Hypersensitivity Reactions in Serious Adverse Events Reported for Paracetamol in the EudraVigilance Database, 2007–2018
Abstract
1. Introduction
2. Materials and Methods
3. Statistical analysis
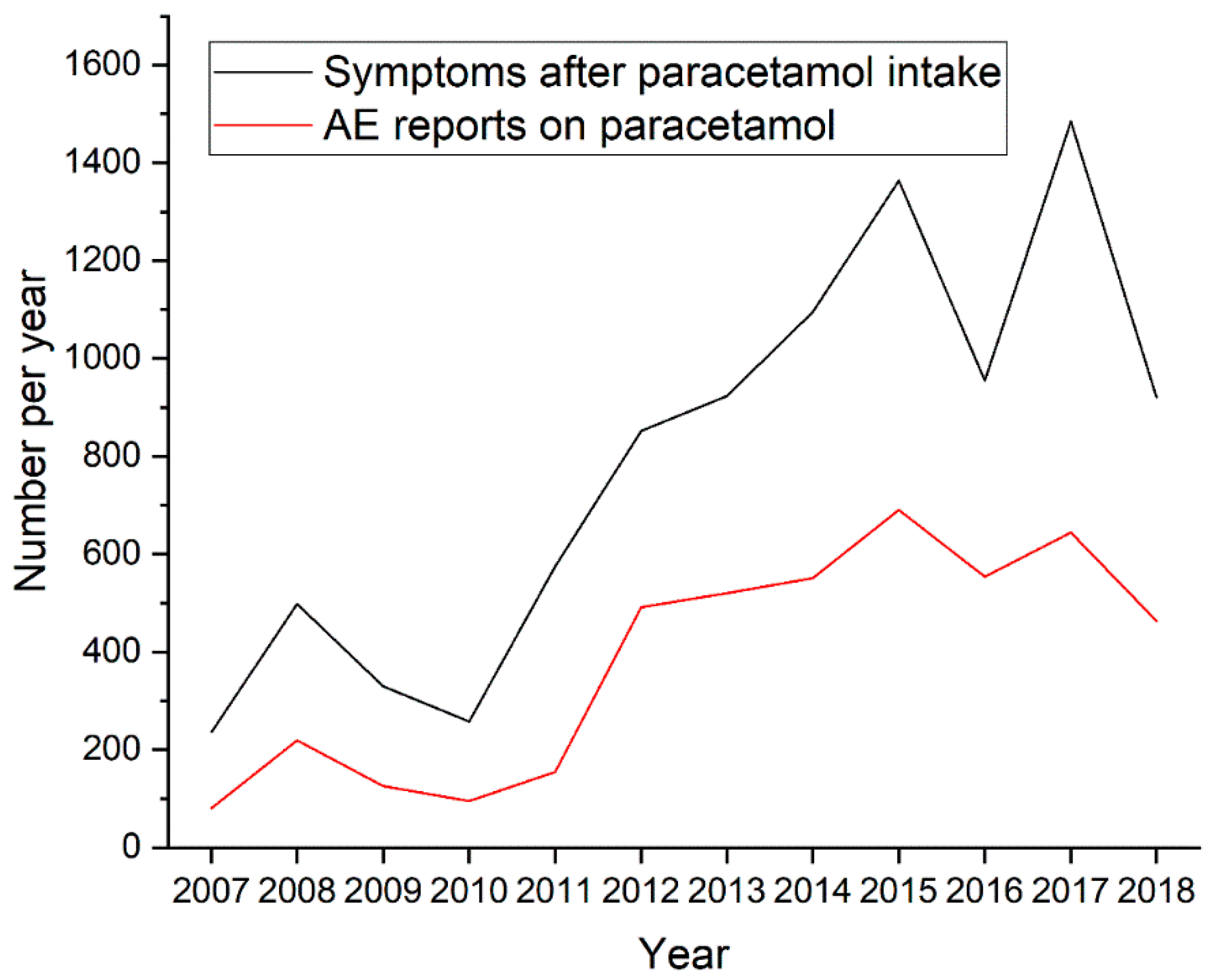
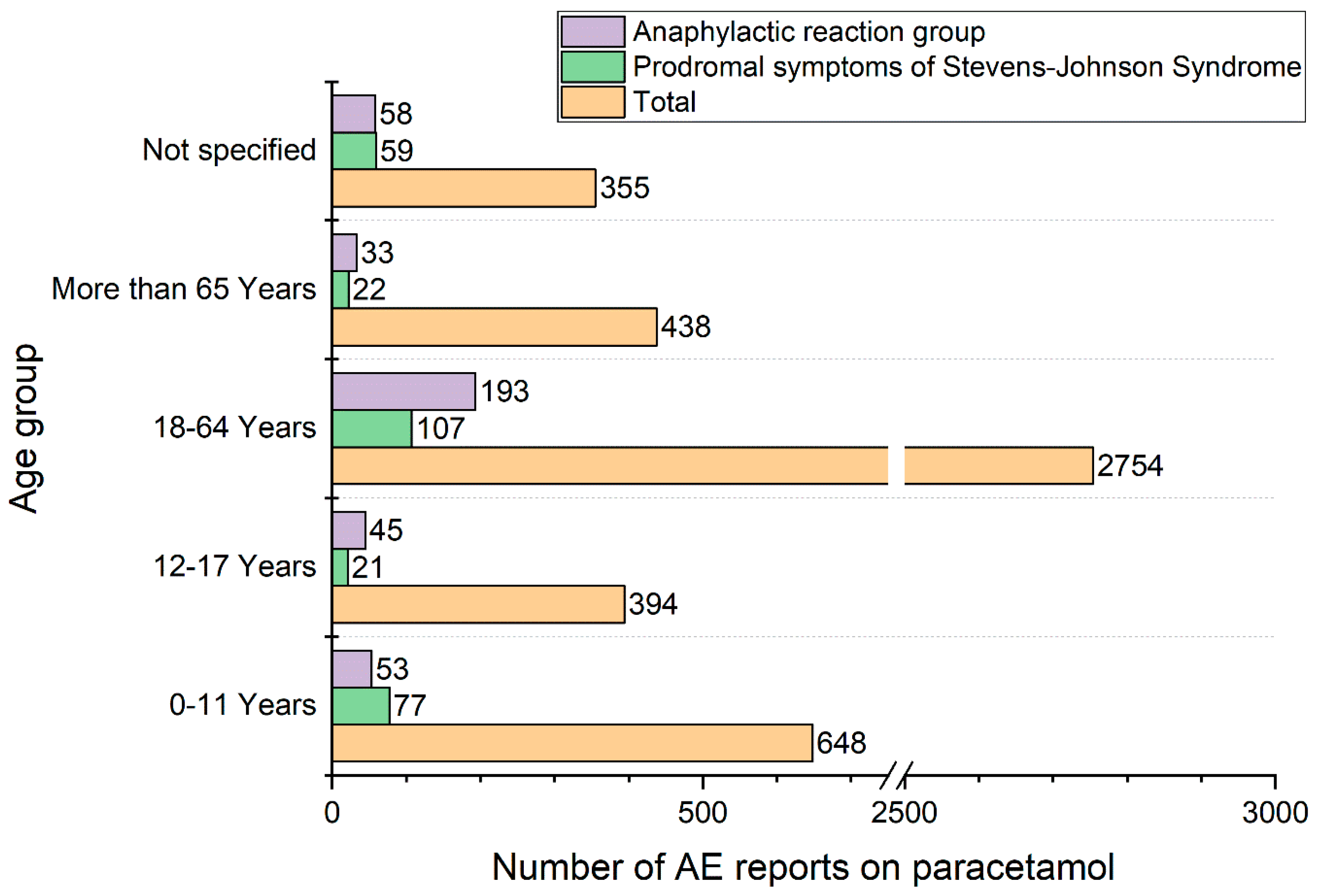
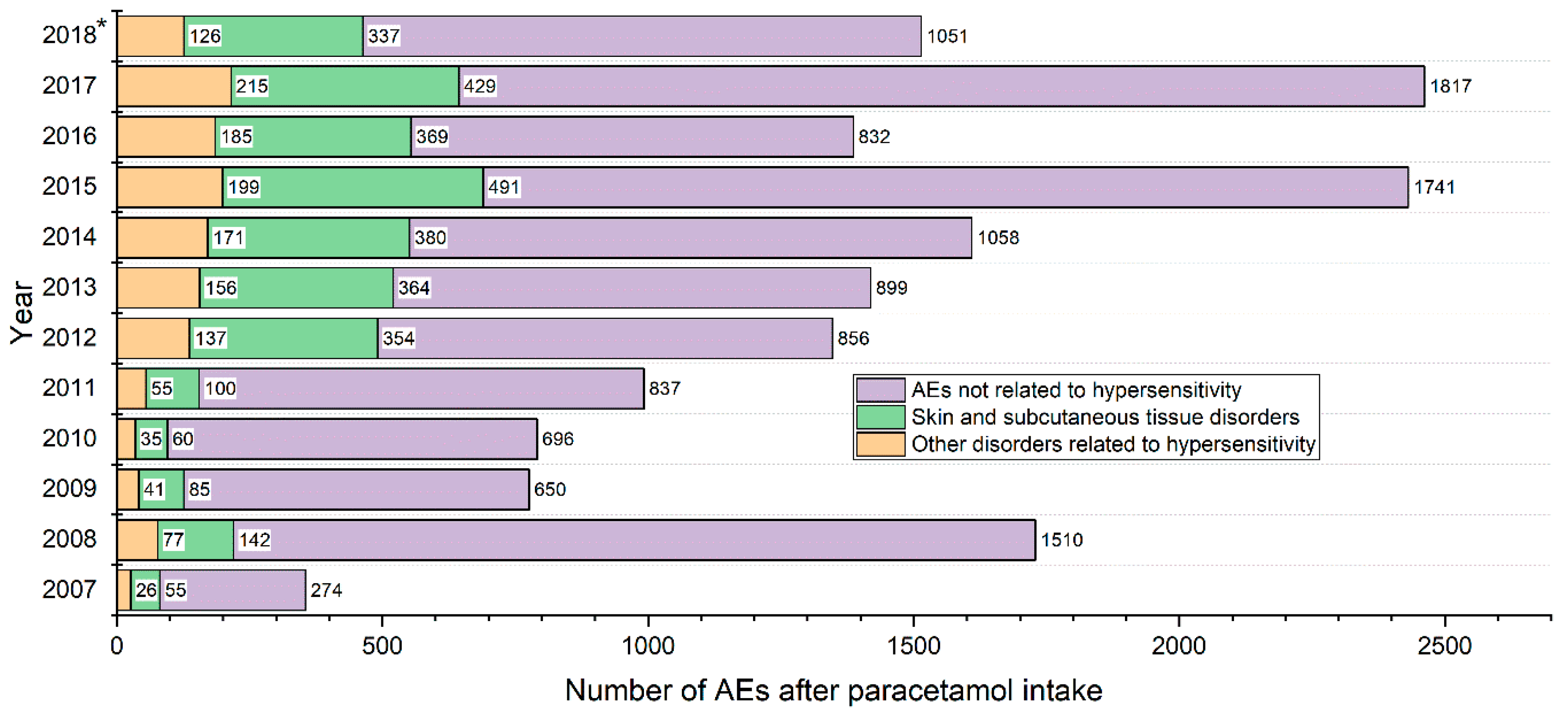
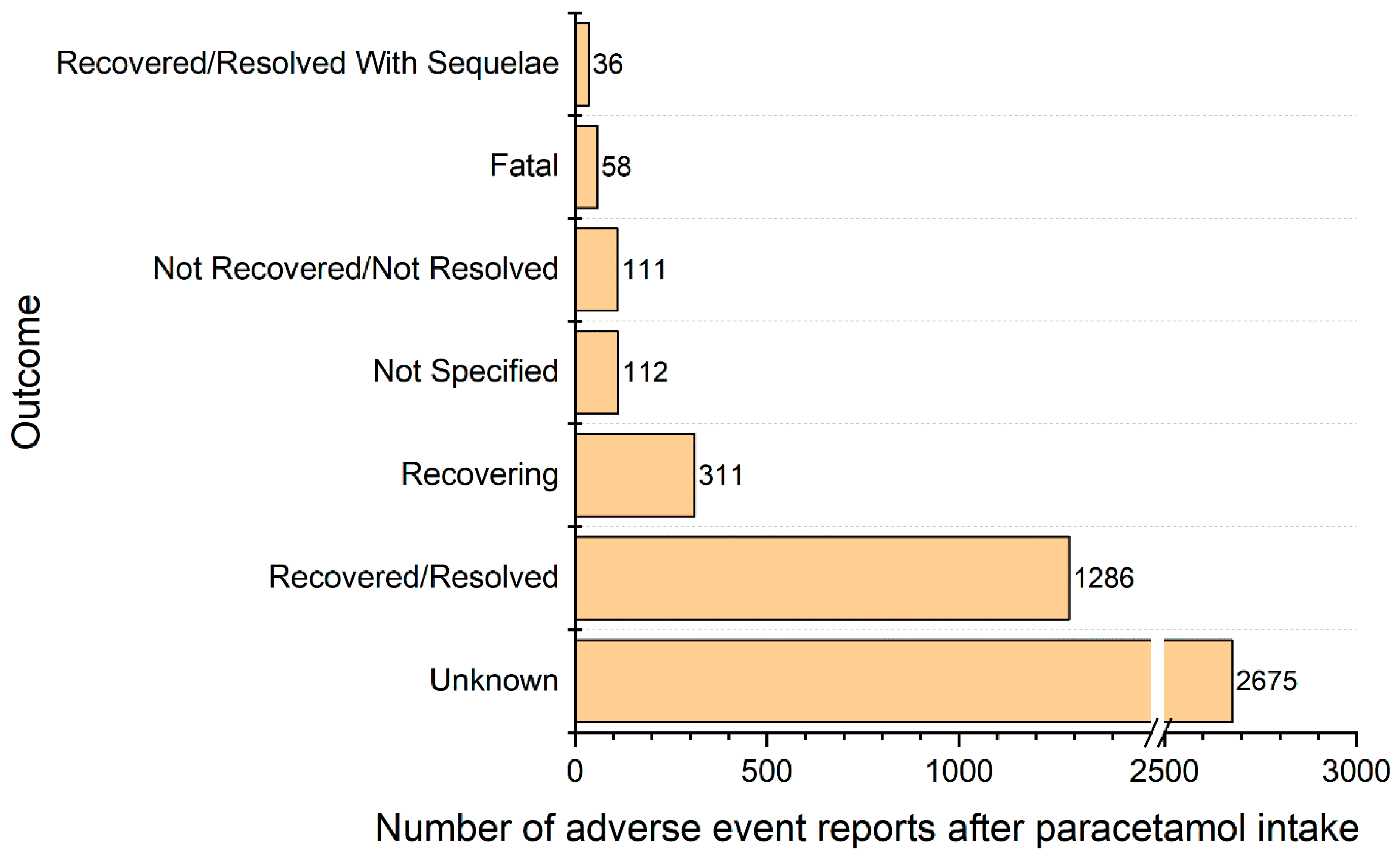
4. Results
Population Characteristic, Presentation of Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jóźwiak-Bebenista, M.; Nowak, J.Z. Paracetamol: Mechanism of action, applications and safety concern. Acta Pol. Pharm. 2014, 71, 11–23. [Google Scholar] [PubMed]
- Dear, J.W.; Antoine, D.J.; Park, B.K. Where are we now with paracetamol? BMJ 2015, 351, h3705. [Google Scholar] [CrossRef]
- Sharma, C.V.; Mehta, V. Paracetamol: Mechanisms and updates. Contin. Educ. Anaesth. Crit. Care Pain 2014, 14, 153–158. [Google Scholar] [CrossRef]
- Drugs.com Paracetamol Side Effects. Available online: https://www.drugs.com/sfx/paracetamol-side-effects.html (accessed on 27 December 2018).
- Craig, D.G.N.; Bates, C.M.; Davidson, J.S.; Martin, K.G.; Hayes, P.C.; Simpson, K.J. Overdose pattern and outcome in paracetamol-induced acute severe hepatotoxicity: Outcome following unintentional paracetamol overdose. Br. J. Clin. Pharmacol. 2011, 71, 273–282. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency EudraVigilance. Available online: https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance (accessed on 27 December2018).
