Understanding Users in the ‘Field’ of Medications
Abstract
:1. Introduction
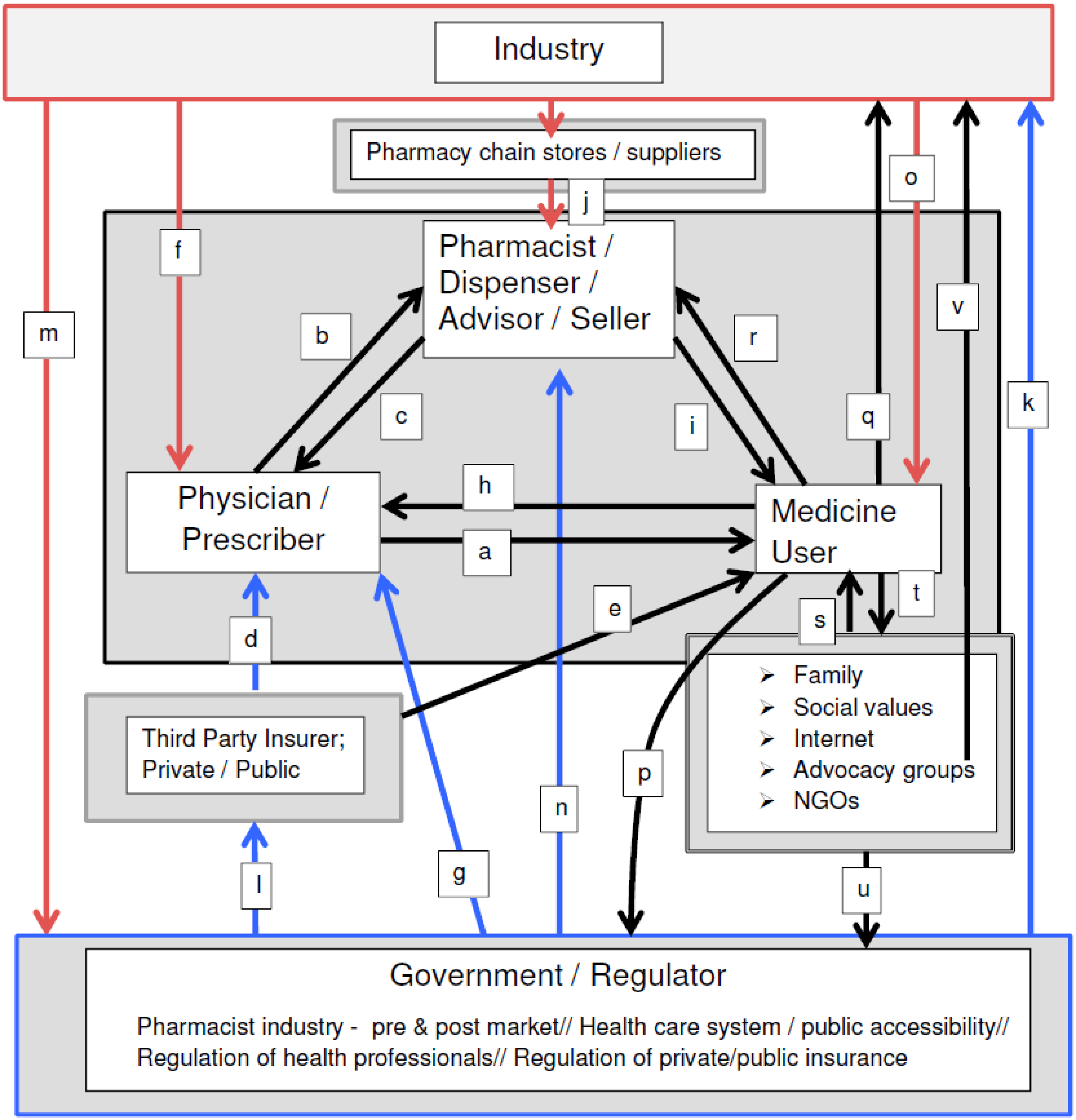
2. The Field of Medications and Its Agents
2.1. Physicians
2.2. Pharmacists
2.3. Government Regulation in the Field of Medications:
“the pharmaceutical industry was and is permitted to have strategic access to, and involvement with, government regulatory policy over and above any other interest group; and more often than other factors, the industry was, and is, decisive in determining regulatory policy outcomes (or lack thereof)”.[28] (p. 873)
2.4. The Pharmaceutical Industry
“The International Federation of Pharmaceutical Manufacturers and Associations advocates policies that encourage discovery of and access to life-saving and life-enhancing medicines to improve the health of people everywhere” [58], and
“PhRMA, the Pharmaceutical Research and Manufacturers of America, represents the country’s leading biopharmaceutical researchers and biotechnology companies. Our members are committed to finding tomorrow’s cures and treatments for some of the most serious diseases such as Cancer, Alzheimer’s Disease, Cystic Fibrosis and Parkinson’s. New medicines are an integral part of the healthcare system, providing doctors and their patients with safe and effective treatment options, extending and improving quality of life”.[59]
2.5. Medicine Users
“one-by-one, activists have attacked structural and legal barriers to access and have advocated for new institutional arrangements and new practices that might make treatment a reality…by promoting generic competition, they have pushed down the costs of ARVs in developing countries ….and have raised global resources for AIDS…relying on the rhetoric of a human right to health, to access to medicines, and to life itself…they have effectively removed structural impediments and leveraged resources to actually increase access to medicines on the ground”….[101] (p. 245)
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Romanow, R.J. Building on Values: The Future of Health Care in Canada—Final Report; Commission on the Future of Health Care in Canada: Saskatoon, SK, Canada, 2002. [Google Scholar]
- Law, J. The Big Pharma; Constable and Robinson: London, UK, 2006. [Google Scholar]
- Canadian Institute for Health Information. Drug Expenditures in Canada 1985–2004; CIHI: Ottawa, ON, Canada, 2005. [Google Scholar]
- Busfield, J. A pill for every ill: Explaining the expansion in medicine use. Soc. Sci. Med. 2010, 70, 934–941. [Google Scholar] [CrossRef] [PubMed]
- IMS Health. The Use of Medicines in the United States. Review of 2010; IMS Institute for Health Care Informatics: Danbury, CT, USA, 2011; Available online: http://www.imshealth.com/en/about-us/news/ims-institute-reports-u.s.-spending-on-medicines-grew-2.3-percent-in-2010,-to-$307.4-billion (accessed on 28 November 2015).
