The Spectrum of Clinical Pharmacy Services in a Non-University Hospital—A Comprehensive Characterization Including a Risk Assessment for Drug-Related Problems and Adverse Drug Reactions
Abstract
1. Introduction
2. Materials and Methods
- i.
- CPS per patient/treatment day [patient chart and pharmacy documentation]
- ii.
- Number of patients involved in CPS per medical department [patient chart and pharmacy documentation]
- iii.
- Receipt of the request for the CPS [pharmacy documentation]
- iv.
- Topic of the CPS [pharmacy documentation]
- v.
- Drug group addressed in CPS [pharmacy documentation]
- vi.
- Time required for CPS [pharmacist interview]
- vii.
- Potential severity of DRP and actually occurring ADR identified during CPS [pharmacist interview]
- viii.
- Probability of occurrence of DRP identified during CPS [pharmacist interview]
- ix.
- Recommendations to solve DRP identified during CPS [pharmacy documentation and pharmacist interview]
- x.
- Acceptance of recommendations to solve DRP identified during CPS [pharmacy documentation and pharmacist interview]
3. Results
3.1. CPS per Patient/Treatment Day
3.2. Number of Patients Involved in CPS per Medical Department
3.3. Receipt of the Request for the CPS
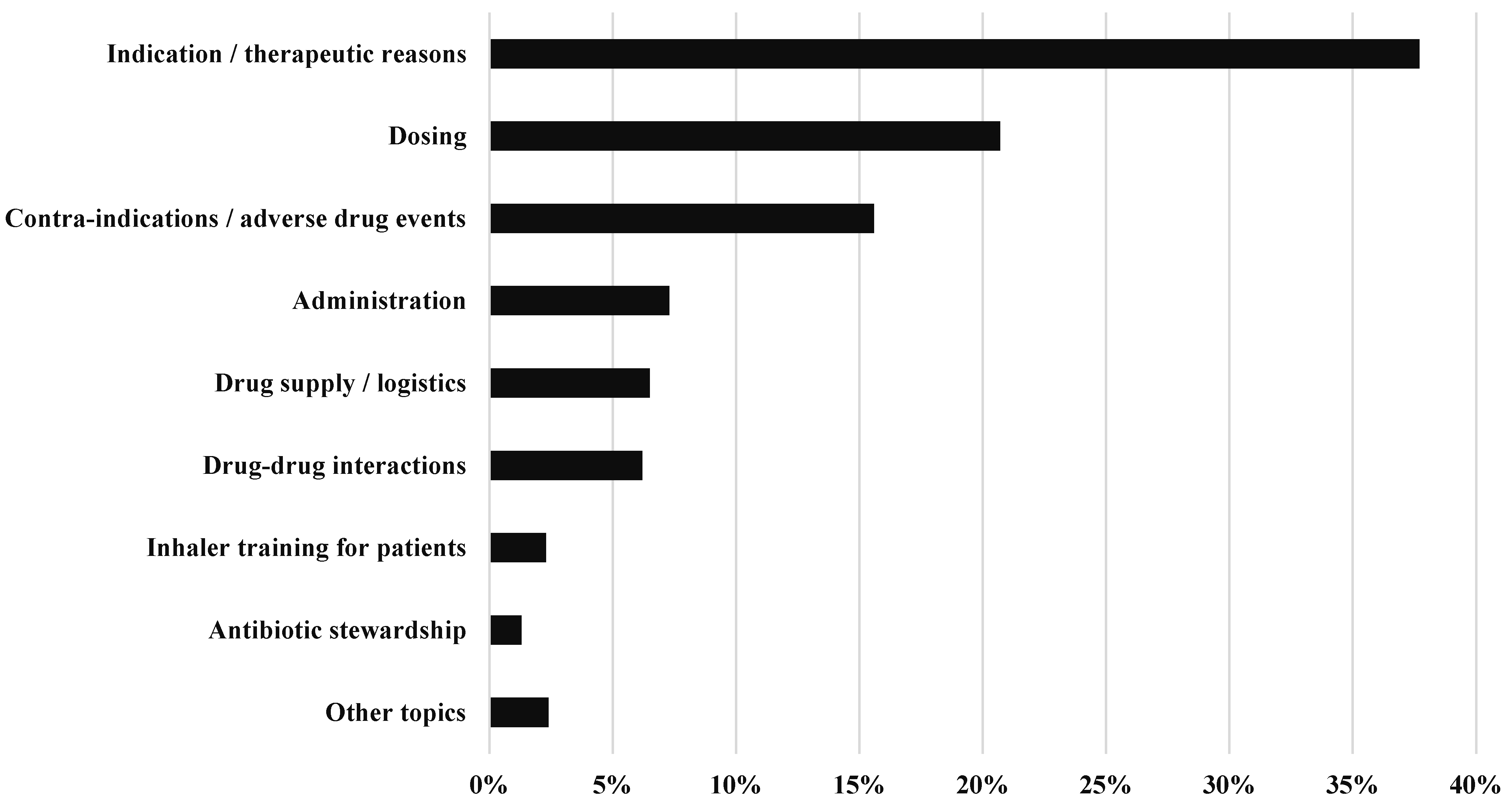
3.4. Topic of the CPS
3.5. Drug Group Addressed in CPS
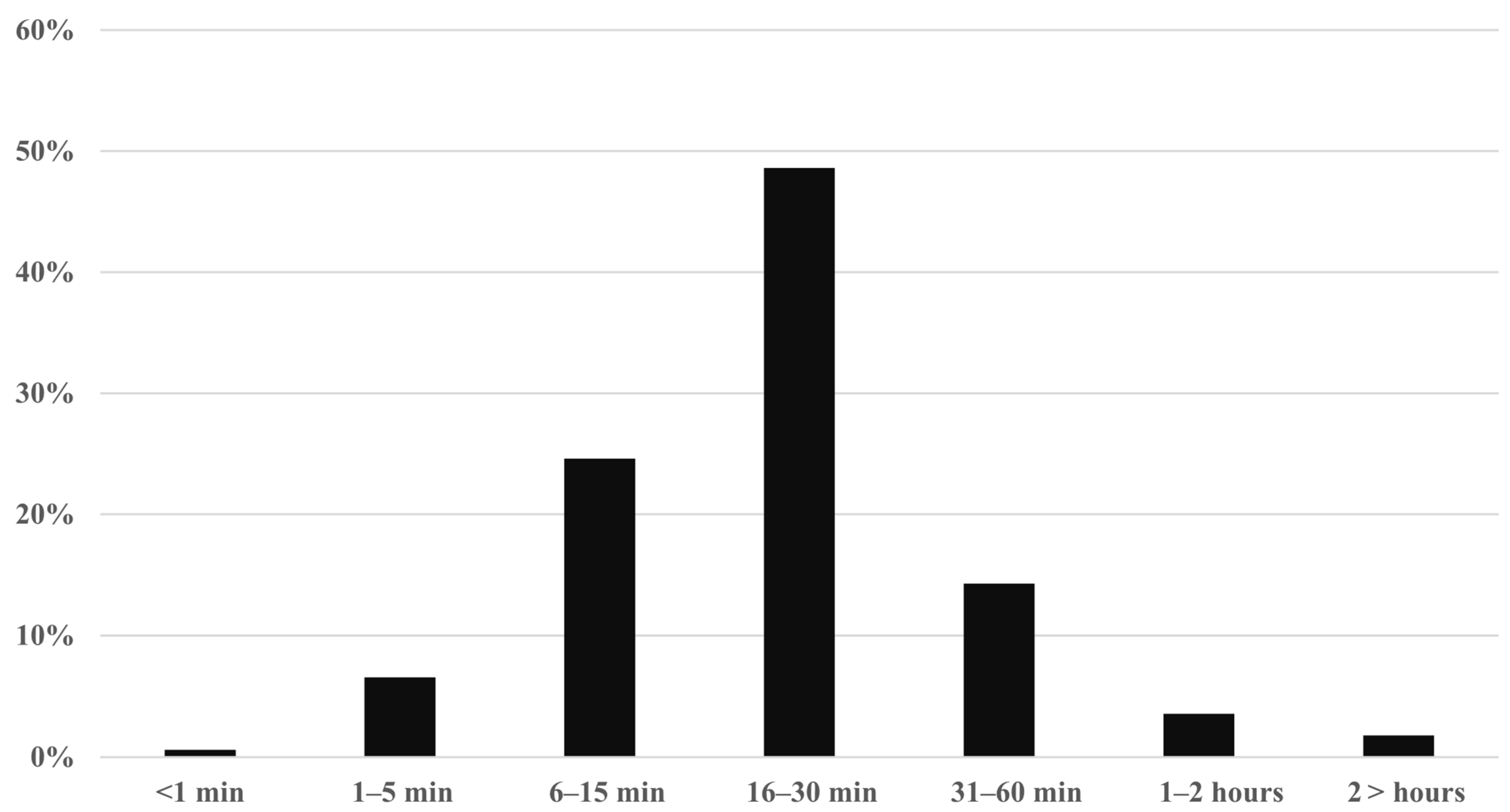
3.6. Time Required for CPS
3.7. Potential Severity of DRP and Actually Occurring ADR Identified During CPS
3.8. Recommendations to Solve DRP Identified During CPS
3.9. Acceptance of Recommendations to Solve DRP Identified During CPS
4. Discussion
4.1. General Considerations
4.2. Main Findings of This Investigation
4.3. Setting
4.4. Methodological Aspects
4.5. Comparison to the Literature
4.6. Future Perspectives
4.7. Limitations and Sources of Potential Bias and Confounders
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| Abbreviation | Full Term |
| ADR | Adverse Drug Reaction |
| CPS | Clinical Pharmacy Services |
| DRP | Drug-Related Problem |
| SOP | Standard Operating Procedure |
| NCC-MERP | National Coordinating Council for Medication Error Reporting and Prevention |
| CPOE | Computerized Physician Order Entry |
| CDSS | Clinical Decision Support System |
References
