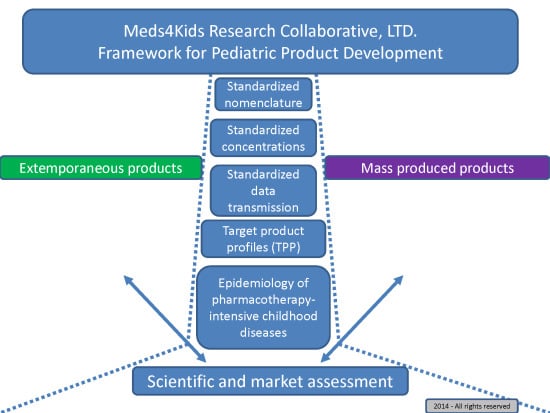
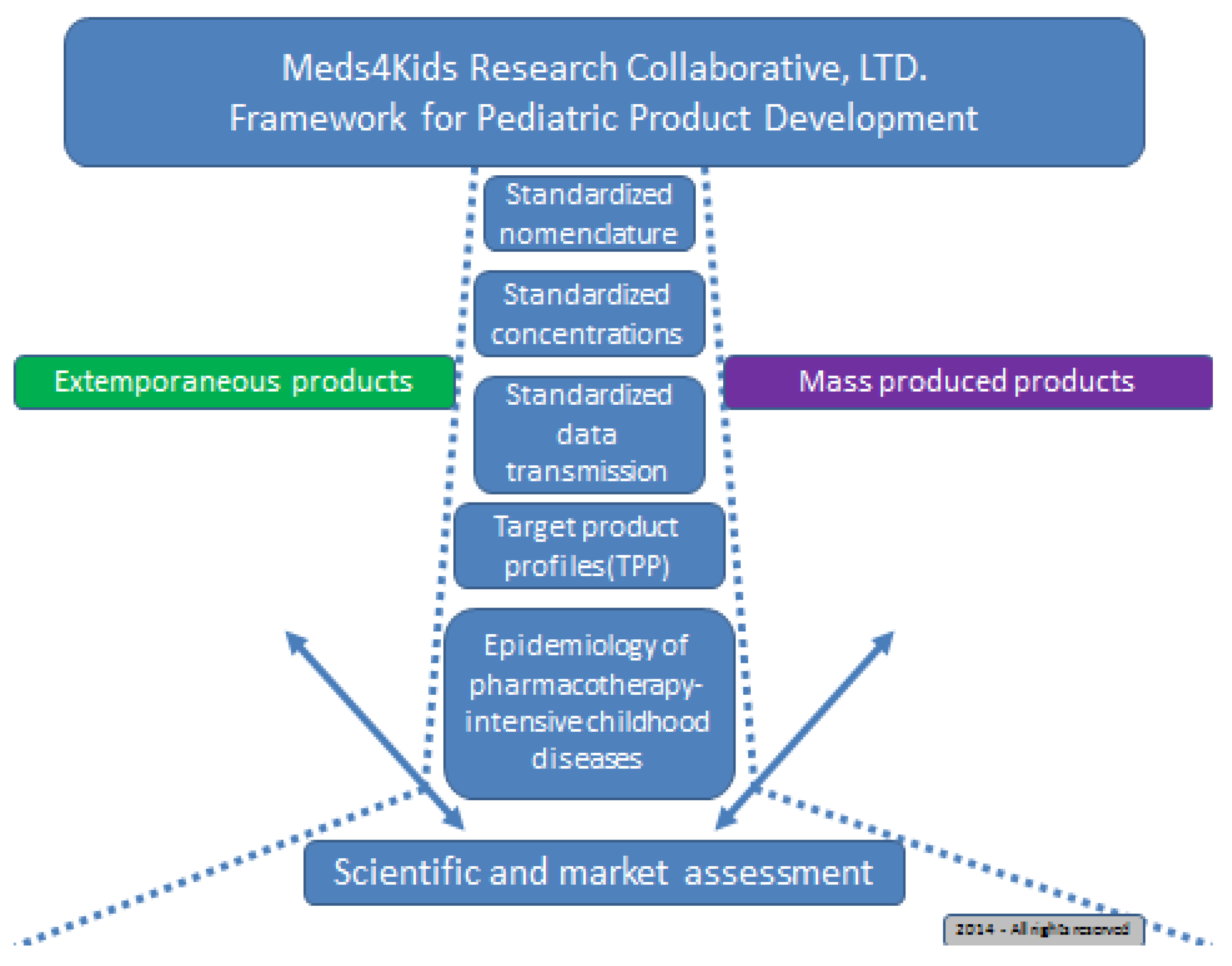
An Algorithm to Identify Compounded Non-Sterile Products that Can Be Formulated on a Commercial Scale or Imported to Promote Safer Medication Use in Children