- The European Parliament and The Council Directive 2001/83/EC of 6 November 2001 on the Community Code Relating to Medicinal Products for Human Use. 2011. Available online: https://ec.europa.eu/health/sites/health/files/files/eudralex/vol–1/dir_2001_83_consol_2012/dir_2001_83_cons_2012_en.pdf (accessed on 27 December 2018).
- European Medicines Agency 2017 Annual Report on EudraVigilance for the European Parliament, the Council and the Commission. Available online: https://www.ema.europa.eu/documents/report/2017-annual-report-eudravigilance-european-parliament-council-commission-reporting-period-1-january_en.pdf (accessed on 27 December 2018).
- Celik, G.; Pichler, W.; Adkinson, N. Drug Allergy. In Middleton’s Allergy: Principles & Practice; Mosby/Elsevier: Philadelphia, PA, USA, 2009; pp. 1205–1226. ISBN 978-0-323-05659-5. [Google Scholar]
- Aronson, J.K.; Ferner, R.E. Clarification of terminology in drug safety. Drug Saf. 2005, 28, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.; Babar, A.; Choudhary, M.; Kutner, M.; Pyrsopoulos, N. Acetaminophen-Induced Hepatotoxicity: A Comprehensive Update. J. Clin. Transl. Hepatol. 2016, 4, 131–142. [Google Scholar]
- Rutkowski, K.; Nasser, S.M.; Ewan, P.W. Paracetamol hypersensitivity: Clinical features, mechanism and role of specific IgE. Int. Arch. Allergy Immunol. 2012, 159, 60–64. [Google Scholar] [CrossRef]
- Rajput, R.; Sagari, S.; Durgavanshi, A.; Kanwar, A. Paracetamol induced Steven-Johnson syndrome: A rare case report. Contemp. Clin. Dent. 2015, 6, 278. [Google Scholar] [CrossRef] [PubMed]
- FDA Drug Safety Communication FDA Warns of Rare but Serious Skin Reactions with the Pain Reliever/Fever Reducer Acetaminophen. Available online: https://www.fda.gov/Drugs/DrugSafety/ucm363041.htm (accessed on 29 November 2018).
- Gyamlani, G.G.; Parikh, C.R. Acetaminophen toxicity: Suicidal vs. accidental. Crit Care 2002, 6, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Blieden, M.; Paramore, L.C.; Shah, D.; Ben-Joseph, R. A perspective on the epidemiology of acetaminophen exposure and toxicity in the United States. Expert Rev. Clin. Pharmacol. 2014, 7, 341–348. [Google Scholar] [CrossRef]
- Allanore, L.; Roujeau, J.-C. Clinic and Pathogenesis of Severe Bullous Skin Reactions: Stevens-Johnson Syndrome, Toxic Epidermal Necrolysis. In Drug Hypersensitivity; Pichler, W.J., Ed.; Karger: Basel, Switzerlands, 2007; pp. 267–277. ISBN 978-3-8055-8269-8. [Google Scholar]
- Sánchez-Borges, M.; Caballero-Fonseca, F.; Capriles-Hulett, A.; González-Aveledo, L. Hypersensitivity Reactions to Nonsteroidal Anti-Inflammatory Drugs: An Update. Pharmaceuticals 2010, 3, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Simons, F.E.R.; Ebisawa, M.; Sanchez-Borges, M.; Thong, B.Y.; Worm, M.; Tanno, L.K.; Lockey, R.F.; El-Gamal, Y.M.; Brown, S.G.; Park, H.-S.; et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ. J. 2015, 8, 1–16. [Google Scholar] [CrossRef]
- Numata, T.; Fukushi, R.; Ito, T.; Tsuboi, R.; Harada, K. Acetaminophen anaphylaxis diagnosed by skin prick test. Allergol. Int. 2016, 65, 490–491. [Google Scholar] [CrossRef] [PubMed]
- Settipane, R.; Schrank, P.; Simon, R.; Mathison, D.; Christiansen, S.; Stevenson, D. Prevalence of cross-sensitivity with acetaminophen in aspirin-sensitive asthmatic subjects. J. Allergy Clin. Immunol. 1995, 96, 480–485. [Google Scholar] [CrossRef]