- Garin, N.; Sole, N.; Lucas, B.; Matas, L.; Moras, D.; Rodrigo-Troyano, A.; Gras-Martin, L.; Fonts, N. Drug related problems in clinical practice: A cross-sectional study on their prevalence, risk factors and associated pharmaceutical interventions. Sci. Rep. 2021, 11, 883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lavan, A.H.; Gallagher, P. Predicting risk of adverse drug reactions in older adults. Ther. Adv. Drug Saf. 2016, 7, 11–22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmidt, S.J.; Wurmbach, V.S.; Lampert, A.; Bernard, S.; HIOPP-6 Consortium; Haefeli, W.E.; Seidling, H.M.; Thürmann, P.A. Individual factors increasing complexity of drug treatment-a narrative review. Eur. J. Clin. Pharmacol. 2020, 76, 745–754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paudyal, V.; Okuyan, B.; Henman, M.C.; Stewart, D.; Fialová, D.; Hazen, A.; Lutters, M.; Oleárová, A.; Weidmann, A.E.; Wirth, F.; et al. Scope, content and quality of clinical pharmacy practice guidelines: A systematic review. Int. J. Clin. Pharm. 2024, 46, 56–69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Powell, J.R.; Cook, J.; Wang, Y.; Peck, R.; Weiner, D. Drug Dosing Recommendations for All Patients: A Roadmap for Change. Clin. Pharmacol. Ther. 2021, 109, 65–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. Guide for Good Prescribing. Available online: https://iris.who.int/bitstream/handle/10665/59001/WHO_DAP_94.11.pdf (accessed on 18 August 2025).
- Simonetti, L.; Lefrant, J.Y.; Cireașă, B.; Poujol, H.; Leguelinel-Blache, G. Pharmacoeconomic and clinical impact of pharmaceutical service in the intensive care unit: A systematic review. Eur. J. Hosp. Pharm. 2024, 32, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, M.; Huang, Y.; Xi, X. Factors influencing clinical pharmacists’ integration into the clinical multidisciplinary care team. Front. Pharmacol. 2023, 14, 1202433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mwakawanga, D.L.; Mutagonda, R.F.; Mlyuka, H.J.; Mikomangwa, W.P.; Kilonzi, M.; Kibanga, W.A.; Marealle, A.I.; Mallya, B.; Katabalo, D.; Sanga, S.; et al. Improving the provision of clinical pharmacy services in low- and middle-income countries: A qualitative study in tertiary health facilities in Tanzania. BMJ Public Health 2025, 3, e001776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hilgarth, H.; Wichmann, D.; Baehr, M.; Kluge, S.; Langebrake, C. Clinical pharmacy services in critical care: Results of an observational study comparing ward-based with remote pharmacy services. Int. J. Clin. Pharm. 2023, 45, 847–856. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bertsche, T.; Pfaff, J.; Schiller, P.; Kaltschmidt, J.; Pruszydlo, M.G.; Stremmel, W.; Walter-Sack, I.; Haefeli, W.E.; Encke, J. Prevention of adverse drug reactions in intensive care patients by personal intervention based on an electronic clinical decision support system. Intensive Care Med. 2010, 36, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Forrey, R.A.; Pedersen, C.A.; Schneider, P.J. Interrater agreement with a standard scheme for classifying medication errors. Am. J. Health Syst. Pharm. 