Abstract
:1. Introduction
2. Why Is off-Label Use of Drugs in Children Still a Problem?
- Lack of specifications required for component development by compounding pharmacies.
- No onsite testing of active ingredients and excipients for purity, potency, content and stability.
- No onsite specifications or testing of product containers and closures.
- Site-to-site variations in compounding procedures, equipment, and the degree of product handling/manipulation.
- Lack of environmental control, which might lead to unintentional contamination and generation of degradation products due to inconsistent exposure to light, temperature and processing controls.
- Lack of testing of finished products for purity, potency, content or stability.
- Stability data for establishing expiry dates of compounded products are derived from published data, where preparation methods likely vary from local methods, or are simply default expiry periods defined by regional pharmacy regulations and “best practices”.
- Published preparation methods provide only a portion of the information needed to consistently prepare a stable potent final product.
- Limited options available to mask bad-tasting active ingredients.
- The dose administration technologies used such as droppers, syringes, scoops, spoons, etc., vary between sites and between prescription fills.
- Weak regulatory oversight.
3. Development of the Algorithm for a Tiered Selection Model for Candidate APIs
3.1. Clinical and Market Considerations
- (1)
- Listed on BPCA priority document (clinical need—broad base);
- (2)
- Available in an injection (API is stable in solution for at least 18–24 months);
- (3)
- Not a federally controlled substance in Canada or the US (minimal paperwork for chain of custody);
- (4)
- Potential applicability for oral adult market (for adult patients that cannot swallow tablets or capsules);
- (5)
- Established indication for any age group in the official labeling of the API (immediate marketability through accelerated ANDA mechanism) and
- (6)
- Off-patent in both Canada and the US, and available in a pharmaceutical grade powder from a reputable supplier.
- The first tier included API molecules that met at least five of the six criteria;
- The second tier fulfilled four criteria;
- The third tier included API molecules not listed by name in the BPCA list but met all others except availability in the United Kingdom; and
- The fourth tier included those available in the UK that could be imported readily for pharmacokinetic (PK), pharmacodynamics (PD), and/or pharmacogenomics (PG) studies in children.
| Generic Name | BCPA Listed? | Injection? | Non-Scheduled? | Oral Adult Market? | Established Adult Indication? | Off Patent? | Dose Form in UK? |
|---|---|---|---|---|---|---|---|
| Baclofen | 1 | 1 | 1 | 1 | 1 | 1 | Yes a |
| Warfarin | 1 | 1 | 1 | 1 | 1 | 1 | Yes b |
| Sildenafil | 1 | 1 | 1 | 1 | 1 | 1 | Yes c |
| l-thyroxine | 1 | 1-lyo | 1 | 1 | 1 | 1 | Yes d |
3.2. Scientific and Production Considerations—Applying BCS and HME
| API Generic Name | BCS Class | Reference |
|---|---|---|
| Metoprolol | II | [15] |
| Clopidogrel | II | [16] |
| Lisinopril | III | [16] |
| Amlodipine | I | [17] |
| Ursodiol | Uncl. | n.d. |
| Bosentan | Uncl. | n.d. |
| Pantoprazole | Uncl. | n.d. |
| Acetazolamide | IV | [17] |
| Spironoloactone | II/IV | [16] |
| Valaciclovir | I/III | [17] |
| Captopril | III | [18] |
| Nifedipine | II | [16] |
| Baclofen | Uncl. | n.d. |
| Warfarin | I | [16] |
| Sildenafil | I | [15] |
| l-thyroxine | III | [16] |
4. Conclusions
Author Contributions
Conflicts of Interest
References and Notes
- Committee on Drugs. Off-label use of drugs in children. Pediatrics 2014, 133, 563–567. [Google Scholar]
- Parrish, R.H., II; Cernak, I. Creating a distinct medicines-use system for children: The time is now. Pharmacy 2015, 3, 72–78. [Google Scholar]
- Laughon, M.M.; Anat, D.; Tripathi, N.; Hornik, C.P.; Cohen-Wolkowiez, M.; Clark, R.H.; Smith, P.B.; Rodriguez, W. Drug labeling and exposure in neonates. JAMA Pediatr. 2014, 168, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Compounding Compendium; The United States Pharmacopeial Convention: Rockville, MD, USA; Available online: http://www.usp.org/sites/default/files/usp_pdf/EN/products/2015_usp_compounding_compendium_feb_2015_toc.pdf (accessed on 21 August 2015).
