Effects of Modifying Supportive Care Medications in Combination Therapy with Pertuzumab, Trastuzumab, and Taxane Anticancer Drugs
Abstract
1. Introduction
2. Materials and Methods
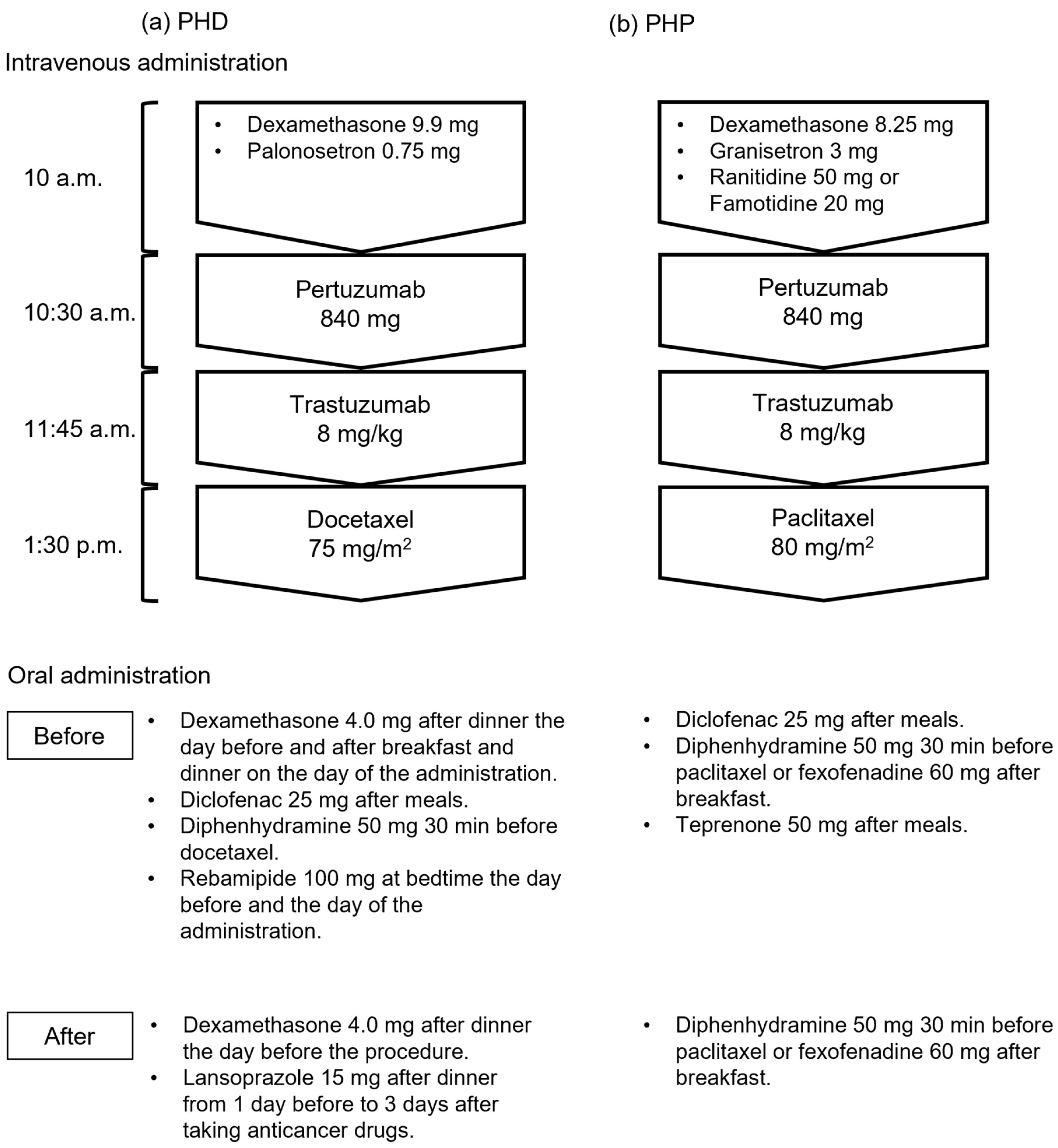
2.1. Patients and Regimens
2.2. Collection of Medical Information
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Patients
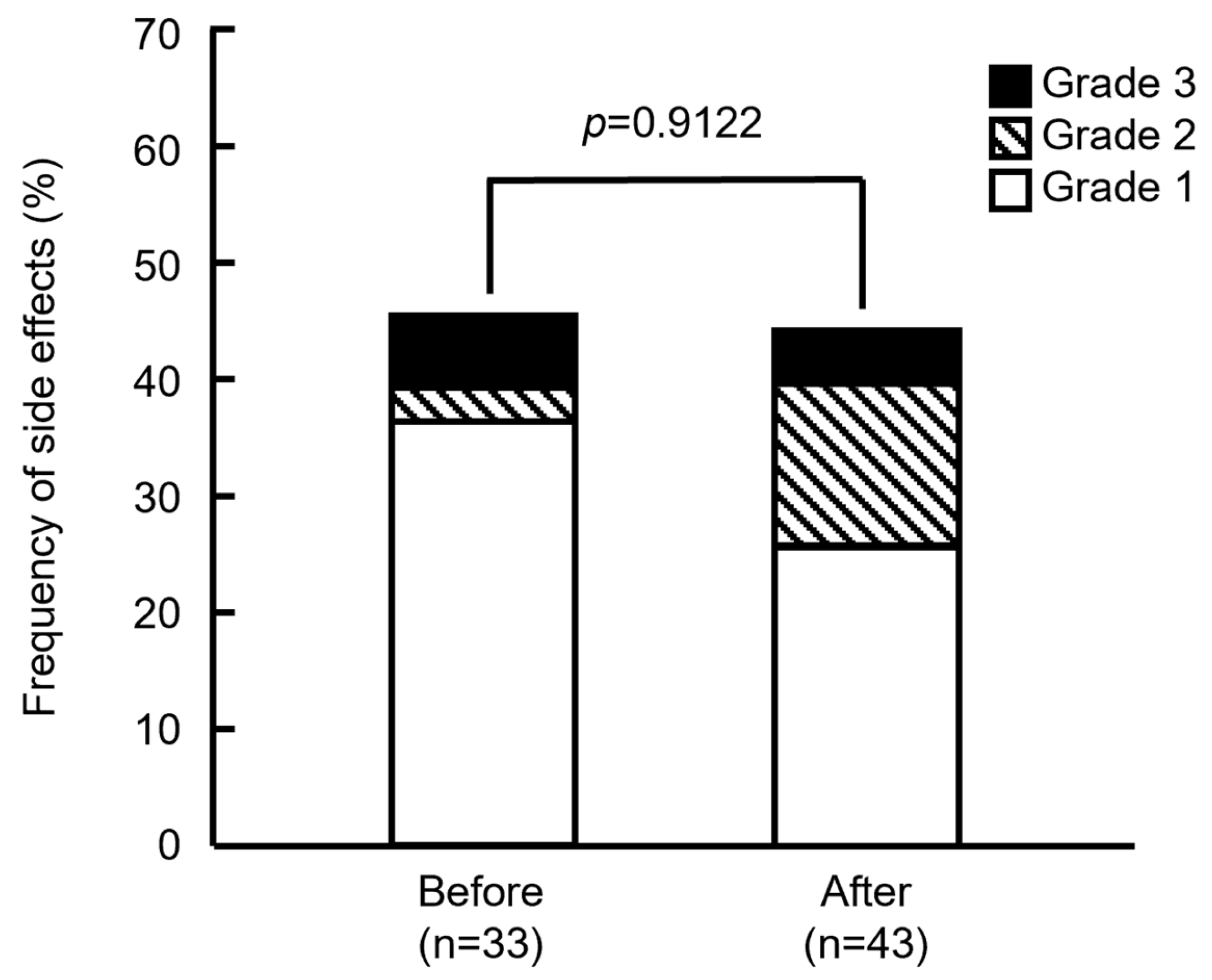
3.2. Frequency of Side Effects Before and After Modification Supportive Care Medications
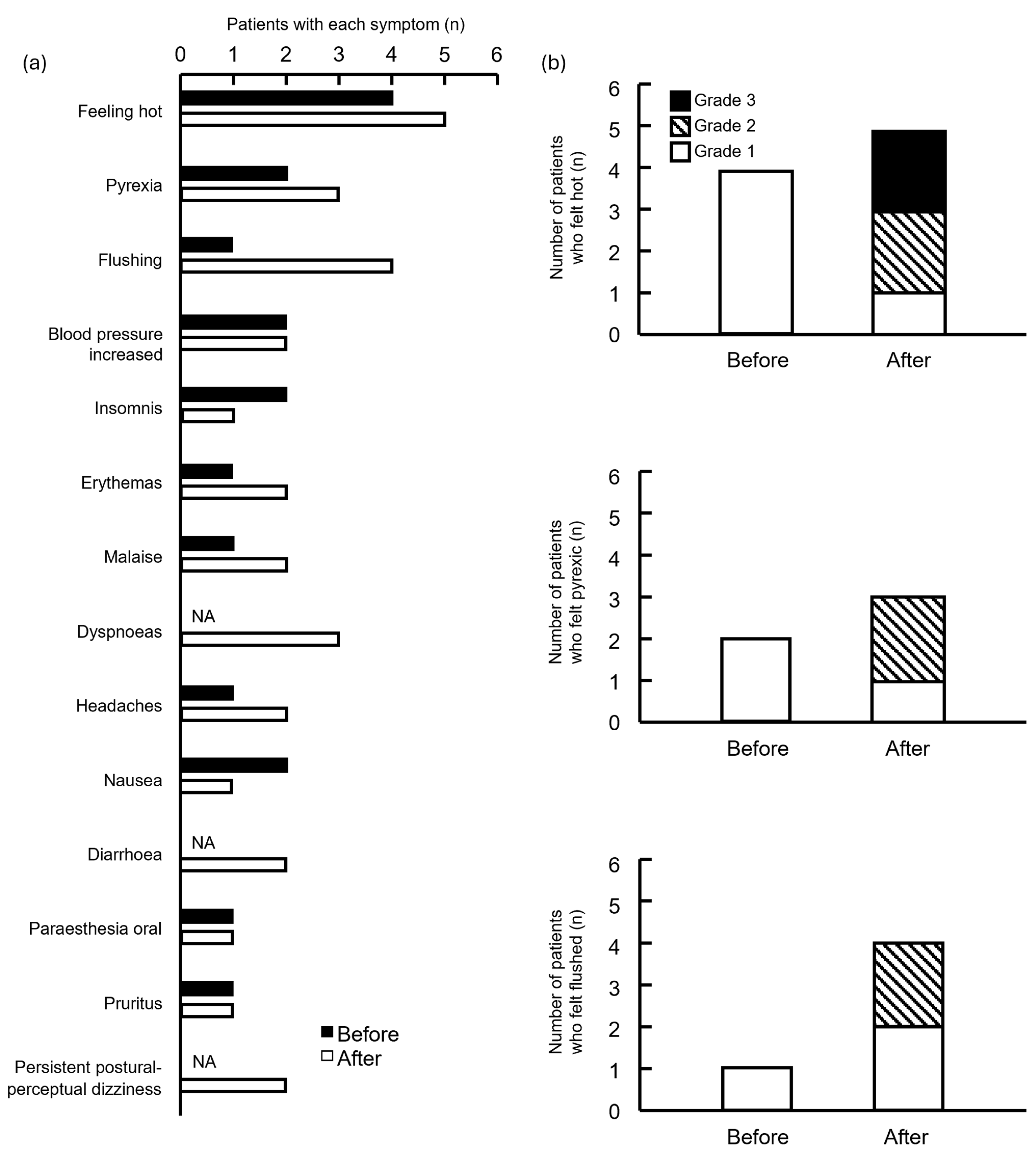
3.3. Symptoms
3.4. Timing of Side Effects
4. Discussion
4.1. Interpretation of Findings
4.2. Limitations
4.3. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PHD | Pertuzumab, Trastuzumab, and Docetaxel |
| PHP | Pertuzumab, Trastuzumab, and Paclitaxel |
| HER2 | Human epidermal growth factor receptor type 2 |
| PER | Pertuzumab |
| TRA | Trastuzumab |
| DTX | Docetaxel |
| PTX | Paclitaxel |
| IRR | Infusion-related reaction |
| BMI | Body mass index |
Appendix A. Description of Side Effects for Each Patient
| Subject ID | Group | Regimen | Timing | Symptoms | Grade |
|---|---|---|---|---|---|
| 1 | Before | PHP | Unknown | Oedema | 1 |
| 2 | Before | PHP | Unknown | Blood pressure increased; Somnolence; Headaches; Nausea | 1 |
| 3 | Before | PHP | DTX/PTX | Blood pressure increased | 2 |
| 4 | Before | PHD | DTX/PTX | Malaise | 1 |
| 5 | Before | PHD | DTX/PTX | Initial insomnia; Pruritus; Erythema; Paraesthesia oral | 3 |
| 6 | Before | PHP | PER/TRA | Somnolence; Feeling abnormal; Tension; Discomfort | 3 |
| 7 | Before | PHP | DTX/PTX | Initial insomnia | 1 |
| 8 | Before | PHP | DTX/PTX | Palpitations; Feeling hot | 1 |
| 9 | Before | PHP | DTX/PTX | Feeling hot | 1 |
| 10 | Before | PHP | DTX/PTX | Pyrexia; Oropharyngeal discomfort | 1 |
| 11 | Before | PHP | DTX/PTX | Hyperhidrosis; Feeling hot | 1 |
| 12 | Before | PHP | DTX/PTX | Flushing | 1 |
