Provision of Bilingual Dispensing Labels to Non-Native English Speakers: An Exploratory Study
Abstract
1. Introduction
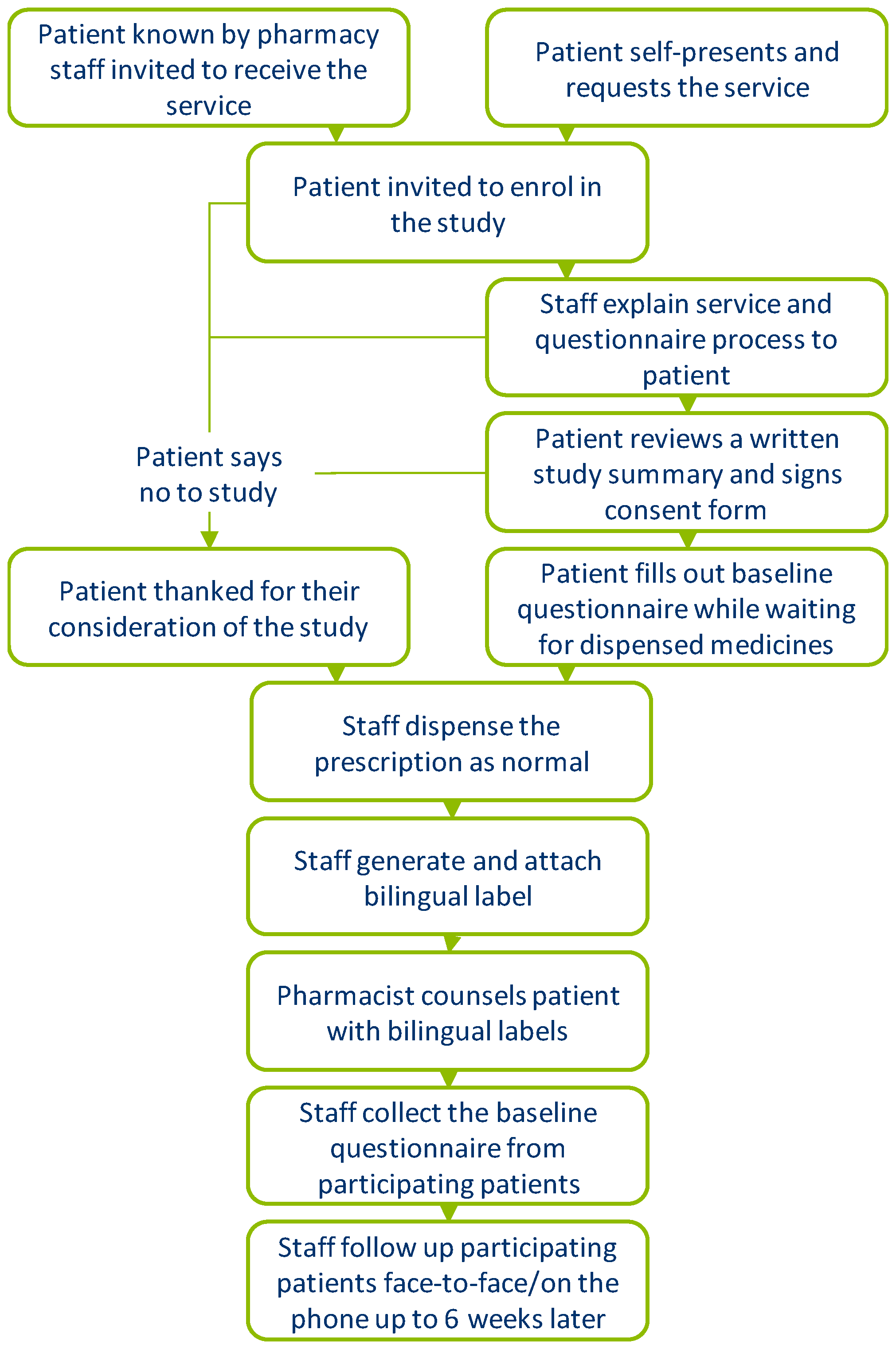
2. Materials and Methods
Data Collection and Analysis
3. Results
3.1. Initial Questionnaire Data
3.2. Follow-Up Questionnaire Data
4. Discussion
Limitations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mira, J.J.; Lorenzo, S.; Guilabert, M.; Navarro, I.; Pérez-Jover, V. A Systematic Review of Patient Medication Error on Self-Administering Medication at Home. Expert Opin. Drug Saf. 2015, 14, 815–838. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.L.; Avery, A.J.; Slavenburg, S.; Royal, S.; Pipe, G.; Lucassen, P.; Pirmohamed, M. Which Drugs Cause Preventable Admissions to Hospital? A Systematic Review. Br. J. Clin. Pharmacol. 2007, 63, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Langley, C.A.; Bush, J. The Aston Medication Adherence Study: Mapping the Adherence Patterns of an Inner-City Population. Int. J. Clin. Pharm. 2014, 36, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Manias, E.; Williams, A. Medication Adherence in People of Culturally and Linguistically Diverse Backgrounds: A Meta-Analysis. Ann. Pharmacother. 2010, 44, 964–982. [Google Scholar] [CrossRef] [PubMed]
- Al Hamid, A.; Aslanpour, Z.; Aljadhey, H.; Ghaleb, M. Hospitalisation Resulting from Medicine-Related Problems in Adult Patients with Cardiovascular Diseases and Diabetes in the United Kingdom and Saudi Arabia. Int. J. Environ. Res. Public Health 2016, 13, 479. [Google Scholar] [CrossRef] [PubMed]