- Jew, R.K.; Soo-Hoo, W.; Erush, S.C. Extemporaneous Formulations for Pediatric, Geriatric, and Special Needs Patients, 2nd ed.; American Society of Health-System Pharmacists: Bethesda, MD, USA, 2010. [Google Scholar]
- Vanchieri, C.; Stith Butler, A.; Knutsen, A. Addressing the Barriers to Pediatric Drug, Development: Workshop Summary Forum on Drug Discovery, Development, and Translation; The National Academies Press: Washington, DC, USA, 2008; Available online: http://www.nap.edu/catalog/11911.html (accessed on 31 March 2015).
- Milne, C. Economic Issues for Making Available Adequate Drug Dosage Forms. In Proceedings of the Pediatric Formulations Initiative Workshop, Potomac, MD, USA, 2011; NIHCD. Available online: http://bpca.nichd.nih.gov/collaborativeefforts/documents/pfi_workshop_11-1-2011.pdf (accessed on 31 March 2015).
- Compounded Non-Sterile Product List. St. Christopher’s Hospital for Children, 2013. (List is available upon request).
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Löbenberg, R.; Amidon, G.L. Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur. J. Pharm. Biopharm. 2000, 5, 3–12. [Google Scholar] [CrossRef]
- Wu, C.Y.; Benet, L.Z. Predicting drug disposition via application of BCS: Transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm. Res. 2005, 22, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Löbenberg, R.; Chacra, N.B.; Stippler, E.S.; Shah, V.P.; DeStefano, A.J.; Hauck, W.W.; Williams, R.L. Toward global standards for comparator pharmaceutical products: Case studies of amoxicillin, metronidazole, and zidovudine in the Americas. AAPS J. 2012, 14, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, S.M.; Amidon, G.L.; Kaul, A.; Lukacova, V.; Vinks, A.; Knipp, G.T. Summary of the National Institute of Child Health and Human Development-Best Pharmaceuticals for Children Act Pediatric Formulation Initiatives Workshop—Pediatric Biopharmaceutics Classification System Working Group. Clin. Ther. 2012, 34, S11–S24. [Google Scholar] [CrossRef] [PubMed]
- Almukainzi, M.; Okumu, A.; Wei, H.; Löbenberg, R. Simulation of in Vitro Dissolution Behavior Using DDDPlus™. AAPS PharmSciTech 2014, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Ramachandran, C.; Bermejo, M.; Yamashita, S.; Yu, L.X.; Amidon, G.L. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol. Pharm. 2006, 3, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, E.; Laosa, O.; Guerra, P.; Duque, B.; Mosquera, B.; Borobia, A.M.; Lei, S.H.; Carcas, A.J.; Frias, J. Acceptability and characteristics of 124 human bioequivalence studies with active substances classified according to the Biopharmaceutic Classification System. Br. J. Clin. Pharmacol. 2010, 70, 694–702. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Expert Committee on Specifications for Pharmaceutical Preparations; WHO Technical Report Series; WHO Press: Geneva, CH, 2013; Available online: http://www.who.int/medicines/areas/quality_safety/quality_assurance/expert_committee/TRS981.pdf (accessed 05 November 2015).