| 13 | Before | PHP | DTX/PTX | Nausea | 1 |
| 14 | Before | PHP | DTX/PTX | Feeling hot | 1 |
| 15 | Before | PHD | DTX/PTX | Pyrexia | 1 |
| 16 | After | PHD | PER/TRA | Flushing | 1 |
| 17 | After | PHD | PER/TRA | Abdominal pain; Headaches | 2 |
| 18 | After | PHD | DTX/PTX | Initial insomnia | 1 |
| 19 | After | PHD | DTX/PTX | Erythema; Diarrhoea | 2 |
| 20 | After | PHD | DTX/PTX | Pyrexia | 1 |
| 21 | After | PHD | PER/TRA | Flushing; Paraesthesia oral; Feeling hot; Erythemas; Pruritus | 2 |
| 22 | After | PHD | DTX/PTX | Scintillating scotoma; Dyspnoea; Flushing | 2 |
| 23 | After | PHD | DTX/PTX | Malaise | 1 |
| 24 | After | PHD | DTX/PTX | Diarrhoea | 1 |
| 25 | After | PHD | DTX/PTX | Persistent postural-perceptual dizziness | 1 |
| 26 | After | PHD | DTX/PTX | Nausea | 2 |
| 27 | After | PHD | Unknown | Feeling hot | 1 |
| 28 | After | PHD | DTX/PTX | Periorbital swelling; Dyspnoea | 1 |
| 29 | After | PHD | DTX/PTX | Flushing | 1 |
| 30 | After | PHD | PER/TRA | Pyrexia; Feeling cold; Feeling hot | 3 |
| 31 | After | PHP | DTX/PTX | Feeling hot | 2 |
| 32 | After | PHD | DTX/PTX | Blood pressure increased | 1 |
| 33 | After | PHD | Unknown | Blood pressure increased; Headaches | 1 |
| 34 | After | PHP | DTX/PTX | Oxygen saturation decreased; Feeling hot; Sinus tachycardia; Pyrexia; Persistent postural-perceptual dizziness; Disorientation; Malaise | 3 |
References
- Chung, C.H. Managing premedications and the risk for reactions to infusional monoclonal antibody therapy. Oncologist 2008, 13, 725–732. [Google Scholar] [CrossRef]
- Cobleigh, M.A.; Vogel, C.L.; Tripathy, D.; Robert, N.J.; Scholl, S.; Fehrenbacher, L.; Wolter, J.M.; Paton, V.; Shak, S.; Lieberman, G.; et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol. 1999, 17, 2639–2648. [Google Scholar] [CrossRef]