- Cousins, D.; Dewsbury, C.; Matthew, L.; Nesbitt, I.; Warner, B.; Chamberlain, J.; Lamont, T.; Willmott, M. PSO/4 Safety in Doses: Medication Safety Incidents in the NHS. Available online: http://webarchive.nationalarchives.gov.uk/20101125232539/http://www.nrls.npsa.nhs.uk/resources/patient-safety-topics/medication-safety/?entryid45=61625&p=3 (accessed on 22 March 2019).
- Office for National Statistics. 2011 Census: Detailed Analysis—English Language Proficiency in England and Wales, Main Language and General Health Characteristics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/language/articles/detailedanalysisenglishlanguageproficiencyinenglandandwales/2013-08-30 (accessed on 22 March 2019).
- Scotland’s Census. Census 2011: Detailed Characteristics on Ethnicity, Identity, Language and Religion in Scotland—Release 3B. Available online: http://www.scotlandscensus.gov.uk/news/census-2011-detailed-characteristics-ethnicity-identity-language-and-religion-scotland-–-0 (accessed on 22 March 2019).
- United States Census Bureau. DP05. ACS Demographic and Housing Estimates 2012–2016 American Community Survey 5-Year Estimates. Available online: https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml (accessed on 22 March 2019).
- Office for National Statistics. Main Language Spoken at Home (Census), Borough. Available online: https://data.london.gov.uk/dataset/main-language-spoken-at-home-borough (accessed on 22 March 2019).