- Dahan, A.; Wolk, O.; Kim, Y.H.; Ramachandran, C.; Crippen, G.M.; Takagi, T.; Bermejo, M.; Amidon, G.L. Purely in silico BCS classification: Science based quality standards for the world’s drugs. Mol. Pharm. 2013, 10, 4378–4390. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Battu, S.K.; McGinity, J.W.; Martin, C. Pharmaceutical applications of hot-melt extrusion—Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.A.; Battu, S.K.; Upadhye, S.B.; Thumma, S.; Crowley, M.M.; Zhang, F.; Martin, C.; McGinity, J.W. Pharmaceutical applications of hot-melt extrusion—Part II. Drug Dev. Ind. Pharm. 2007, 33, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Boateng, J.S.; Snowden, M.J. A Review of Hot-Melt Extrusion: Process Technology to Pharmaceutical Products. ISRN Pharm. 2012, 436763. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, H.K.; Fotaki, N.; Klein, S. Paediatric Biopharmaceutics. Adv. Drug Deliv. Rev. 2014, 73, 102–126. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, H.K.; European Paediatric Formulation Initiative (EUPFI). Paediatric Biopharmaceutics Classification System: Current status and future decisions. Int. J. Pharm. 2014, 469, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Tannergren, C.; Bergendal, A.; Lennermas, H.; Abrahamsson, B. Toward an increased understanding of the barriers to colonic drug absorption in humans: Implications for early controlled release candidate assessment. Mol. Pharm. 2009, 6, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.T.; Daniels, S.R.; Hayman, L.L.; Maahs, D.M.; McCrindle, B.W.; Mitsnefes, M.; Zachariah, J.P.; Urbina, E.M.; American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Update: Ambulatory blood pressure monitoring in children and adolescents: A scientific statement from the American Heart Association. Hypertension 2014, 63, 1116–1135. [Google Scholar] [CrossRef] [PubMed]
- The Netherlands. Public Assessment Report for paediatric studies submitted in accordance with Article 45 of Regulation (EC) No1901/2006, as amended. Metoprolol succinate. Available online: www.sukl.cz/file/76992_1_1 (accessed 05 November 2015).
- Allen, L.V.; Erickson, M.A. Stability of labetalol hydrochloride, metoprolol tartrate, verapamil hydrochloride, and spironolactone with hydrochlorothiazide in extemporaneously compounded oral liquids. Am. J. Health Syst. Pharm. 1996, 53, 2304–2309. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatt-Mehta, V.; MacArthur, R.B.; Löbenberg, R.; Cies, J.J.; Cernak, I.; II, R.H.P. An Algorithm to Identify Compounded Non-Sterile Products that Can Be Formulated on a Commercial Scale or Imported to Promote Safer Medication Use in Children. Pharmacy 2015, 3, 284-294. https://doi.org/10.3390/pharmacy3040284
Bhatt-Mehta V, MacArthur RB, Löbenberg R, Cies JJ, Cernak I, II RHP. An Algorithm to Identify Compounded Non-Sterile Products that Can Be Formulated on a Commercial Scale or Imported to Promote Safer Medication Use in Children. Pharmacy. 2015; 3(4):284-294. https://doi.org/10.3390/pharmacy3040284
Chicago/Turabian StyleBhatt-Mehta, Varsha, Robert B. MacArthur, Raimar Löbenberg, Jeffrey J. Cies, Ibolja Cernak, and Richard H. Parrish II. 2015. "An Algorithm to Identify Compounded Non-Sterile Products that Can Be Formulated on a Commercial Scale or Imported to Promote Safer Medication Use in Children" Pharmacy 3, no. 4: 284-294. https://doi.org/10.3390/pharmacy3040284
APA StyleBhatt-Mehta, V., MacArthur, R. B., Löbenberg, R., Cies, J. J., Cernak, I., & II, R. H. P. (2015). An Algorithm to Identify Compounded Non-Sterile Products that Can Be Formulated on a Commercial Scale or Imported to Promote Safer Medication Use in Children. Pharmacy, 3(4), 284-294. https://doi.org/10.3390/pharmacy3040284