Impact of Pharmacist Interventions in a Portuguese Hospital: A Study Using the CLEO Multidimensional Tool
Abstract
1. Introduction
2. Materials and Methods
2.1. CLEO Tool
2.2. Study Design
2.3. Registration Routine
2.4. Data Collection and Classification
2.5. Ethical Considerations
2.6. Data Analysis
3. Results
3.1. Patient Characteristics
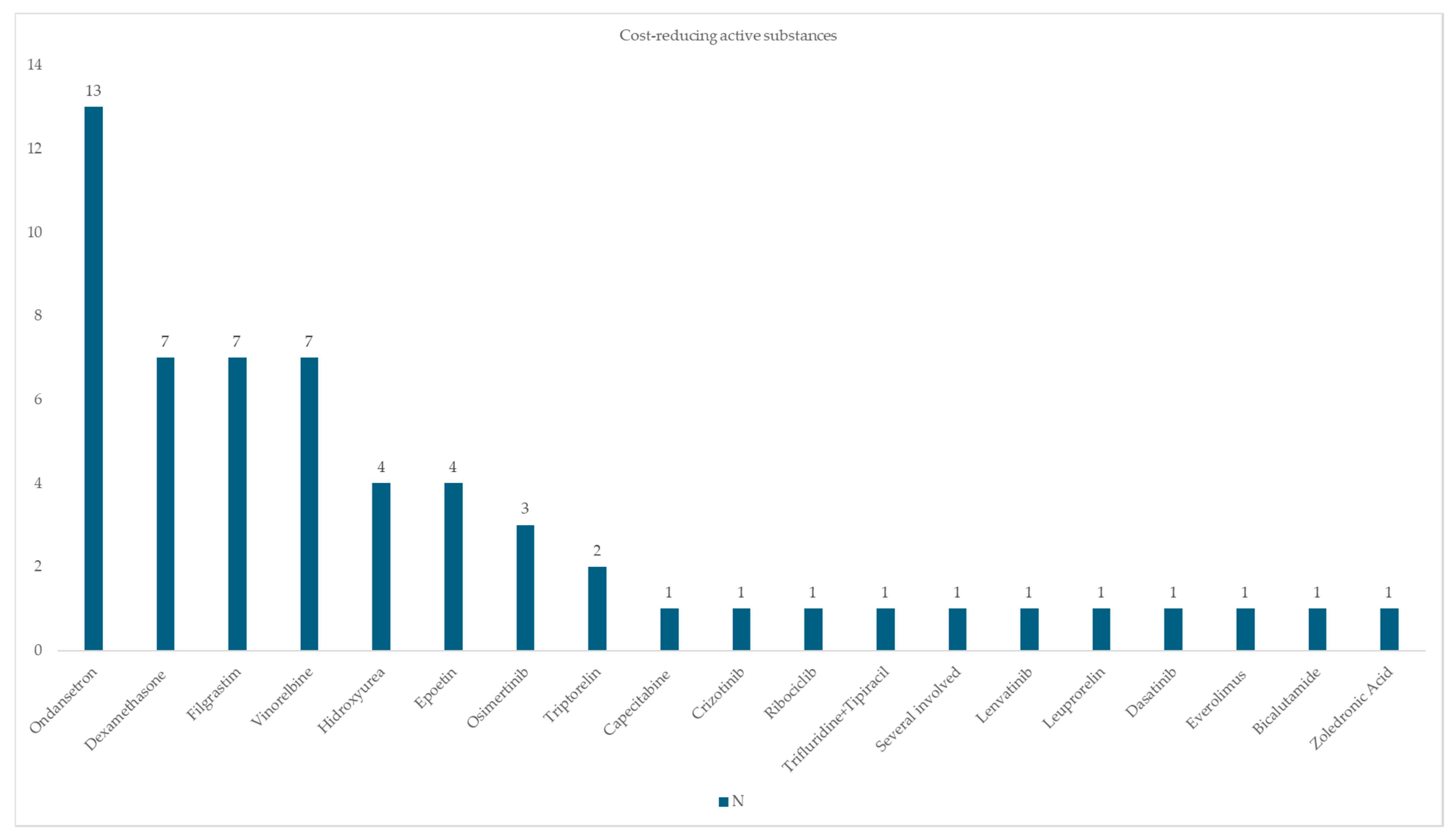
3.2. Active Substances Involved in Interventions
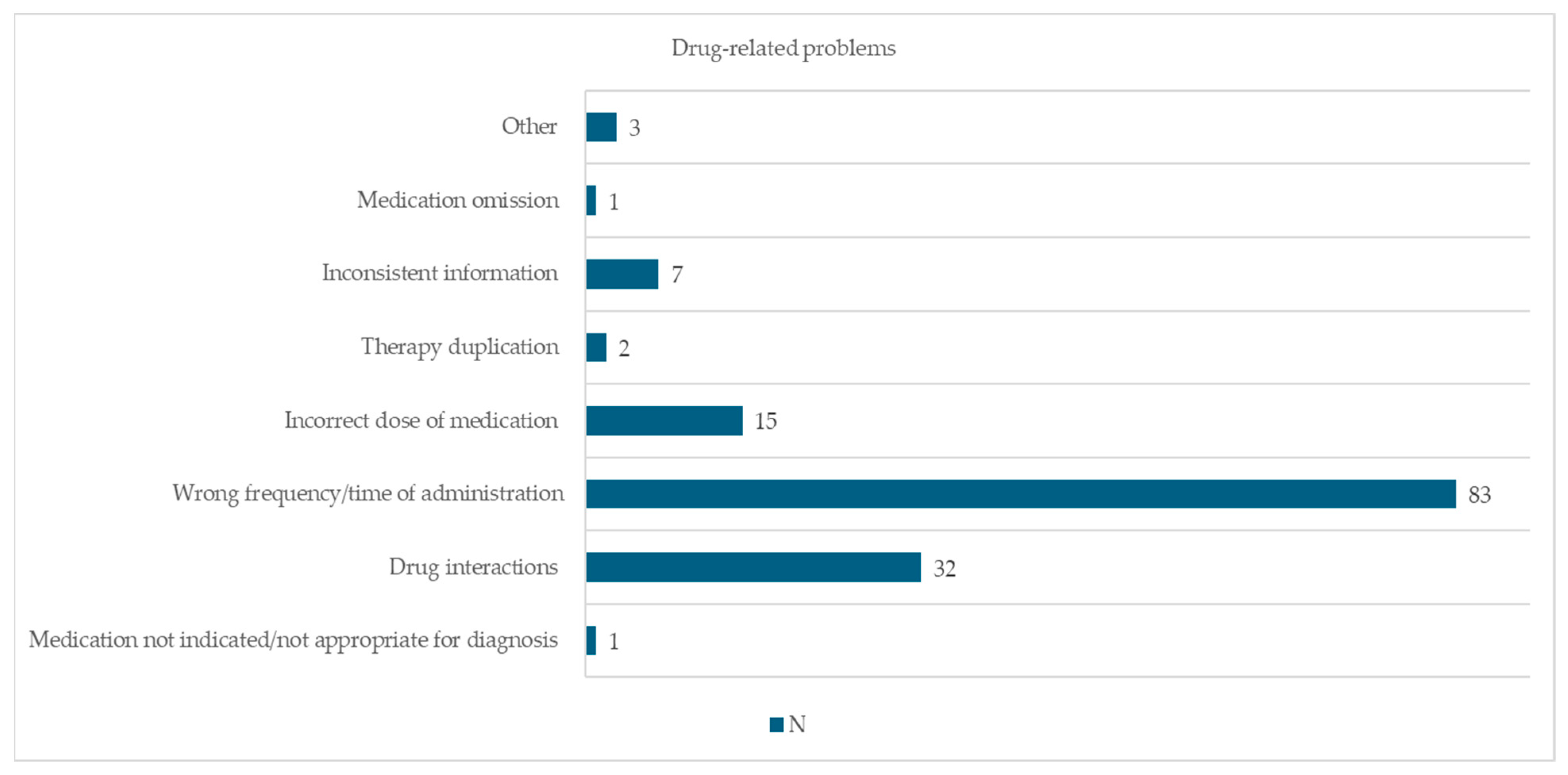
3.3. Drug-Related Problems and Actions Taken