- Steering Committee of Women and Health Protection. Introduction. In The Push to Prescribe: Woman and Canadian Drug Policy; Rochon Ford, A., Saibil, D., Eds.; Women’s Press: Toronto, ON, Canada, 2010; pp. 1–16. [Google Scholar]
- Holloway, K. Uneasy subjects: Medical students’ conflicts over the pharmaceutical industry. Soc. Sci. Med. 2014, 114, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Milewa, T. Health care, consumerism and the politics of identity. In The New Sociology of Health Service; Gabe, J., Calnan, M., Eds.; Routledge: London, UK, 2009; pp. 161–176. [Google Scholar]
- Crawford, R. Healthism and the medicalization of everyday life. Health 2006, 10, 401–420. [Google Scholar] [CrossRef] [PubMed]
- Rose, N. Powers of Freedom: Reframing Political Thought; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Foucault, M. Technologies of the self. In Technologies of the Self: A Seminar with Michel Foucault; Martin, L.H., Gutman, H., Hutton, P.H., Eds.; The University of Massachusetts Press: Amherst, MA, USA, 1988; pp. 16–49. [Google Scholar]
- Ryan, K.; Bissell, P.; Morgall Traulsen, J. The work of Michel Foucault: Relevance to pharmacy practice. Int. J. Pharm. Pract. 2004, 12, 43–52. [Google Scholar] [CrossRef]
- Ballantyne, P.J.; Mirza, R.M.; Austin, Z.; Boon, H.S.; Fisher, J.A. Becoming old as a “pharmaceutical person”: Negotiation of health and medicines by Canadian immigrant and non-immigrant older adults. Can. J. Aging 2011, 30, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Dew, K.; Chamberlain, K.; Hodgetts, D.; Norris, P.; Radley, A.; Gabe, J. Home as a hybrid centre of medication practice. Soc. Health Illn. 2014, 36, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.M.; Eerdekens, M.; Lindenmayer, J.P.; Keith, S.J.; Lesem, M.; Karcher, K. Long-acting injectable Risperidone: Efficacy and safety of the first long-acting atypical antipsychotic. Am. J. Psychia 2003, 160, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Anheier, H.K.; Gerhards, J.; Romo, F.P. Forms of Capital and Social Structure in Cultural Fields: Examining Bourdieu’s Social Topography. Am. J. Sociol. 1995, 100, 859–903. [Google Scholar] [CrossRef]
- Lumme-Sandt, K.; Virtanen, P. Older people in the field of medication. Sociol. Health Illn. 2002, 24, 285–304. [Google Scholar] [CrossRef]
- Abraham, J. Pharmaceuticalization of society in context: Theoretical, empirical and health dimensions. Sociology 2010, 44, 603–622. [Google Scholar] [CrossRef]
- Light, D.W. The rhetoric and realities of community health care: The limits of countervailing powers to meet the health care needs of the twenty-first century. J. Health Politics Policy Law 1997, 22, 105–145. [Google Scholar]
- Harten, C.; Ballantyne, P.J. Impact of cost-sharing within provincial drug benefit programs: A review. J. Pharm. Finance Econ. Policy 2004, 13, 35–53. [Google Scholar] [CrossRef]