- Chaudary, R. Language Barriers and Patient Care in Community Pharmacies MPharm Project (Unit U22192) (Supervisor: Dr. Helena Herrera). Master’s Thesis, University of Portsmouth, Portsmouth, England, 2016. [Google Scholar]
- Schwappach, D.L.B.; Meyer Massetti, C.; Gehring, K. Communication Barriers in Counselling Foreign-Language Patients in Public Pharmacies: Threats to Patient Safety? Int. J. Clin. Pharm. 2012, 34, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, M.; Renfrew, M.R.; Green, A.R.; Lopez, L.; Tan-McGrory, A.; Brach, C.; Betancourt, J.R. Identifying and Preventing Medical Errors in Patients with Limited English Proficiency: Key Findings and Tools for the Field. J. Healthc. Qual. 2014, 36, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.; Gany, F.; Rosenfeld, P.; Carrasquillo, O.; Sharif, I.; Behar, E.; Ambizas, E.; Patel, P.; Schwartz, L.; Mangione, R. Access to Multilingual Medication Instructions at New York City Pharmacies. J. Urban Health 2007, 84, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, E.S.; Raynor, T.D.; de Jong-van den Berg, L.T. Accessing Medication Information by Ethnic Minorities: Barriers and Possible Solutions. Pharm. World Sci. 2003, 25, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Tsang, B.; Thornley, S. Language Barriers in the Community Pharmacy: A Survey of Northern and Western Auckland. J. Prim. Health Care 2011, 3, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, M.; Tomany-Korman, S.; Flores, G. Language Barriers to Prescriptions for Patients With Limited English Proficiency: A Survey of Pharmacies. Pediatrics 2007, 120, e225–e235. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.C.; Sarkar, U.; Chen, A.H.; Schillinger, D.; Wolf, M.S. Evaluation of Language Concordant, Patient-Centered Drug Label Instructions. J. Gen. Intern. Med. 2012, 27, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Bautista, M.A.C.; Tan, N.C.; Tang, W.E.; Tay, S.; Tan, A.S.L.; Pouliot, A.; Saffari, S.E.; Chei, C.-L.; Vaillancourt, R. Bilingual Text With or Without Pictograms Improves Elderly Singaporeans’ Understanding of Prescription Medication Labels. Gerontologist 2017, 59, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Riley, M.B.; Boyington, D.; Johnston, P.; Trochez, K.; Jennings, C.; Mashburn, J.; Kripalani, S. Development of a Patient-Centered Bilingual Prescription Drug Label. J. Health Commun. 2013, 18, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Divi, C.; Koss, R.G.; Schmaltz, S.P.; Loeb, J.M. Language Proficiency and Adverse Events in US Hospitals: A Pilot Study. Int. J. Qual. Heal. Care 2007, 19, 60–67. [Google Scholar] [CrossRef] [PubMed]
- The National Archives on behalf of HM Government. The Medicines (Labelling) Amendment Regulations 1992, No. 3273, Whole Instrument. Available online: http://www.legislation.gov.uk/uksi/1992/3273/made (accessed on 22 March 2019).
- European Commission. Final—Revision 14.2. Guideline on the Packaging Information of Medicinal Products for Human Use Authorised by the Union. Available online: https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-2/2015-02_packaging.pdf (accessed on 22 March 2019).
- The National Archives on behalf of HM Government. The Human Medicines Regulations 2012. No. 1916, PART 13, CHAPTER 1, Requirements for Packaging and Package Leaflets Relating to Medicinal Products, Regulation 266. Available online: http://www.legislation.gov.uk/uksi/2012/1916/part/13/chapter/1/crossheading/requirements-for-packaging-and-package-leaflets-relating-to-medicinal-products/made (accessed on 22 March 2019).
- Eur-Lex Access to European Union Law. Directive 2004/27/EC of the European Parliament and of the Council of 31 March 2004 Amending Directive 2001/83/EC on the Community Code Relating to Medicinal Products for Human Use (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32004L0027 (accessed on 22 March 2019).