- Lee, Q. Hypersensitivity to antipyretics: Pathogenesis, diagnosis and management. Hong Kong Med. J. 2017, 23, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, M. Do Women Have More Adverse Drug Reactions? Am. J. Clin. Dermatol. 2001, 2, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Kando, J.C.; Yonkers, K.A.; Cole, J.O. Gender as a Risk Factor for Adverse Events to Medications. Drugs 1995, 50, 1–6. [Google Scholar] [CrossRef]
- Rydberg, D.M.; Mejyr, S.; Loikas, D.; Schenck-Gustafsson, K.; von Euler, M.; Malmström, R.E. Sex differences in spontaneous reports on adverse drug events for common antihypertensive drugs. Eur. J. Clin. Pharmacol. 2018, 74, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Toki, T.; Ono, S. Spontaneous Reporting on Adverse Events by Consumers in the United States: An Analysis of the Food and Drug Administration Adverse Event Reporting System Database. Drugs Real World Outcomes 2018, 5, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Stergiopoulos, S.; Brown, C.A.; Felix, T.; Grampp, G.; Getz, K.A. A Survey of Adverse Event Reporting Practices Among US Healthcare Professionals. Drug Saf. 2016, 39, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Cranswick, N.; Coghlan, D. Paracetamol efficacy and safety in children: The first 40 years. Am. J. Ther. 2000, 7, 135–141. [Google Scholar] [CrossRef]
- Eccles, R. Efficacy and safety of over-the-counter analgesics in the treatment of common cold and flu. J. Clin. Pharmacy Ther. 2006, 31, 309–319. [Google Scholar] [CrossRef]
- Rojas-Pérez-Ezquerra, P.; Sánchez-Morillas, L.; Gómez-Traseira, C.; Gonzalez-Mendiola, R.; Alcorta Valle, A.R.; Laguna-Martinez, J. Selective hypersensitivity reactions to acetaminophen: A 13-case series. J. Allergy Clin. Immunol. Pract. 2014, 2, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Roberts, G.; Worm, M.; Bilò, M.B.; Brockow, K.; Fernández Rivas, M.; Santos, A.F.; Zolkipli, Z.Q.; Bellou, A.; Beyer, K.; et al. Anaphylaxis: Guidelines from the European Academy of Allergy and Clinical Immunology. Allergy 2014, 69, 1026–1045. [Google Scholar] [CrossRef]
- Creamer, D.; Walsh, S.A.; Dziewulski, P.; Exton, L.S.; Lee, H.Y.; Dart, J.K.G.; Setterfield, J.; Bunker, C.B.; Ardern-Jones, M.R.; Watson, K.M.T.; et al. U.K. guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults 2016. Br. J. Dermatol. 2016, 174, 1194–1227. [Google Scholar] [CrossRef]
- Feldmeyer, L.; Heidemeyer, K.; Yawalkar, N. Acute Generalized Exanthematous Pustulosis: Pathogenesis, Genetic Background, Clinical Variants and Therapy. Int. J. Mol. Sci. 2016, 17, 1214. [Google Scholar] [CrossRef]
- Sidoroff, A. Acute Generalized Exanthematous Pustulosis. In Chemical Immunology and Allergy; French, L.E., Ed.; S. KARGER AG: Basel, Switzerlands, 2012; Volume 97, pp. 139–148. ISBN 978-3-8055-9970-2. [Google Scholar]
- Chen, Y.-C.; Cho, Y.-T.; Chang, C.-Y.; Chu, C.-Y. Drug reaction with eosinophilia and systemic symptoms: A drug-induced hypersensitivity syndrome with variable clinical features. Dermatol. Sin. 2013, 31, 196–204. [Google Scholar] [CrossRef]
- Porebski, G. In Vitro Assays in Severe Cutaneous Adverse Drug Reactions: Are They Still Research Tools or Diagnostic Tests Already? Int. J. Mol. Sci. 2017, 18, 1737. [Google Scholar] [CrossRef]