- Williams, S.J.; Martin, P.; Gabe, J. The pharmaceuticalization of society? A framework for analysis. Soc. Health Illn. 2011, 33, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Lexchin, J.; Bero, L.; Djulbegovic, B.; Clark, O. Pharmaceutical industry sponsorship and research outcome and quality: Systematic review. BMJ 2003, 326, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Sismondo, S. Pharmaceutical company funding and its consequences: A qualitative systematic review. Contemp. Clin. Trials 2008, 29, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Mintzes, B.; Lexchin, J.; Sutherland, J.M.; Beaulieu, M.D.; Wilkes, M.S.; Durrieu, G.; Reynolds, E. Pharmaceutical sales representatives and patient safety: A comparative, prospective study of information quality in Canada, France, and the United States. J. Gen. Intern. Med. 2013, 28, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, S.H.; Greene, J.A. A historical perspective of pharmaceutical promotion and physician education. JAMA 2008, 300, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Fugh-Berman, A.; Ahari, S. Following the script: How drug reps make friends and influence doctors. PLoS Med. 2007, 4, e150. [Google Scholar] [CrossRef] [PubMed]
- Padamsee, T.J. The pharmaceutical corporation and the ‘good work’ of managing women’s bodies. Soc. Sci. Med. 2011, 72, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J. Sociology of pharmaceuticals development and regulation: A realist empirical research programme. Soc. Health Illn. 2008, 30, 869–885. [Google Scholar] [CrossRef] [PubMed]
- Silversides, A. Lifting the curtain on the drug approval process. In The Push to Prescribe: Woman and Canadian Drug Policy; Rochon Ford, A., Saibil, D., Eds.; Women’s Press: Toronto, ON, Canada, 2010; pp. 115–138. [Google Scholar]
- Fergusson, D.; Doucette, S.; Cranley Grass, K.; Shapiro, S.; Healy, D.; Herbert, P.; Hutton, B. Association between suicide attempts and selective serotonin reuptake inhibitors: Systematic review of randomised controlled trials. BMJ 2005, 330, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.J.; Campen, D.; Hui, R.; Spence, M.; Cheetham, C.; Levy, G.; Shoor, S.; Ray, W.A. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: Nested case control study. Lancet 2005, 365, 475–481. [Google Scholar] [CrossRef]
- Herxheimer, A.; Mintzes, B. Anti-depressants and adverse effects in young patients: Uncovering the evidence. CMAJ 2004, 170, 487–489. [Google Scholar] [PubMed]
- Abraham, J.; Davis, C. Risking public safety: Experts, the medical profession and ‘acceptable’ drug injury. Health Risk Soc. 2005, 7, 379–395. [Google Scholar] [CrossRef]
- Stafford, R.S. Regulating off-label drug use: Re-thinking the role of the FDA. NEJM 2008, 358, 1427–1429. [Google Scholar] [CrossRef] [PubMed]