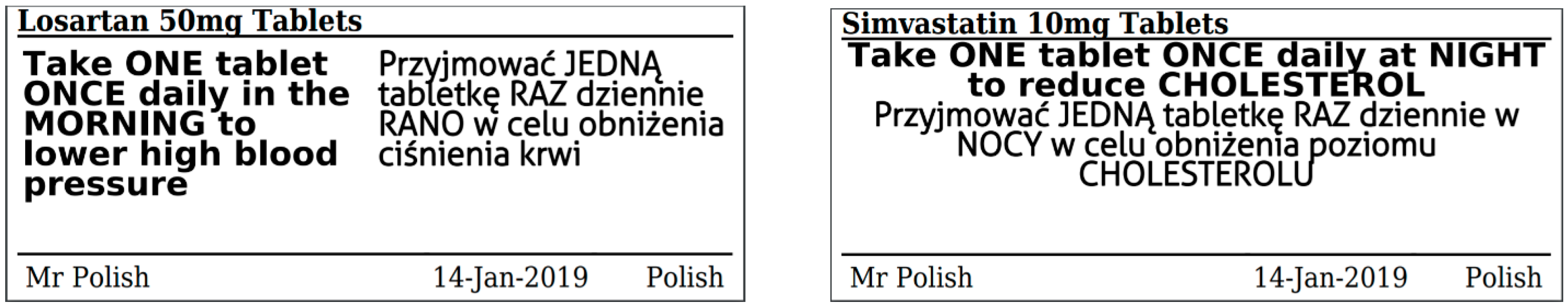
| Characteristic | Frequency |
|---|---|
| Gender | |
| Male | 58 (38%) |
| Female | 92 (61%) |
| Age group | |
| 18–25 | 7 (5%) |
| 26–40 | 40 (27%) |
| 41–60 | 37 (25%) |
| Over 60 | 64 (42%) |
| Length of time in the U.K. | |
| Less than 1 years | 8 (5%) |
| 1–5 years | 22 (15%) |
| 6–10 years | 29 (19%) |
| More than 10 years | 89 (59%) |
| Native language | |
| Arabic | 51 (34%) |
| Bengali | 31 (21%) |
| Gujarati | 14 (9%) |
| Hindi | 14 (9%) |
| Polish | 9 (6%) |
| Punjabi (Gurmukhi) | 18 (12%) |
| Somali | 4 (3%) |
| Tamil | 10 (7%) |
| “Always” or “Most of the Time” | “Sometimes” | “Rarely” or “Never” | |
|---|---|---|---|
| Do you read the information on the labels of your medicines (example shown below)? | 36% (n = 55) | 37% (n = 56) | 26% (n = 40) |
| How often do you understand this information? * | 38% (n = 57) | 41% (n = 62) | 21% (n = 31) |
| Does someone else help you to understand this information? | 39% (n = 59) | 38% (n = 58) | 23% (n = 34) |
| How often would you say that you take the right amount of your medicines (for example, the right number of puffs from the inhaler, the right volume of liquid from a bottle or the right number of tablets) and at the right time, as specified by the label? † | 58% (n = 88) | 27% (n = 41) | 11% (n = 16) |
| “Yes” | “No” | “Not Sure” | |
|---|---|---|---|
| Do you think the translated information helped you to take your medicines at the right time as specified on the label? | 82% (n = 105) | 8% (n = 10) | 10% (n = 13) |
| Do you think the translated information helped you to use the right amount of medicine, as specified on the medication label (for example, the right number of puffs from the inhaler, the right volume of liquid from a bottle, or the right number of tablets)? | 83% (n = 106) | 6% (n = 7) | 12% (n = 15) |
| After reading the translated information, did you notice you were taking your medicines in a way other than that specified on the medication label? * | 62% (n = 46) | 30% (n = 22) | 8% (n = 6) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, H.; Alsaif, M.; Khan, G.; Barnes, N.; Rutter, P. Provision of Bilingual Dispensing Labels to Non-Native English Speakers: An Exploratory Study. Pharmacy 2019, 7, 32. https://doi.org/10.3390/pharmacy7010032
Herrera H, Alsaif M, Khan G, Barnes N, Rutter P. Provision of Bilingual Dispensing Labels to Non-Native English Speakers: An Exploratory Study. Pharmacy. 2019; 7(1):32. https://doi.org/10.3390/pharmacy7010032
Chicago/Turabian StyleHerrera, Helena, Murtada Alsaif, Ghalib Khan, Nicola Barnes, and Paul Rutter. 2019. "Provision of Bilingual Dispensing Labels to Non-Native English Speakers: An Exploratory Study" Pharmacy 7, no. 1: 32. https://doi.org/10.3390/pharmacy7010032
APA StyleHerrera, H., Alsaif, M., Khan, G., Barnes, N., & Rutter, P. (2019). Provision of Bilingual Dispensing Labels to Non-Native English Speakers: An Exploratory Study. Pharmacy, 7(1), 32. https://doi.org/10.3390/pharmacy7010032