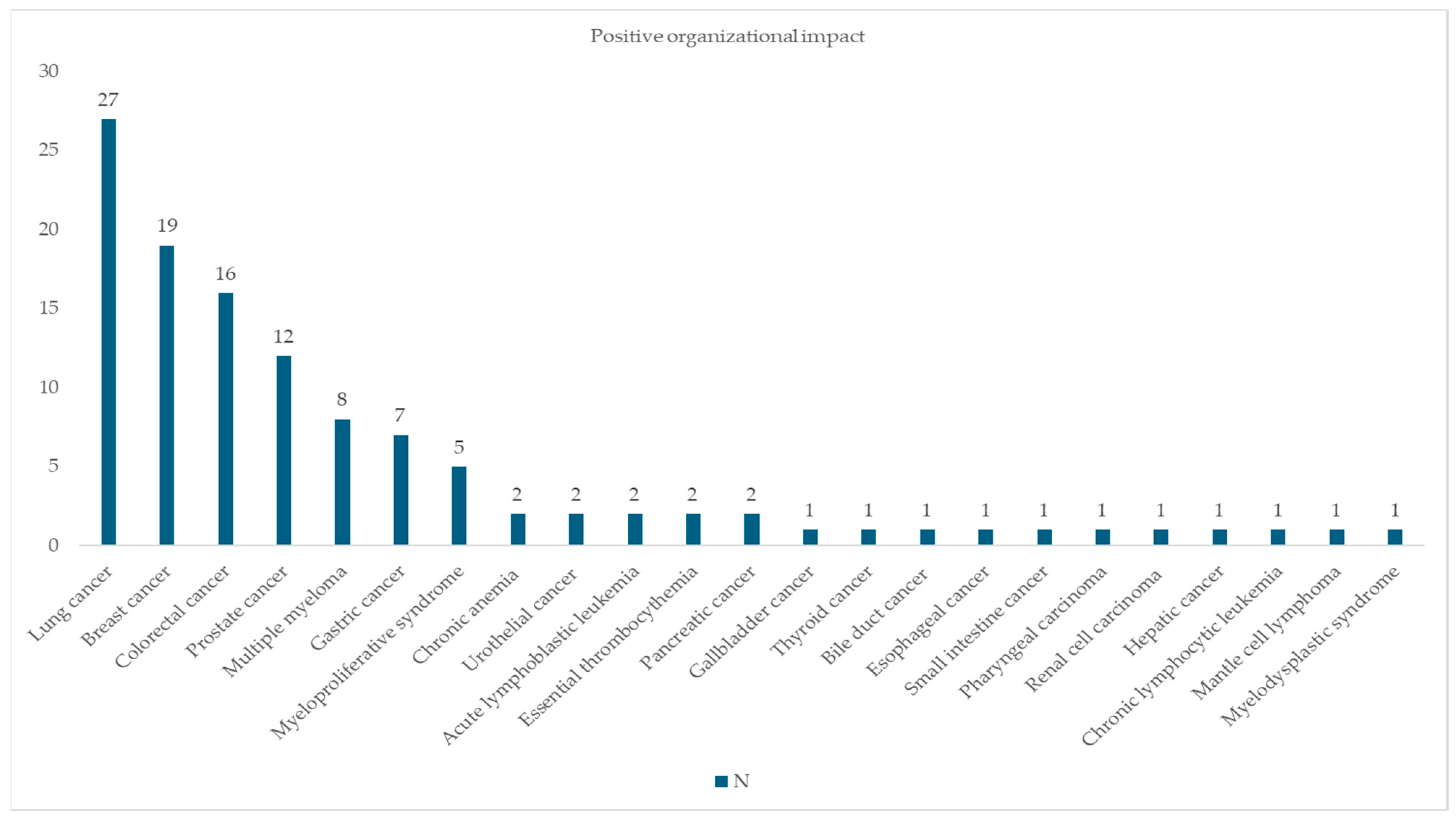
3.4. CLEO Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atey, T.M.; Peterson, G.M.; Salahudeen, M.S.; Bereznicki, L.R.; Wimmer, B.C. Impact of pharmacist interventions provided in the emergency department on quality use of medicines: A systematic review and meta-analysis. Emerg. Med. J. 2023, 40, 120–127. [Google Scholar] [CrossRef]
- Alshehri, A.A.; Jalal, Z.; Cheema, E.; Haque, M.S.; Jenkins, D.; Yahyouche, A. Impact of the pharmacist-led intervention on the control of medical cardiovascular risk factors for the primary prevention of cardiovascular disease in general practice: A systematic review and meta-analysis of randomised controlled trials. Br. J. Clin. Pharmacol. 2020, 86, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceutical Society of Australia. Guidelines for Pharmacists Performing Clinical Interventions; Pharmaceutical Society of Australia: Deakin West, Australia, 2018; Available online: https://www.ppaonline.com.au/wp-content/uploads/2019/01/PSA-Clinical-Interventions-Guidelines.pdf (accessed on 7 June 2025).
