Current Clinical Practice for the Use of Hypnotics to Manage Primary Insomnia in Adults in a Tertiary Hospital in Saudi Arabia: An Audit Study
Abstract
1. Introduction
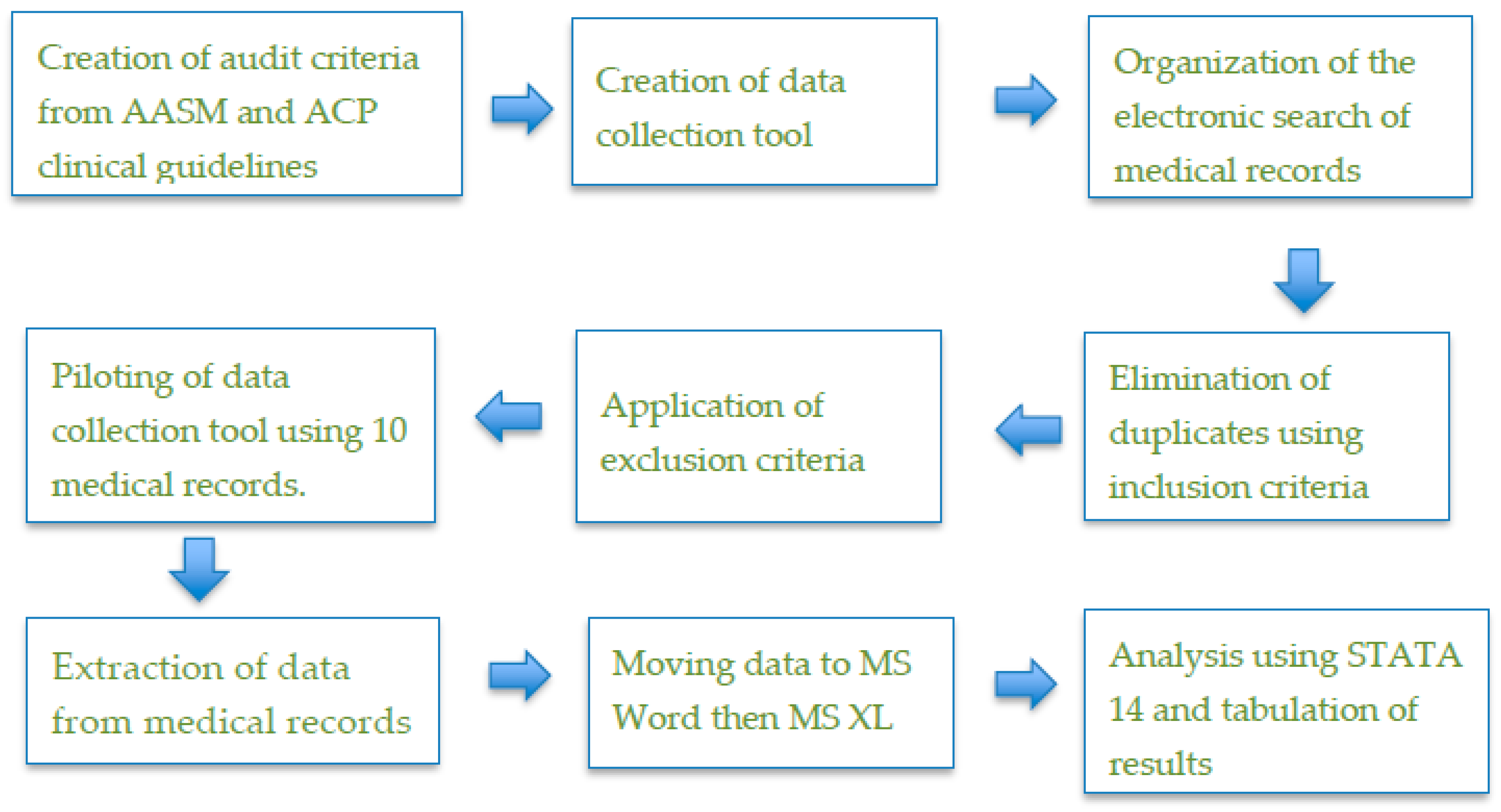
2. Materials and Methods
- not only prescribed BZDs or Z-drugs but also had clear documentation of their use;
- given CBT-I as the initial treatment (documented);
- prescribed these drugs when physicians decided to use pharmacological treatment;
- initially prescribed the lowest licensed dosage of BZDs or Z-drugs;
- prescribed the drugs specifically for the maximum recommended duration (4–5 weeks);
- on long-term use of the medicines and were reviewed by their doctors every few weeks or on a monthly basis, and then every 6 months (documented).
3. Results
- 75% (379) of patients were prescribed BZDs or Z-drugs, and of these, 48% (182) had clearly documented indications for their use.
- 61% (307) of patients received some form of medication for insomnia; none were offered or received CBT-I as a first-line treatment; 59% (182) used BZDs or Z-drugs as first treatment, of which 65% (118) began with the lowest recommended dose; 51% (93) of all prescriptions complied with the recommended duration.
- After being prescribed BZDs or Z-drugs and beginning medication use, no patient was reviewed by the physician for the effects of long-term use.