- Eugale, T.; Buckeridge, D.L.; Verma, A.; Winslade, N.E.; Benedetti, A.; Hanley, J.A.; Tamblyn, R. Association of off-label drug use and adverse drug events in an adult population. JAMA Intern. Med. 2016, 176, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Pound, P.; Britten, N.; Morgan, M.; Yardley, L.; Pope, C.; Daker-White, G.; Campbell, R. Resisting medicines: A synthesis of qualitative studies of medicine taking. Soc. Sci. Med. 2005, 61, 133–155. [Google Scholar] [CrossRef] [PubMed]
- Mintzes, B.; Barer, M.; Kravitz, R.L.; Bassett, K.; Lexchin, J.; Kazanjian, A.; Evans, R.G.; Pan, R.; Marion, S.A. How does direct-to-consumer advertising (DTCA) affect prescribing? A survey in primary care environments with and without legal DTCA. CMAJ 2003, 169, 405–412. [Google Scholar] [PubMed]
- Stevenson, F.A.; Leontowitch, M.; Duggan, C. Over-the-counter medicines: Professional expertise and consumer discourses. Soc. Health Illn. 2008, 30, 913–928. [Google Scholar] [CrossRef] [PubMed]
- United Kingdom Department of Health. Choosing Health through Pharmacy: A Programme for Pharmaceutical Public Health 2005–2015; Department of Health: London, UK, 2005. [Google Scholar]
- Pharmacy Council of New Zealand. Pharmacist Prescribers. Available online: http://www.pharmacycouncil.org.nz/cms_display.php?sn=232&st=1 (accessed on 27 November 2015).
- Canadian Pharmacists Association Pharmacists’ Expanded Scope of Practice. Available online: http://www.pharmacists.ca/index.cfm/pharmacy-in-canada/scope-of-practice-canada/ (accessed on 27 November 2015).
- Hepler, D.D.; Strand, L.M. Opportunities and responsibilities in pharmaceutical care. Am. J. Pharm. Ed. 1989, 53, 7S–15S. [Google Scholar]
- Jesson, P.; Bissell, P. Public health and pharmacy: A critical review. Crit. Public Health 2006, 16, 159–169. [Google Scholar] [CrossRef]
- Mead, N.; Bower, P. Patient-centredness: A conceptual framework and review of the empirical literature. Soc. Sci. Med. 2000, 51, 1087–1110. [Google Scholar] [CrossRef]
- Bush, J.; Langley, C.A.; Wilson, K.A. The corporatization of community pharmacy: Implications for service provision, the public health function and pharmacy’s claims to professional status in the United Kingdom. Res. Soc. Admin. Pharm. 2009, 5, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.E. Corporate control and professional prerogative: An unresolved tension for pharmacists. Res. Soc. Admin. Pharm. 2009, 5, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Richardson, E.; Pollock, A.M. Community pharmacists: From dispensing to diagnosis. BMJ 2010, 340. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.G.; McMahon, M.; Mitton, C.; Roughead, E.; Kirk, R.; Kanavos, P.; Menon, D. Centralized drug review processes in Australia, Canada, New Zealand, and the United Kingdom. Health Affairs 2006, 25, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. Patented Medicines Prices Review Board. Available online: http://www.canada.ca/en/patented-medicine-prices-review/index.html (accessed on 27 November 2015).