2007, 64, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, N.; Wei, L.; Ghaleb, M.; Pasut, E.; Leschiutta, S.; Rossi, P.; Troncon, M.G. Evaluation of the implementation of a clinical pharmacy service on an acute internal medicine ward in Italy. BMC Health Serv. Res. 2018, 18, 259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drovandi, A.; Robertson, K.; Tucker, M.; Robinson, N.; Perks, S.; Kairuz, T. A systematic review of clinical pharmacist interventions in paediatric hospital patients. Eur. J. Pediatr. 2018, 177, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Zehra, A.; Munawar, R.; Gul, W.; Fatima, T.; Khan, Y.; Jaffery, R.; Babar, Z.U. Economic evaluation of clinical pharmacy service using integrated health system in tertiary care hospital. Expert Rev. Pharmacoecon. Outcomes Res. 2024, 24, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.R.; Andersen, S.E.; Rasmussen, M.; Honoré, P.H. Clinical pharmacist service in the acute ward. Int. J. Clin. Pharm. 2013, 35, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- McCarney, R.; Warner, J.; Iliffe, S.; van Haselen, R.; Griffin, M.; Fisher, P. The Hawthorne Effect: A randomised, controlled trial. BMC Med. Res. Methodol. 2007, 7, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jussim, L.; Harber, K.D. Teacher expectations and self-fulfilling prophecies: Knowns and unknowns, resolved and unresolved controversies. Pers. Soc. Psychol. Rev. 2005, 9, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Lagreula, J.; Maes, F.; Wouters, D.; Quennery, S.; Dalleur, O. Optimizing pharmacists’ detection of prescribing errors: Comparison of on-ward and central pharmacy services. J. Clin. Pharm. Ther. 2021, 46, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.K.; Bach, O.; Mosch, K.; Bertsche, T. A Clinical Pharmacy Service to Prevent Drug-Drug Interactions and Potentially Inappropriate Medication: A Consecutive Intervention Study in Older Intermediate Care Patients of a Regional Hospital. Pharmacy 2025, 13, 60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Givens, J.; Dull, R. Association Between an Enhanced Clinical Pharmacy Service and Patient Experience in Hospitalized Adults: A Cohort Study. Hosp. Pharm. 2024, 60, 154–162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sa, E.; Cho, Y.; Suh, S.Y.; Park, T.E.; Rhie, S.J. Clinical Outcomes Associated with the Implementation of a Dedicated Clinical Pharmacy Service in a Resource-Limited Neurocritical Intensive Care Unit. Hosp. Pharm. 