- Cook-Bruns, N. Retrospective analysis of the safety of Herceptin immunotherapy in metastatic breast cancer. Oncology 2001, 61 (Suppl. S2), 58–66. [Google Scholar] [CrossRef]
- Roselló, S.; Blasco, I.; García Fabregat, L.; Cervantes, A.; Jordan, K. ESMO Guidelines Committee. Management of infusion reactions to systemic anticancer therapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2017, 28 (Suppl. S4), iv100–iv118. [Google Scholar] [CrossRef]
- Ishida, S.; Masuguchi, K.; Kawashiri, T.; Tsuji, T.; Watanabe, H.; Akiyoshi, S.; Kubo, M.; Masuda, S.; Egashira, N. Effects of diluent volume and administration time on the incidence of anaphylaxis following docetaxel therapy in breast cancer. Biol. Pharm. Bull. 2020, 43, 663–668. [Google Scholar] [CrossRef]
- Boulanger, J.; Boursiquot, J.N.; Cournoyer, G.; Lemieux, J.; Masse, M.S.; Almanric, K.; Guay, M.P. Comité de l’évolution des pratiques en oncologie. Management of hypersensitivity to platinum- and taxane-based chemotherapy: Cepo review and clinical recommendations. Curr. Oncol. 2014, 21, e630–e641. [Google Scholar] [CrossRef]
- Verweij, J.; Clavel, M.; Chevalier, B. Paclitaxel (Taxol) and docetaxel (Taxotere): Not simply two of a kind. Ann. Oncol. 1994, 5, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Goto, E.; Hata, T.; Nishihara, M.; Neo, M.; Iwamoto, M.; Kimura, K.; Goto, M.; Rikitake, Y. Preventive effect of dexamethasone premedication on the development of infusion-related reaction in breast cancer patients receiving trastuzumab. Br. J. Clin. Pharmacol. 2023, 89, 2102–2112. [Google Scholar] [CrossRef] [PubMed]
- Nakagaki, S.; Matsunuma, R.; Yamaguchi, K.; Hayami, R.; Tsuneizumi, M. The preferred premedication order to prevent infusion reactions in patients with breast cancer receiving pertuzumab plus trastuzumab and docetaxel. J. Adv. Med. Med. Res. 2021, 33, 24–30. [Google Scholar] [CrossRef]