- UK Department of Health. Pharmaceutical Price Regulation Scheme. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/282523/Pharmaceutical_Price_Regulation.pdf (accessed on 27 November 2015).
- US Congress. Medicare Prescription Drug Price Negotiation Act of 2007. Available online: https://www.congress.gov/bill/110th-congress/house-bill/4 (accessed on 27 November 2015).
- Bauschke, R. Regulatory agencies, pharmaceutical information and the Internet: A European perspective. Health Policy 2012, 104, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Mulinari, S. Regulating drug information in Europe: A pyrrhic victory for pharmaceutical industry critics? Soc. Health Illn. 2013, 35, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Puzzanghera, J.; Masunaga, S. Pfizer and Allergan’s $160 billion pharmaceutical merger puts new twist on tax-avoiding inversions. Los Angeles Times. 23 November 2015. Available online: http://www.post-gazette.com/business/healthcare-business/2015/11/23/Pfizer-Allergan-160-million-merger-forms-world-s-largest-drugmaker/stories/201511230138 (accessed on 27 November 2015).
- Pollack, A. Transforming the critique of Big Pharma. Biosocieties 2011, 6, 106–118. [Google Scholar] [CrossRef]
- Lexchin, J. How safe are new drugs? Market withdrawal of drugs approved in Canada between 1990 and 2000. Open Med. 2014, 8, e14–e19. [Google Scholar] [PubMed]
- McGee, S. Daraprim ‘Profiteering’ Controversy Lifts Lid on Soaring Cost of Prescription Drugs. Guardian UK. 27 September 2015. Available online: http://readersupportednews.org/opinion2/277-75/32624-daraprim-profiteering-controversy-lifts-lid-on-soaring-cost-of-prescription-drugs (accessed on 27 November 2015).
- International Federation of Pharmaceutical Manufacturers and Associations. About IFPMA. Available online: http://www.ifpma.org/about-ifpma/welcome.html (accessed on 27 November 2015).
- Pharmaceutical Research and Manufacturers of America. About PhRMA. Available online: http://www.phrma.org/about (accessed on 27 November 2015).
- Ebeling, M. ‘Get with the program!’ Pharmaceutical marketing, symptom checklists and self-diagnosis. Soc. Sci. Med. 2011, 73, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Barker, K.K. Listening to Lyrica: Contested illnesses and pharmaceutical determinism. Soc. Sci. Med. 2011, 73, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Sharfstein, J.M.; North, M.; Serwint, J.R. Over the counter but no longer under the radar—Paediatric cough and cold medications. N. Engl. J. Med. 2007, 357, 2321–2324. [Google Scholar] [CrossRef] [PubMed]
- Chaar, B.; Kwong, K. Direct-to-consumer advertising: Australian pharmacists’ experiences with non-prescription medicines. Int. J. Pharm. Pract. 2010, 18, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mintzes, B. Ask your doctor: Women and direct-to-consumer advertising. In The Push to Prescribe: Woman and Canadian Drug Policy; Rochon Ford, A., Saibil, D., Eds.; Women’s Press: Toronto, ON, Canada, 2010; pp. 17–46. [Google Scholar]
- Batt, S. Who pays the piper? Industry funding of patients’ groups. In The Push to Prescribe: Woman and Canadian Drug Policy; Rochon Ford, A., Saibil, D., Eds.; Women’s Press: Toronto, ON, Canada, 2010; pp. 67–92. [Google Scholar]
- Lofgren, H. Pharmaceuticals and the consumer movement: The ambivalences of ‘patient power’. Aust. Health Rev. 2004, 28, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A. IMS: US Prescription Drug Spending Jumped 13 pct. in 2014. Associated Press, 14 April 2015. Available online: http://finance.yahoo.com/news/ims-us-prescription-drug-spending-040233602.html# (accessed on 27 November 2015).
