, predictive values and likelihood ratios. Emerg. Med. 1999, 11, 175–181. [Google Scholar] [CrossRef]
- Chan, A.H.Y.; Stewart, A.W.; Harrison, J.; Black, P.N.; Mitchell, E.A.; Foster, J.M. Electronic adherence monitoring device performance and patient acceptability: A randomized control trial. Expert Rev. Med. Devices 2017, 14, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Brooke, S. SUS—A retrospective. J. Usability Stud. 2013, 8, 29–40. [Google Scholar]
- Rummel, B. System Usability Scale—jetzt auch auf Deutsch. 2015. Available online: https://experience.sap.com/files/System_Usability_Scale_A4_DE.doc (accessed on 28 June 2018). (In German).
- Bangor, A.; Kortum, P.; Miller, J. Determining what individual SUS scores mean: Adding an adjective rating scale. J. Usability Stud. 2009, 4, 114–123. [Google Scholar]
- Polymeris, A.A.; Albert, V.; Hersberger, K.E.; Engelter, S.T.; Schaedelin, S.; Arnet, I. Protocol for MAAESTRO: Electronic Monitoring and improvement of Adherence to direct oral Anticoagulant treatment-a randomized crossover study of an Educational and reminder-based intervention in ischemic STROke patients under polypharmacy. Front. Neurol. 2018, 9, 1134. [Google Scholar] [CrossRef] [PubMed]
- Hartman, L.; Lems, W.F.; Boers, M. Outcome measures for adherence data from a medication event monitoring system: A literature review. J. Clin. Pharm. Ther. 2019, 44, 1–5. [Google Scholar] [CrossRef]
- Herzer, M.; Ramey, C.; Rohan, J.; Cortina, S. Incorporating electronic monitoring feedback into clinical care: A novel and promising adherence promotion approach. Clin. Child. Psychol. Psy. 2012, 17, 505–518. [Google Scholar] [CrossRef]
- van Bruggen, R.; Gorter, K.; Stolk, R.P.; Zuithoff, P.; Klungel, O.H.; Rutten, G.E.H.M. Refill adherence and polypharmacy among patients with type 2 diabetes in general practice. Pharmacoepidemiol. Drug Saf. 2009, 18, 983–991. [Google Scholar] [CrossRef]
- Zeller, A.; Schroeder, K.; Peters, T.J. An adherence self-report questionnaire facilitated the differentiation between nonadherence and nonresponse to antihypertensive treatment. J. Clin. Epidemiol. 2008, 61, 282–288. [Google Scholar] [CrossRef]
- Schmitz, J.M.; Sayre, S.L.; Stotts, A.L.; Rothfleisch, J.; Mooney, M.E. Medication compliance during a smoking cessation clinical trial: A brief intervention using MEMS feedback. J. Behav. Med. 2005, 28, 139–147. [Google Scholar] [CrossRef]
- Sulaiman, I.; Seheult, J.; MacHale, E.; Boland, F.; O’Dwyer, S.M.; Rapcan, V. A method to calculate adherence to inhaled therapy that reflects the changes in clinical features of asthma. Ann. Am. Thorac. Soc. 2016, 13, 1894–1903. [Google Scholar] [CrossRef]
- Arnet, I.; Walter, P.N.; Hersberger, K.E. Polymedication Electronic Monitoring System (POEMS)—A new technology for measuring adherence. Front. Pharm. 2013, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- De Bleser, L.; Vincke, B.; Dobbels, F. A new electronic monitoring device to measure medication adherence: Usability of the Helping Hand™. Sensors 2010, 10, 1535–1552. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.M.; Smith, L.; Usherwood, T.; Sawyer, S.M.; Rand, C.S.; Reddel, H.K. The reliability and patient acceptability of the SmartTrack device: A new electronic monitor and reminder device for metered dose inhalers. J. Asthma 2012, 49, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Shtrichman, R.; Conrad, S.; Schimo, K.; Shachar, R.; Machluf, E.; Mindal, E. Use of a digital medication management system for effective assessment and enhancement of patient adherence to therapy (ReX): Feasibility study. JMIR Hum. Factors 2018, 5, e10128. [Google Scholar] [CrossRef] [PubMed]