- Eriksson, T. The CLEO assessment tool for pharmacist interventions. Eur. J. Hosp. Pharm. 2021, 28, 181. [Google Scholar] [CrossRef] [PubMed]
- Vo, H.T.; Charpiat, B.; Chanoine, S.; Juste, M.; Roubille, R.; Rose, F.X.; Conort, O.; Allenet, B.; Bedouch, P.; Working Group “Valorization of Pharmacist Interventions” of the French Society of Clinical Pharmacy. CLEO: A multidimensional tool to assess clinical, economic and organisational impacts of pharmacists’ interventions. Eur. J. Hosp. Pharm. 2021, 28, 193–200. [Google Scholar] [CrossRef]
- Reinau, D.; Furrer, C.; Stämpfli, D.; Bornand, D.; Meier, C.R. Evaluation of drug-related problems and subsequent clinical pharmacists’ interventions at a Swiss university hospital. J. Clin. Pharm. Ther. 2019, 44, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.T.; Nguyen, K.H.P.; Le, H.B.; Pham, H.T.; Nguyen, H.T.; Nguyen, N.T.B.; Dong, P.T.X.; Dang, T.N.D.; Pham, V.T.T.; Nguyen, D.T.; et al. Translation and validation of the CLEO tool in Vietnamese to assess the significance of pharmacist interventions. Int. J. Clin. Pharm. 2024, 47, 119–127. [Google Scholar] [CrossRef]
- Clarenne, J.; Mongaret, C.; Vermorel, C.; Bosson, J.L.; Gangloff, S.C.; Lambert-Lacroix, S.; Bedouch, P. Characteristics of hospital pharmacist interventions and their clinical, economic and organizational impacts: A five-year observational study on the French National Observatory. Int. J. Clin. Pharm. 2024, 47, 403–411. [Google Scholar] [CrossRef]
- Kaboli, P.; Hoth, A.; McClimon, B.; Schnipper, J. Clinical pharmacists and inpatient medical care: A systematic review. Arch. Intern. Med. 2006, 166, 955–964. [Google Scholar] [CrossRef]
- Zecchini, C.; Vo, T.H.; Chanoine, S.; Lepelley, M.; Laramas, M.; Lemoigne, A.; Allenet, B.; Federspiel, I.; Bedouch, P. Clinical, economic and organizational impact of pharmacist interventions on injectable antineoplastic prescriptions: A prospective observational study. BMC Health Serv. Res. 2020, 20, 113. [Google Scholar] [CrossRef]
- Bond, C.A.; Raehl, C.L. 2006 national clinical pharmacy services survey: Clinical pharmacy services, collaborative drug management, medication errors, and pharmacy technology. Pharmacotherapy 2008, 28, 1–13. [Google Scholar] [CrossRef]
- Leape, L.L.; Cullen, D.J.; Clapp, M.D.; Burdick, E.; Demonaco, H.J.; Erickson, J.I.; Bates, D.W. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA 1999, 282, 267–270. [Google Scholar] [CrossRef]
- Machado, S.; Falcão, F.; Cavaco, A.M.D. Documentation and classification of hospital pharmacist interventions: A scoping review. Br. J. Clin. Pharmacol. 2023, 90, 722–739. [Google Scholar] [CrossRef]
- Charpiat, B.; Conort, O.; Juste, M.; Rose, F.X.; Roubille, R.; Bedouch, P.; Allenet, B. The French Society of Clinical Pharmacy ACT-IP© project: Ten years onward, results and prospects. Pharm. Hosp. Clin. 2015, 50, 15–24. [Google Scholar] [CrossRef]
- Stämpfli, D.; Baumgartner, P.; Boeni, F.; Bedouch, P.; Lampert, M.L.; Hersberger, K.E. Translation and validation of a tool to assess the impact of clinical pharmacists’ interventions. Int. J. Clin. Pharm. 2019, 41, 56–64. [Google Scholar] [CrossRef]