- Hirschler, B. Bumper haul of expensive new drugs heads to U.S. and Europe. Reuters, 20 November 2015. Available online: http://finance.yahoo.com/news/bumper-haul-expensive-drugs-heads-185324287.html (accessed on 27 November 2015).
- Kilwein, J.H. Commentary: The noncompliance blame game. J Clin. Pharm. Ther. 2001, 26, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Britten, N. Lay views of drugs and medicines: Orthodox and unorthodox accounts. In Modern Medicine—Lay Perspectives and Experiences; Williams, S.J., Calnan, M., Eds.; UCL Press: London, UK, 1996; pp. 48–73. [Google Scholar]
- Benson, J.; Britten, N. Patients’ decisions about whether or not to take antihypertensive drugs: Qualitative study. BMJ 2002, 325, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, P.; Holme Hansen, E.; Morgall Traulsen, J.M.; Eskildsen, K. Changes in self-concept while using SSRI antidepressants. Qual. Health Res. 2002, 12, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Hansson Scherman, M; Löwhagen, O. Drug compliance and identity: Reasons for non-compliance. Experiences of medication from persons with asthma/allergy. Patient Educ. Couns. 2004, 54, 3–9. [Google Scholar]
- Hansen, D.L.; Holstein, B.E.; Holme Hansen, E. “I’d rather not take it, but…”: Young women’s perceptions of medicines. Qual. Health Res. 2009, 19, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.E.; Ballantyne, P.J.; Hawker, G. Older adults living with osteoarthritis: Theorizing the impact of age and gender on lay-professional negotiations around medicine use. Can. J. Aging 2012, 31, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Venn, S.; Arber, S. Understanding older peoples’ decisions about the use of sleeping medications: Issues of control and autonomy. Soc. Health Illn. 2012, 34, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- Sandell, K.; Bornäs, H. Functional numbness instead of feelings as a direction: Young adults’ experiences of antidepressant use. Sociology 2015, 1–16. [Google Scholar]
- Brijnath, B.; Antoniades, J. “I’m running my depression”: Self-management of depression in neoliberal Australia. Soc. Sci. Med. 2016, 152, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martin, E. The pharmaceutical person. Biosocieties 2006, 1, 273–287. [Google Scholar] [CrossRef]
- Carder, P.C.; Vuckovic, N.; Green, C.A. Negotiating medications: Patient perceptions of long-term medication use. J. Clin. Pharm. Ther. 2003, 28, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Angell, B.; Bolden, G.B. Justifying medication decisions in mental health care: Psychiatrists’ accounts for treatment recommendations. Soc. Sci. Med. 2015, 138, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Reason, B.; Terner, M.; McKeag, A.M.; Tipper, B.; Webster, G. The impact of polypharmacy on the health of Canadian seniors. Fam. Pract. 2012, 29, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.A.; Poon, I.; Nair, M. Clinical consequences of polypharmacy in the elderly: Expect the unexpected, think the unthinkable. Expert Opin. Drug Saf. 2007, 6, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Persaud, M.; Holdoyd-Leduc, J.M. Anticholinergic medications in the older adult: A hidden burden. Can. Geriatr. Soc. J. 2014, 4, 4–7. [Google Scholar]
- Mallet, L.; Spinewine, A.; Huang, A. The challenge of managing drug interactions in elderly people. Lancet 2007, 370, 185–191. [Google Scholar] [CrossRef]
- Health Quality Ontario. Looking for Balance: Antipsychotic Medication Use in Ontario Long-Term Care Homes; Queen’s Printer for Ontario: Toronto, ON, Canada, 2015; Available online: http://www.hqontario.ca/public-reporting/theme-reports/looking-for-balance (accessed on 03 April 2016).