- Bionghi, N.; Daftary, A.; Maharaj, B.; Msibi, Z.; Amico, K.R.; Friedland, G. Pilot evaluation of a second-generation electronic pill box for adherence to Bedaquiline and antiretroviral therapy in drug-resistant TB/HIV co-infected patients in KwaZulu-Natal, South Africa. BMC Infect. Dis. 2018, 18, 171. [Google Scholar] [CrossRef]
- Blaschke, T.F.; Osterberg, L.; Vrijens, B.; Urquhart, J. Adherence to medications: Insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Ann. Rev. Pharm. Toxicol. 2012, 52, 275–301. [Google Scholar] [CrossRef]
- Hensel, B.K.; Demiris, G.; Courtney, K.L. Defining obtrusiveness in home telehealth technologies. J. Am. Med. Inform. Assoc. 2006, 13, 428–431. [Google Scholar] [CrossRef]
- Haberer, J.; Kahane, J.; Kigozi, I.; Emenyonu, N.; Hunt, P.; Martin, J. Real-time adherence monitoring for HIV antiretroviral therapy. AIDS Behav. 2010, 14, 1340–1346. [Google Scholar] [CrossRef]
- Burnier, M. Is there a threshold for medication adherence? Lessons learnt from electronic monitoring of drug adherence. Front. Pharm. 2019, 9, 1540. [Google Scholar] [CrossRef]
- Gould, O.N.; Todd, L.; Irvine-Meek, J. Adherence devices in a community sample: How are pillboxes used? Can. Pharm. J. 2009, 142, 28–35. [Google Scholar] [CrossRef]

| Conditions [Number of Smart Cards] | ||||||
|---|---|---|---|---|---|---|
| Events (Number) | Field [n = 20] | Purse [n = 2] | Dropped (Recorded before Dropping) [n = 20] | Dropped (Recorded after Dropping) [n = 20] | Post Mail [n = 2] | Refrigerator [n = 20] |
| diary-recorded | 847 | 83 | 827 | 20 | 22 | 560 |
| electronically-stored | 812 | 85 | 827 | 20 | 22 | 533 |
| electronically-stored +/− 5 min | 804 | 83 | 827 | 19 | 22 | 518 |
| supernumerary electronically-stored (false positive) | 5 | 2 | 0 | 0 | 0 | 15 |
| not electronically-stored although diary-recorded (false negative) | 44 | 0 | 0 | 0 | 0 | 27 |
| Sensitivity | 94.9% | 100% | - | 95% | - | 92.5% |
| Specificity | 99.4% | 97.6% | - | - | - | 97.3% |
| Recovery | - | - | 100% | - | 100% | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnet, I.; Rothen, J.-P.; Albert, V.; Hersberger, K.E. Validation of a Novel Electronic Device for Medication Adherence Monitoring of Ambulatory Patients. Pharmacy 2019, 7, 155. https://doi.org/10.3390/pharmacy7040155
Arnet I, Rothen J-P, Albert V, Hersberger KE. Validation of a Novel Electronic Device for Medication Adherence Monitoring of Ambulatory Patients. Pharmacy. 2019; 7(4):155. https://doi.org/10.3390/pharmacy7040155
Chicago/Turabian StyleArnet, Isabelle, Jean-Pierre Rothen, Valerie Albert, and Kurt E. Hersberger. 2019. "Validation of a Novel Electronic Device for Medication Adherence Monitoring of Ambulatory Patients" Pharmacy 7, no. 4: 155. https://doi.org/10.3390/pharmacy7040155
APA StyleArnet, I., Rothen, J.-P., Albert, V., & Hersberger, K. E. (2019). Validation of a Novel Electronic Device for Medication Adherence Monitoring of Ambulatory Patients. Pharmacy, 7(4), 155. https://doi.org/10.3390/pharmacy7040155