- Inada, Y.; Nanai, A.; Tomohiro, K.; Tomoko, T.; Mariko, O.; Takashi, C.; Hitoshi, A.; Atsushi, Y. Preventive effect of infusion reaction by changing the administration order of dexamethasone in preoperative and postoperative adjuvant chemotherapy in pertuzumab, trastuzumab, and docetaxel combination therapy. J. Jpn. Soc. Hosp. Pharm. 2022, 58, 776–780. [Google Scholar]
- Nakamura, S.; Ando, M.; Masuda, N.; Aogi, K.; Ino, H.; Iwata, H.; Tokuda, Y.; Yamamoto, N.; Kasai, H.; Takeuchi, M.; et al. Randomized Phase II study of primary systemic chemotherapy and trastuzumab for operable HER2 positive breast cancer. Clin. Breast Cancer 2012, 12, 49–56. [Google Scholar] [CrossRef]
- Thompson, L.M.; Eckmann, K.; Boster, B.L.; Hess, K.R.; Michaud, L.B.; Esteva, F.J.; Hortobágyi, G.N.; Barnett, C.M. Incidence, risk factors, and management of infusion-related reactions in breast cancer patients receiving trastuzumab. Oncologist 2014, 19, 228–234. [Google Scholar] [CrossRef]
- Tokuda, Y.; Suzuki, Y.; Ohta, M.; Saito, Y.; Kubota, M.; Tajima, T.; Umemura, S.; Osamura, R.Y. Compassionate use of humanized anti-HER2/neu protein, trastuzumab for metastatic breast cancer in Japan. Breast Cancer 2001, 8, 310–315. [Google Scholar] [CrossRef]
- Shimada, H.; Ogami, M.; Suzuki, Y.; Mitsuhashi, S.; Hozumi, Y.; Suzuki, M. Survey of risk factors affecting infusion reaction in patients receiving trastuzumab monotherapy. J. Jpn. Soc. Hosp. Pharm. 2022, 58, 1298–1302. [Google Scholar]
- Nitz, U.A.; Gluz, O.; Christgen, M.; Grischke, E.M.; Augustin, D.; Kuemmel, S.; Braun, M.; Potenberg, J.; Kohls, A.; Krauss, K.; et al. De-escalation strategies in HER2-positive early breast cancer (EBC): Final analysis of the WSG-ADAPT HER2+/HR- phase II trial: Efficacy, safety, and predictive markers for 12 weeks of neoadjuvant dual blockade with trastuzumab and pertuzumab ± weekly paclitaxel. Ann. Oncol. 2017, 28, 2768–2772. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, Y.; Tsujimoto, M.; Yamamoto, K.; Shimizu, R.; Kosaka, T.; Sakaguchi, K.; Dobuchi, N.; Nishiguchi, K.; Shikata, K. Risk factors for infusion reactions in patients with breast cancer administered trastuzumab therapy. Biol. Pharm. Bull. 2023, 46, 964–968. [Google Scholar] [CrossRef]
- Lee, C.; Gianos, M.; Klaustermeyer, W.B. Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Ann. Allergy Asthma Immunol. 2009, 102, 179–187, quiz 187–179, 222. [Google Scholar] [CrossRef]
- Bayrak Durmaz, M.S.; Unutmaz, D.G.; Demir, M.; Goksel, O.; Dursun, A.B.; Bavbek, S. Hypersensitivity reactions to taxanes: A multicenter study for outcomes and safety of rapid drug desensitization. Allergy Asthma Immunol. Res. 2024, 16, 142–153. [Google Scholar] [CrossRef]
- Gunaydin, C.; Bilge, S.S. Effects of nonsteroidal anti-inflammatory drugs at the molecular level. Eurasian J. Med. 2018, 50, 116–121. [Google Scholar] [CrossRef]
- Chen, J.S.; Alfajaro, M.M.; Chow, R.D.; Wei, J.; Filler, R.B.; Eisenbarth, S.C.; Wilen, C.B. Non-steroidal anti-inflammatory drugs dampen the cytokine and antibody response to SARS-CoV-2 infection. J. Virol. 2021, 95, e00014-21. [Google Scholar] [CrossRef]
- Pintea, I.; Petricau, C.; Dumitrascu, D.; Muntean, A.; Branisteanu, D.C.; Branisteanu, D.E.; Deleanu, D. Hypersensitivity reactions to monoclonal antibodies: Classification and treatment approach (Review). Exp. Ther. Med. 2021, 22, 949. [Google Scholar] [CrossRef]
- Winkler, U.; Jensen, M.; Manzke, O.; Schulz, H.; Diehl, V.; Engert, A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood 1999, 94, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, E.; Sloan, S.; Narayan, K.; Hay, C.A.; Smith, P.; Engler, F.; Jeeninga, R.; Smits, S.; Trevejo, J.; Shriver, Z.; et al. Safety and efficacy of monoclonal antibody VIS410 in adults with uncomplicated influenza A infection: Results from a