- Filc, D. The medical text: Between biomedicine and hegemony. Soc. Sci. Med. 2004, 59, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Salter, B.; Zhou, Y.; Datta, S. Hegemony in the marketplace of biomedical innovation: Consumer demand and stem cell science. Soc. Sci. Med. 2015, 131, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R. Health as meaningful practice. Health: Interdiscip. J. Soc. Study Health Illn. Med. 2006, 10, 401–420. [Google Scholar]
- Noerreslet, M.; Jemec, G.B.E.; Traulsen, J.M. Involuntary autonomy: Patients’ perceptions of physicians, conventional medicines and risks in the management of atopic dermatitis. Soc. Sci. Med. 2009, 69, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.J.; Ward, K.J.; O’Rourke, A.J. The ‘expert patient’: Empowerment of medical dominance? The case of weight loss, pharmaceutical drugs and the internet. Soc. Sci. Med. 2005, 60, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.J.; Ward, K. Health identities: From expert patient to resisting consumer. Health: Interdiscip. J. Soc. Study Health Illn. Med. 2006, 10, 461–479. [Google Scholar] [CrossRef] [PubMed]
- Varul, M.A. Talcott Parsons, the sick role and chronic illness. Body Soc. 2010, 16, 72–94. [Google Scholar] [CrossRef]
- Stephens, E.C.; Johnson, M.M.S. Dr. Mom and other influences on youger and older adults’ OTC medication purchases. J. Appl. Gerontol. 2000, 19, 441–459. [Google Scholar] [CrossRef]
- Kata, A. A post-modern Pandora’s box: Anti-vaccination misinformation on the Internet. Vaccine 2010, 28, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, D.; Sang, Y. Facebook as a platform for health information and communication: A case study of a diabetes group. J. Med. Syst. 2013, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F. New forms of citizenship and sociopolitical inclusion: Accessing antiretroviral therapy in Rio de Janeiro favela. Soc. Health Illn. 2008, 30, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Lyttleton, C.; Beesey, A.; Sitthikriengkrai, M. Expanding community through ARV provision in Thailand. AIDS Care 2007, 19 (Suppl. 1), S44–S53. [Google Scholar] [CrossRef] [PubMed]
- Nyugen, V.K.; Ako, C.Y.; Niamba, P.; Sylla, A.; Tiendrébéogo, I. Adherence as therapeutic citizenship: Impact of the history of access to antiretroviral drugs on adherence to treatment. AIDS 2007, 21 (Suppl. 5), S31–S35. [Google Scholar]
- Rabinow, P. Artificiality and enlightenment: From sociobiology to biosociality. In Incorporations; Crary, J., Kwinter, S., Eds.; Zone: New York, NY, USA, 2005. [Google Scholar]
- Baker, B.A. Placing access to essential medicines on the human rights agenda. In The Power of Pills. Social, Ethical and Legal Issues in Drug Development, Marketing and Pricing; Cohen, J.C.C., Illingworth, P., Schuklenk, U., Eds.; Pluto Press: London, UK, 2006; Chapter 22. [Google Scholar]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballantyne, P.J. Understanding Users in the ‘Field’ of Medications. Pharmacy 2016, 4, 19. https://doi.org/10.3390/pharmacy4020019
Ballantyne PJ. Understanding Users in the ‘Field’ of Medications. Pharmacy. 2016; 4(2):19. https://doi.org/10.3390/pharmacy4020019
Chicago/Turabian StyleBallantyne, Peri J. 2016. "Understanding Users in the ‘Field’ of Medications" Pharmacy 4, no. 2: 19. https://doi.org/10.3390/pharmacy4020019
APA StyleBallantyne, P. J. (2016). Understanding Users in the ‘Field’ of Medications. Pharmacy, 4(2), 19. https://doi.org/10.3390/pharmacy4020019