- 44 % (134) of patients were given anti-histamines or anti-histamine analgesics (combination of paracetamol and diphenhydramine) for insomnia.
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olfson, M.; King, M.; Schoenbaum, M. Benzodiazepine use in the United States. JAMA Psychiatry 2015, 72, 136–142. [Google Scholar] [CrossRef] [PubMed]
- NICE. Hypnotics. 2015. Available online: https://www.nice.org.uk/advice/ktt6 (accessed on 20 June 2016).
- Schutte-Rodin, S.; Broch, L.; Buysse, D.; Dorsey, C.; Sateia, M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J. Clin. Sleep Med. 2008, 4, 487–504. [Google Scholar] [PubMed]
- Sateia, M.J.; Buysse, D.J.; Krystal, A.D.; Neubauer, D.N.; Heald, J.L. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 307–349. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Baglioni, C.; Bassetti, C.; Bjorvatn, B.; Dolenc Groselj, L.; Ellis, J.G.; Espie, C.A.; Garcia-Borreguero, D.; Gjerstad, M.; Gonçalves, M.; et al. European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 2017, 26, 675–700. [Google Scholar] [CrossRef] [PubMed]
- NINC. Guidance on the Use of Zaleplon, Zolpidem and Zopiclone for the Short Term Management of Insomnia: Technology Appraisal Guidance. 2004. Available online: https://www.nice.org.uk/guidance/ta77/chapter/1-Guidance (accessed on 15 June 2016).
- Qaseem, A.; Kansagara, D.; Forciea, M.A.; Cooke, M.; Denberg, T.D. Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2016, 165, 125–133. [Google Scholar] [CrossRef] [PubMed]
- ISS. The first Guidelines for the Assessment and Management of Patients with Sleep Disorders. Available online: http://www.irishsleepsociety.org/guidelines.htm (accessed on 12 July 2016).
- Kapil, V.; Green, J.L.; Le Lait, C.; Wood, D.M.; Dargan, P.I. Misuse of benzodiazepines and Z-drugs in the UK. Br. J. Psychiatry 2014, 205, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Siriwardena, A.N.; Qureshi, M.Z.; Dyas, J.V.; Middleton, H.; Orner, R. Magic bullets for insomnia? Patients’ use and experiences of newer (Z drugs) versus older (benzodiazepine) hypnotics for sleep problems in primary care. Br. J. Gen. Pract. 2008, 58, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Bhardwaj, R. Audit on use of hypnotics for insomnia in comparison with national institute of clincal excellence guidelines. Eur. Psychiatry 2010, 25, 991. [Google Scholar] [CrossRef]
- Cadogan, C.; Ryder, S. An audit of prescribing practices for benzodiazepines and Z-drugs. Ir. Med. J. 2015, 108, 84–86. [Google Scholar]
- Bahammam, A.S. Sleep medicine in Saudi Arabia: Current problems and future challenges. Ann. Thorac. Med. 2011, 6, 3–10. [Google Scholar] [CrossRef]
- BaHammam, A.S.; Al-Jahdali, H.; AlHarbi, A.S.; AlOtaibi, G.; Asiri, S.M.; AlSayegh, A. Saudi regulations for the accreditation of sleep medicine physicians and technologists. Ann. Thorac. Med. 2013, 8, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.A.; Al-Ghamdy, Y.S.; Al-Haddad, N.S.; Abdelgadir, M.H.; Tawfik, M.H. Integration of mental health care into primary care. Preliminary observations of continuing implementation phase. Saudi Med. J. 2001, 22, 899–906. [Google Scholar] [PubMed]
- Koenig, H.G.; Al Zaben, F.; Sehlo, M.G.; Khalifa, D.A.; Al Ahwal, M.S.; Qureshi, N.A.; Al-Habeeb, A.A. Mental health care in Saudi Arabia: Past, present and future. OJPSYCH 2014, 4, 113. [Google Scholar] [CrossRef]
- Al Ghamdy, Y.S.; Qureshi, N.A.; Abdel Ghadir, M.H.; Al Habeeb, T.A.; Ahmad, S.A. Psychotropic Drugs Prescriptions in Al-Qassim Region, Saudi Arabia. East. Mediterr. Health J. 1999, 5, 27–34. [Google Scholar]
- Altokhais, T.I.; Al-Obaid, O.A.; Kattan, A.E.; Amer, Y.S.; CPG Collaborative Groups. Assessment of implementability of an adapted clinical practice guideline for surgical antimicrobial prophylaxis at a tertiary care university hospital. J. Eval. Clin. Pract. 2017, 23, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Albejaidi, F.M. Healthcare system in Saudi Arabia: An analysis of structure, total quality management and future challenges. JAPSS 2010, 2, 794–818. [Google Scholar]
- Benjamin, A. Audit: How to do it in practice. BMJ 2008, 336, 1241–1245. [Google Scholar] [CrossRef]
- Flegel, K. Tertiary hospitals must provide general care. Can. Med. Assoc. 2015, 187, 235. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 14; StataCorp LP: College Station, TX, USA, 2015. [Google Scholar]
- Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction; Substance Abuse and Mental Health Services Administration (US): Rockville, MD, USA, 2004.