- Duwez, M.; Chanoine, S.; Lepelley, M.; Vo, T.H.; Pluchart, H.; Mazet, R.; Allenet, B.; Pison, C.; Briault, A.; Saint-Raymond, C.; et al. Clinical evaluation of pharmacists’ interventions on multidisciplinary lung transplant outpatients’ management: Results of a 7-year observational study. BMJ Open 2020, 10, e041563. [Google Scholar] [CrossRef]
- Novais, T.; Reallon, E.; Martin, J.; Barral, M.; Krolak-Salmon, P.; Coste, M.H.; Zenagui, H.; Garnier-Crussard, A.; Hoegy, D.; Mouchoux, C. Clinical impact of an individualised clinical pharmacy programme into the memory care pathway of older people: An observational study. Int. J. Clin. Pharm. 2024, 46, 889–898. [Google Scholar] [CrossRef]
- Durand, A.; Gillibert, A.; Membre, S.; Mondet, L.; Lenglet, A.; Mary, A. Acceptance Factors for In-Hospital Pharmacist Interventions in Daily Practice: A Retrospective Study. Front. Pharmacol. 2022, 13, 811289. [Google Scholar] [CrossRef]
- Vo, T.H.; Charpiat, B.; Catoire, C.; Juste, M.; Roubille, R.; Rose, F.X.; Chanoine, S.; Bosson, J.L.; Conort, O.; Allenet, B.; et al. Tools for Assessing Potential Significance of Pharmacist Interventions: A Systematic Review. Drug Saf. 2016, 39, 131–146. [Google Scholar] [CrossRef]
- Seden, K.; Kirkham, J.J.; Kennedy, T.; Lloyd, M.; James, S.; Mcmanus, A.; Ritchings, A.; Simpson, J.; Thornton, D.; Gill, A.; et al. Cross-sectional study of prescribing errors in patients admitted to nine hospitals across North West England. BMJ Open 2013, 3, e002036. [Google Scholar] [CrossRef] [PubMed]
- Brixey, J.; Johnson, T.R.; Zhang, J. Evaluating a medical error taxonomy. Proc. AMIA Symp. 2002, 71–75. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC2244554/ (accessed on 17 March 2025).
- Santos, H.; Iglésias, P.; Fernández-Llimós, F.; Faus, M.J.; Rodrigues, L.M. Second Consensus of Granada on pharmacotherapy failure. Cross-cultural translation from Spanish to Portuguese (European). Acta Med. Port. 2004, 17, 59–66. [Google Scholar] [CrossRef]
- Knez, L.; Laaksonen, R.; Duggan, C.; Nijjar, R. Evaluation of clinical interventions made by pharmacists in cancer services. Pharm. J. 2025, 284, 2009. [Google Scholar] [CrossRef]
- Coutsouvelis, J.; Siderov, J.; Tey, A.Y.; Bortz, H.D.; O’Connor, S.R.; Rowan, G.D.; Vasileff, H.M.; Page, A.T.; Percival, M.A. The impact of pharmacist-led strategies implemented to reduce errors related to cancer therapies: A systematic review. J. Pharm. Pract. Res. 2020, 50, 466–480. [Google Scholar] [CrossRef]
- Bates, D.W.; Spell, N.; Cullen, D.J.; Burdick, E.; Laird, N.; Petersen, L.A.; Small, S.D.; Sweitzer, B.J.; Leape, L.L. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA 1997, 277, 307–311. [Google Scholar] [CrossRef] [PubMed]

| Variable Patients | N | % |
|---|---|---|
| Sex | ||
| Male | 58 | 40.3 |
| Female | 86 | 59.7 |
| Age group (Years) | ||
| <18 years | 0 | 0 |
| 18–64 years | 47 | 32.6 |
| 65–85 years | 93 | 64.6 |
| >85 years | 4 | 2.8 |
| Base Pathology | ||
| Acute lymphoblastic leukemia | 5 | 3.5 |
| Acute myeloid leukemia | 1 | 0.7 |
| Bile duct cancer | 1 | 0.7 |
| Breast cancer | 29 | 20.1 |
| Chronic anemia | 2 | 1.4 |
| Chronic lymphocytic leukemia | 1 | 0.7 |
| Colorectal cancer | 16 | 11.1 |
| Esophageal cancer | 2 | 1.4 |