2024, 2, 132–138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Canning, M.L.; Barras, M.; McDougall, R.; Yerkovich, S.; Coombes, I.; Sullivan, C.; Whitfield, K. Defining quality indicators, pharmaceutical care bundles and outcomes of clinical pharmacy service delivery using a Delphi consensus approach. Int. J. Clin. Pharm. 2024, 46, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Colleran, M.; Melanophy, G.; Bermingham, M. Enhancing the clinical pharmacy service of a large teaching hospital: Development of a new clinical prioritisation tool. Explor. Res. Clin. Soc. Pharm. 2023, 12, 100335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bertsche, T.; Niemann, D.; Mayer, Y.; Ingram, K.; Hoppe-Tichy, T.; Haefeli, W.E. Prioritising the prevention of medication handling errors. Pharm. World Sci. 2008, 30, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.L.; Levy, B.; Gryzlak, B.; Xu, Y.; Chrischilles, E.; Dawson, J.; Vander Weg, M.; Christensen, A.; James, P.; Polgreen, L. Cluster-Randomized Trial to Evaluate a Centralized Clinical Pharmacy Service in Private Family Medicine Offices. Circ. Cardiovasc. Qual. Outcomes. 2018, 11, e004188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zube, O.; Schlüter, W.; Dicken, J.; Hensen, J.; Bertsche, T. The Spectrum of Clinical Pharmacy Services in a Non-University Hospital—A Comprehensive Characterization Including a Risk Assessment for Drug-Related Problems and Adverse Drug Reactions. Pharmacy 2025, 13, 164. https://doi.org/10.3390/pharmacy13060164
Zube O, Schlüter W, Dicken J, Hensen J, Bertsche T. The Spectrum of Clinical Pharmacy Services in a Non-University Hospital—A Comprehensive Characterization Including a Risk Assessment for Drug-Related Problems and Adverse Drug Reactions. Pharmacy. 2025; 13(6):164. https://doi.org/10.3390/pharmacy13060164
Chicago/Turabian StyleZube, Olaf, Wiebke Schlüter, Johanna Dicken, Jan Hensen, and Thilo Bertsche. 2025. "The Spectrum of Clinical Pharmacy Services in a Non-University Hospital—A Comprehensive Characterization Including a Risk Assessment for Drug-Related Problems and Adverse Drug Reactions" Pharmacy 13, no. 6: 164. https://doi.org/10.3390/pharmacy13060164
APA StyleZube, O., Schlüter, W., Dicken, J., Hensen, J., & Bertsche, T. (2025). The Spectrum of Clinical Pharmacy Services in a Non-University Hospital—A Comprehensive Characterization Including a Risk Assessment for Drug-Related Problems and Adverse Drug Reactions. Pharmacy, 13(6), 164. https://doi.org/10.3390/pharmacy13060164