- Ohayon, M.M.; Roth, T. Place of chronic insomnia in the course of depressive and anxiety disorders. J. Psychiatr. Res. 2003, 37, 9–15. [Google Scholar] [CrossRef]
- Schaeffer, J. Poor Documentation: Why It Happens and How to Fix It. For The Record. 2016. Available online: https://www.fortherecordmag.com/archives/0516p12.shtml (accessed on 22 May 2017).
- Stepanski, E.J. Hypnotics should not be considered for the initial treatment of chronic insomnia. Con. JCSM 2005, 1, 125–128. [Google Scholar] [PubMed]
- Organization, W.H. Mental Health Atlas Country Profile. 2014. Available online: http://www.who.int/mental_health/evidence/atlas/profiles-2014/sau.pdf (accessed on 28 October 2018).
- Essential Medicines and Health Products Information Portal. Ministry of Health Formulary (MOHF) Drug List, 2nd Revised ed. 2014. Available online: http://apps.who.int/medicinedocs/en/d/Js23157en/ (accessed on 20 June 2016).
- Gunja, N. The clinical and forensic toxicology of Z-drugs. J. Med. Toxicol. 2013, 9, 155–162. [Google Scholar] [CrossRef]
- James, W. Hypnotics and the risks of dementia. JCSM 2017, 13, 837. [Google Scholar] [CrossRef] [PubMed]
- George, C. Perspectives on the management of insomnia in patients with chronic respiratory disorders. Sleep 2000, 23, S31–S35. [Google Scholar] [PubMed]
| Patient Number |
|---|
| Prescribing physicians’ numbers |
| Patients who were prescribed these medications had clearly documented indications for use (YES/NO) |
| Patients received CBT-I as the initial treatment (documented) (YES/NO) |
| Patients were prescribed BZDs or Z-drugs if pharmacological treatments were considered (YES/NO) |
| Patients were initially prescribed the lowest licensed dosage (for adults or elderly) of BZDs or Z-drugs (YES/NO) |
| Patients were prescribed BZDs or Z-drugs for 4‒5 weeks (YES/NO) |
| Patients on long-term use of medicines were reviewed by their doctors every few weeks or monthly and then every 6 months (Documented) (YES/NO) |
| To treat chronic insomnia, anti-histamine or anti-histamine/ analgesic medicines were used (YES/NO) |
| Indicators | Records | Count Yes | Percentage % |
|---|---|---|---|
| Patients who received BZDs or Z-drugs had clearly documented indications for use | 379 | 182 | 48 |
| Patients received CBT-I as the initial treatment (documented) | 307 | 0 | 0 |
| Patients were prescribed BZDs or Z-drugs if pharmacological treatments were considered | 307 | 182 | 59 |
| Patients were initially prescribed the lowest licensed dosage of prescribed BZDs or Z-drugs | 182 | 118 | 65 |
| Patients were prescribed BZDs or Z-drugs for 4‒5 weeks | 182 | 93 | 51 |
| Patients on long-term use of medicines were reviewed by their doctors every few weeks or monthly, and then every 6 months | 89 | 0 | 0 |
| To treat chronic insomnia, anti-histamine or anti-histamine analgesic medicines were used | 307 | 134 | 44 |
| Number of Criteria Met | Count | Percentage (%) |
|---|---|---|
| Met 0 criteria | 13 | 4.2 |
| Met 1 criterion | 105 | 34.2 |
| Met any 2 criteria | 47 | 15.3 |
| Met any 3 criteria | 69 | 22.5 |
| Met any 4 criteria | 62 | 20.2 |
| Met any 5 criteria | 11 | 3.6 |
| Total | 307 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobia, A.; Ryan, K.; Grant, D.; BaHammam, A. Current Clinical Practice for the Use of Hypnotics to Manage Primary Insomnia in Adults in a Tertiary Hospital in Saudi Arabia: An Audit Study. Pharmacy 2019, 7, 15. https://doi.org/10.3390/pharmacy7010015
Dobia A, Ryan K, Grant D, BaHammam A. Current Clinical Practice for the Use of Hypnotics to Manage Primary Insomnia in Adults in a Tertiary Hospital in Saudi Arabia: An Audit Study. Pharmacy. 2019; 7(1):15. https://doi.org/10.3390/pharmacy7010015
Chicago/Turabian StyleDobia, Ali, Kath Ryan, Daniel Grant, and Ahmed BaHammam. 2019. "Current Clinical Practice for the Use of Hypnotics to Manage Primary Insomnia in Adults in a Tertiary Hospital in Saudi Arabia: An Audit Study" Pharmacy 7, no. 1: 15. https://doi.org/10.3390/pharmacy7010015
APA StyleDobia, A., Ryan, K., Grant, D., & BaHammam, A. (2019). Current Clinical Practice for the Use of Hypnotics to Manage Primary Insomnia in Adults in a Tertiary Hospital in Saudi Arabia: An Audit Study. Pharmacy, 7(1), 15. https://doi.org/10.3390/pharmacy7010015