- Porębski, G.; Czarnobilska, E.; Bosak, M. Cytotoxic-based assays in delayed drug hypersensitivity reactions induced by antiepileptic drugs. Pol. Arch. Med. Wewn. 2015, 125, 823–834. [Google Scholar] [CrossRef][Green Version]
- Rolfes, L.; van Hunsel, F.; van der Linden, L.; Taxis, K.; van Puijenbroek, E. The Quality of Clinical Information in Adverse Drug Reaction Reports by Patients and Healthcare Professionals: A Retrospective Comparative Analysis. Drug Saf. 2017, 40, 607–614. [Google Scholar] [CrossRef]
- Inácio, P.; Cavaco, A.; Airaksinen, M. The value of patient reporting to the pharmacovigilance system: A systematic review: The value of patient reporting to the pharmacovigilance system. Br. J. Clin. Pharmacol. 2017, 83, 227–246. [Google Scholar] [CrossRef]
| Adverse Event | n | % of Reports | Adverse Event | n | % of Reports |
|---|---|---|---|---|---|
| Angioedema | 1108 | 24.1% | Stevens-Johnson syndrome | 129 | 2.8% |
| Rash | 847 | 18.5% | Anaphylactic shock | 115 | 2.5% |
| Urticaria | 480 | 10.5% | Fixed eruption | 83 | 1.8% |
| Orbital or periorbital oedema | 390 | 8.5% | Rash generalised | 76 | 1.7% |
| Head, neck or respiratory tract oedema excluding orbital or periorbital area | 319 | 7.0% | Lip swelling | 75 | 1.6% |
| Hypersensitivity; Drug hypersensitivity | 316 | 6.9% | Swelling face | 72 | 1.6% |
| Eye swelling | 263 | 5.7% | Rash erythematous | 65 | 1.4% |
| Anaphylactic reaction | 246 | 5.4% | Cough | 65 | 1.4% |
| Erythema | 173 | 3.8% | Blister | 58 | 1.3% |
| Face oedema | 163 | 3.6% | Maculo-papular rash | 53 | 1.2% |
| Oedema in other than head or unspecified localisation | 97 | 2.1% |
| Reaction Groups | Healthcare Professional | Non-Healthcare Professional | Not Specified/Missing | Total |
|---|---|---|---|---|
| Skin and subcutaneous tissue disorders | 2657 | 493 | 16 | 3166 |
| Eye disorders | 532 | 116 | 2 | 650 |
| Immune system disorders | 559 | 159 | 4 | 722 |
| General disorders and administration site conditions | 230 | 0 | 0 | 230 |
| Vascular disorders | 178 | 37 | 1 | 216 |
| Respiratory, thoracic and mediastinal disorders | 172 | 53 | 0 | 225 |
| Gastrointestinal disorders | 136 | 29 | 2 | 167 |
| Reproductive system and breast disorders | 9 | 15 | 0 | 24 |
| Ear and labyrinth disorders | 4 | 4 | 0 | 8 |
| Investigations | 2 | 1 | 0 | 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popiołek, I.; Piotrowicz-Wójcik, K.; Porebski, G. Hypersensitivity Reactions in Serious Adverse Events Reported for Paracetamol in the EudraVigilance Database, 2007–2018. Pharmacy 2019, 7, 12. https://doi.org/10.3390/pharmacy7010012
Popiołek I, Piotrowicz-Wójcik K, Porebski G. Hypersensitivity Reactions in Serious Adverse Events Reported for Paracetamol in the EudraVigilance Database, 2007–2018. Pharmacy. 2019; 7(1):12. https://doi.org/10.3390/pharmacy7010012
Chicago/Turabian StylePopiołek, Iwona, Katarzyna Piotrowicz-Wójcik, and Grzegorz Porebski. 2019. "Hypersensitivity Reactions in Serious Adverse Events Reported for Paracetamol in the EudraVigilance Database, 2007–2018" Pharmacy 7, no. 1: 12. https://doi.org/10.3390/pharmacy7010012
APA StylePopiołek, I., Piotrowicz-Wójcik, K., & Porebski, G. (2019). Hypersensitivity Reactions in Serious Adverse Events Reported for Paracetamol in the EudraVigilance Database, 2007–2018. Pharmacy, 7(1), 12. https://doi.org/10.3390/pharmacy7010012