| Essential thrombocythemia | 2 | 1.4 |
| Gallbladder cancer | 1 | 0.7 |
| Gastric cancer | 7 | 4.9 |
| Hepatic cancer | 1 | 0.7 |
| Lung cancer | 29 | 20.1 |
| Mantle cell lymphoma | 1 | 0.7 |
| Monoclonal gammopathy | 1 | 0.7 |
| Multiple myeloma | 13 | 9.0 |
| Myelodysplastic Syndrome | 3 | 2.1 |
| Myeloproliferative Syndrome | 5 | 3.5 |
| Pancreatic cancer | 2 | 1.4 |
| Pharyngeal carcinoma | 2 | 1.4 |
| Prostate Cancer | 15 | 10.4 |
| Renal Cell Carcinoma | 1 | 0.7 |
| Small intestine cancer | 1 | 0.7 |
| Tyroid cancer | 1 | 0.7 |
| Urothelial cancer | 2 | 1.4 |
| Total | 144 | 100 |
| Pharmacotherapeutic Group | Active Substance | N | % |
|---|---|---|---|
| Serotonin (5-HT3) Antagonists | Ondansetron | 28 | 19.4 |
| Immunostimulants | Filgrastim | 20 | 13.9 |
| Antianemic preparations | Epoetin | 5 | 3.5 |
| Corticosteroids for systemic use | Dexamethasone | 18 | 12.5 |
| Tyrosine Kinase inhibitors | Cabozantinib | 1 | 0.7 |
| Crizotinib | 1 | 0.7 | |
| Dabrafenib | 1 | 0.7 | |
| Dasatinib | 4 | 2.8 | |
| Imatinib | 2 | 1.4 | |
| Ibrutinib | 1 | 0.7 | |
| Lenvatinib | 3 | 2.1 | |
| Osimertinib | 3 | 2.1 | |
| Ribociclib | 2 | 1.4 | |
| Antineoplastic agents, cytotoxic | Chlorambucil | 1 | 0.7 |
| Docetaxel + Cyclophosphamide | 1 | 0.7 | |
| Hidroxyurea | 5 | 3.5 | |
| Vinorelbine | 8 | 5.6 | |
| Antimetabolite | Capecitabine | 7 | 4.9 |
| Trifluridine + Tipiracil | 2 | 1.4 | |
| Antiandrogens | Bicalutamide | 4 | 2.8 |
| Several involved (apalutamide, ciproterone, bicalutamide, leuprorelin) 1 | 1 | 0.7 | |
| Enzalutamide | 2 | 1.4 | |
| Immunosuppressants | Lenalidomide | 2 | 1.4 |
| Talidomide | 4 | 2.8 | |
| Gonadotropin-Releasing Hormone analogs | Triptorelin | 2 | 1.4 |
| Leuprorelin | 3 | 2.1 | |
| Anti-estrogens | Tamoxifen | 5 | 3.5 |
| Bisphosphonates | Zoledronic acid | 2 | 1.4 |
| Antidiabetics | Various oral antidiabetics | 1 | 0.7 |
| Antidyslipidemic | Ezetimibe | 1 | 0.7 |
| Immunostimulating agent | Interferon alfa 2a | 1 | 0.7 |
| B-cell lymphoma (BCL)-2 inhibitor | Venetoclax | 1 | 0.7 |
| Aromatase Inhibitor | Letrozol | 1 | 0.7 |
| Mammalian target of rapamycin (mTOR) kinase inhibitors | Everolimus | 1 | 0.7 |
| Total | 144 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, S.; Jesus, M.; Faria, S.; Machado, S.; Morgado, M. Impact of Pharmacist Interventions in a Portuguese Hospital: A Study Using the CLEO Multidimensional Tool. Pharmacy 2025, 13, 143. https://doi.org/10.3390/pharmacy13050143
Silva S, Jesus M, Faria S, Machado S, Morgado M. Impact of Pharmacist Interventions in a Portuguese Hospital: A Study Using the CLEO Multidimensional Tool. Pharmacy. 2025; 13(5):143. https://doi.org/10.3390/pharmacy13050143
Chicago/Turabian StyleSilva, Sofia, Mafalda Jesus, Sandra Faria, Sara Machado, and Manuel Morgado. 2025. "Impact of Pharmacist Interventions in a Portuguese Hospital: A Study Using the CLEO Multidimensional Tool" Pharmacy 13, no. 5: 143. https://doi.org/10.3390/pharmacy13050143
APA StyleSilva, S., Jesus, M., Faria, S., Machado, S., & Morgado, M. (2025). Impact of Pharmacist Interventions in a Portuguese Hospital: A Study Using the CLEO Multidimensional Tool. Pharmacy, 13(5), 143. https://doi.org/10.3390/pharmacy13050143