randomized, double-blind, phase-2, placebo-controlled study. EBiomedicine 2019, 40, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Stebbing, J.; Baranau, Y.; Baryash, V.; Manikhas, A.; Moiseyenko, V.; Dzagnidze, G.; Zhavrid, E.; Boliukh, D.; Stroyakovskii, D.; Pikiel, J.; et al. CT-P6 compared with reference trastuzumab for HER2-positive breast cancer: A randomised, double-blind, active-controlled, phase 3 equivalence trial. Lancet Oncol. 2017, 18, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Pegram, M.D.; Bondarenko, I.; Zorzetto, M.M.C.; Hingmire, S.; Iwase, H.; Krivorotko, P.V.; Lee, K.S.; Li, R.K.; Pikiel, J.; Aggarwal, R.; et al. PF-05280014 (a trastuzumab biosimilar) plus paclitaxel compared with reference trastuzumab plus paclitaxel for HER2-positive metastatic breast cancer: A randomised, double-blind study. Br. J. Cancer 2019, 120, 172–182. [Google Scholar] [CrossRef]
- Tan, A.R.; Im, S.A.; Mattar, A.; Colomer, R.; Stroyakovskii, D.; Nowecki, Z.; De Laurentiis, M.; Pierga, J.Y.; Jung, K.H.; Schem, C.; et al. Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection plus chemotherapy in HER2-positive early breast cancer (FeDeriCa): A randomised, open-label, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2021, 22, 85–97. [Google Scholar] [CrossRef]
- Shrimp, F.; Behrens, D.; Ochieng, P. Delayed Trastuzumb (Herceptin)-Related ARDS. Chest 2016, 150, 525A. [Google Scholar] [CrossRef]
- Mezzano, V.; Giavina-Bianchi, P.; Picard, M.; Caiado, J.; Castells, M. Drug desensitization in the management of hypersensitivity reactions to monoclonal antibodies and chemotherapy. BioDrugs 2014, 28, 133–144. [Google Scholar] [CrossRef]

| Patients | Before (n = 33) | After (n = 43) | p | |
|---|---|---|---|---|
| Female | 33 (100) | 43 (100) | ||
| Age (years) | 49.5 (32.3–65.7) | 59.8 (40.1–79.2) | <0.0001 *,a | |
| Body Weight | 56.0 (40.8–79.6) | 55.8 (42.4–84.5) | 0.8217 a | |
| Body Mass Index | 22.0 (16.8–32.9) | 23.1 (17.2–34.1) | 0.7060 a | |
| Stage | I–III | 29 (87.9) | 41 (95.3) | 0.3940 c |
| IV | 4 (12.1) | 2 (4.65) | ||
| Purpose of chemotherapy (Stage I–III) | Neoadjuvant | 23 (69.7) | 23 (53.5) | 0.0439 *,b |
| Adjuvant | 6 (18.2) | 18 (41.9) | ||
| History of anthracycline therapy | Yes | 6 (18.2) | 16 (37.2) | 0.0698 b |
| ER status | Positive | 18 (54.5) | 23 (53.5) | 0.9270 b |
| Negative | 15 (45.5) | 20 (46.5) | ||
| PgR status | Positive | 12 (36.4) | 20 (46.5) | 0.3745 b |
| Negative | 21 (63.6) | 23 (53.5) | ||
| Allergy | Yes | 20 (60.6) | 26 (60.5) | 0.9901 b |
| No | 13 (39.4) | 17 (39.5) | ||
| Regimen | PHD | 5 (15.2) | 39 (90.7) | <0.0001 *,b |
| PHP | 28 (84.8) | 4 (9.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takagi, M.; Maeda, S.; Maeda, M.; Fujio, Y.; Hirobe, S. Effects of Modifying Supportive Care Medications in Combination Therapy with Pertuzumab, Trastuzumab, and Taxane Anticancer Drugs. Pharmacy 2025, 13, 168. https://doi.org/10.3390/pharmacy13060168
Takagi M, Maeda S, Maeda M, Fujio Y, Hirobe S. Effects of Modifying Supportive Care Medications in Combination Therapy with Pertuzumab, Trastuzumab, and Taxane Anticancer Drugs. Pharmacy. 2025; 13(6):168. https://doi.org/10.3390/pharmacy13060168
Chicago/Turabian StyleTakagi, Mina, Shinichiro Maeda, Makiko Maeda, Yasushi Fujio, and Sachiko Hirobe. 2025. "Effects of Modifying Supportive Care Medications in Combination Therapy with Pertuzumab, Trastuzumab, and Taxane Anticancer Drugs" Pharmacy 13, no. 6: 168. https://doi.org/10.3390/pharmacy13060168
APA StyleTakagi, M., Maeda, S., Maeda, M., Fujio, Y., & Hirobe, S. (2025). Effects of Modifying Supportive Care Medications in Combination Therapy with Pertuzumab, Trastuzumab, and Taxane Anticancer Drugs. Pharmacy, 13(6), 168. https://doi.org/10.3390/pharmacy13060168






