Real-World Evidence Assessing the Safety of Administering Intravenous Rituximab Biosimilar in the First Cycle and Subcutaneous Rituximab in Subsequent Cycles in B-Cell Lymphoma Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Study Outcomes and Data Collection
2.3. Statistical Analysis
2.4. Use of Generative Artificial Intelligence (GenAI)
3. Results
3.1. Baseline Characteristics
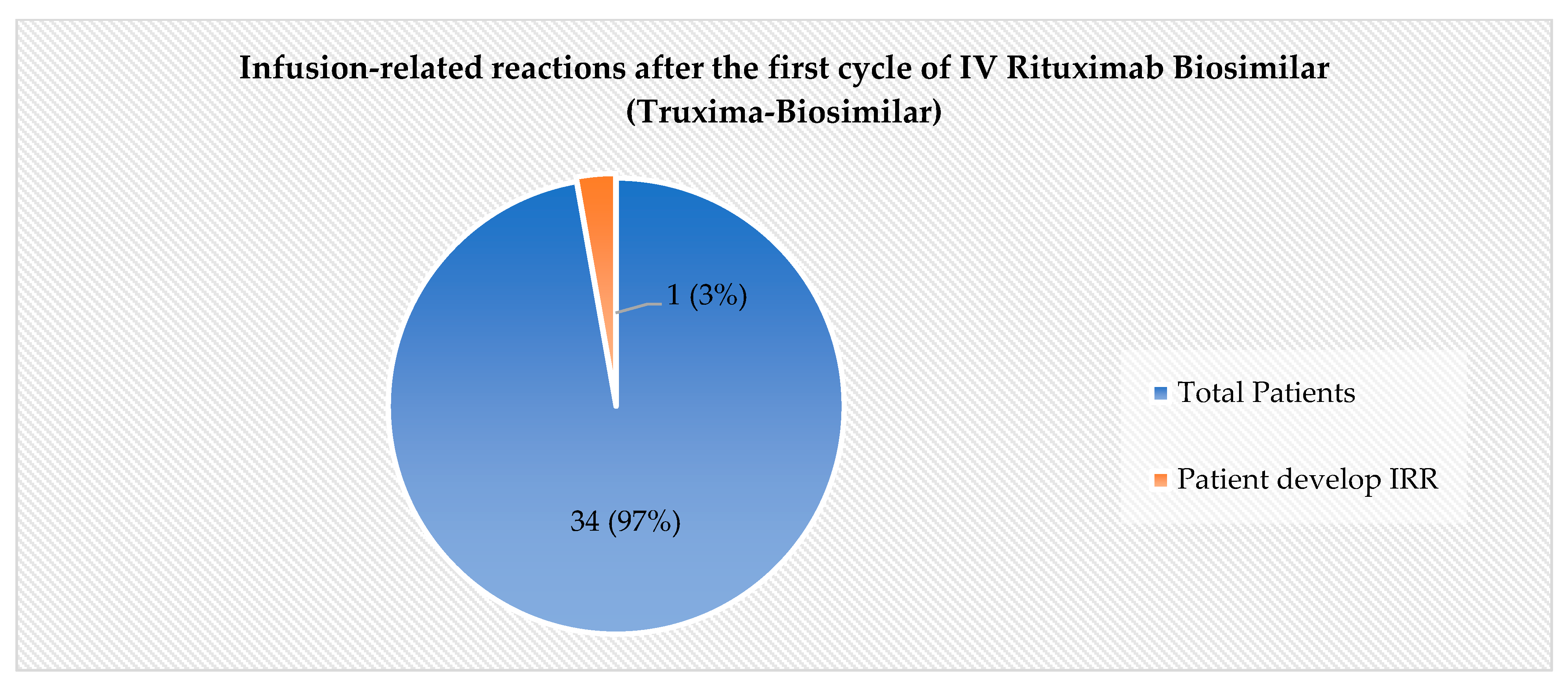
3.2. The Incidence of IRRs
3.3. Effectiveness Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.A.; Aseeri, M.A.; Alshamrani, M.A.; Alnatsheh, A.H.; Alhamdan, H.S. Emerging Role of Biosimilars in Oncology-Hematology in Saudi Arabia: A Practical Perspective. Glob. J. Qual. Saf. Healthc. 2019, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, A.R.; Al-Samil, A.M.; Alsayyari, A.; Yousef, C.C.; Khan, M.A.; Alhamdan, H.S.; Al-Jedai, A. The landscape of biosimilars in Saudi Arabia: Preparing for the next decade. Expert Opin. Biol. Ther. 2023, 23, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Yousef, C.C.; Khan, M.A.; Almodaimegh, H.; Alshamrani, M.; Al-Foheidi, M.; AlAbdalkarim, H.; AlJedai, A.; Naeem, A.; Abraham, I.; Alfoheidy, M.O. Cost-efficiency analysis of conversion to biosimilar filgrastim for supportive cancer care and resultant expanded access analysis to supportive care and early-stage HER2+ breast cancer treatment in Saudi Arabia: Simulation study. J. Med. Econ. 2023, 26, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.; Barrett, M.; Foà, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Truxima as Biosimilar to Rituxan for Non-Hodgkin’s Lymphoma | FDA. 2025. Available online: https://www.fda.gov/drugs/fda-approves-truxima-biosimilar-rituxan-non-hodgkins-lymphoma (accessed on 25 March 2025).
- RUXIENCETM (rituximab-pvvr) Prescribing Information. 2025. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761103s000lbl.pdf (accessed on 25 March 2025).
- RIABNITM (rituximab-arrx) Prescribing Information. 2025. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761140s000lbl.pdf (accessed on 25 March 2025).
- Welslau, M.; Kubuschok, B.; Topaly, J.; Otremba, B.; Wolff, T.; Bryn, G. REFLECT: Prospective multicenter non-interventional study evaluating the effectiveness and safety of Sandoz rituximab (SDZ-RTX; Rixathon®) in combination with CHOP for the treatment of patients with previously untreated CD20-positive diffuse large B-cell lymphoma. Ther. Adv. Hematol. 2023, 14, 20406207231183765. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, W.; Moreira, I.; Kanakasetty, G.B.; Munhoz, E.; Echeveste, M.A.; Giri, P.; Castro, N.; Pereira, J.; Akria, L.; Alexeev, S.; et al. Rituximab biosimilar and reference rituximab in patients with previously untreated advanced follicular lymphoma (ASSIST-FL): Primary results from a confirmatory phase 3, double-blind, randomised, controlled study. Lancet Haematol. 2017, 4, e350–e361. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Merli, F.; Mihaljević, B.; Mercadal, S.; Siritanaratkul, N.; Solal-Céligny, P.; Boehnke, A.; Berge, C.; Genevray, M.; Zharkov, A.; et al. Efficacy and safety of subcutaneous rituximab versus intravenous rituximab for first-line treatment of follicular lymphoma (SABRINA): A randomised, open-label, phase 3 trial. Lancet Haematol. 2017, 4, e272–e282. [Google Scholar] [CrossRef] [PubMed]
- Lugtenburg, P.; Avivi, I.; Berenschot, H.; Ilhan, O.; Marolleau, J.P.; Nagler, A.; Rueda, A.; Tani, M.; Turgut, M.; Osborne, S.; et al. Efficacy and safety of subcutaneous and intravenous rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in first-line diffuse large B-cell lymphoma: The randomized MabEase study. Haematologica 2017, 102, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- MabThera Summary of Product Characteristics. 2025. Available online: https://www.ema.europa.eu/en/documents/product-information/mabthera-epar-product-information_en.pdf (accessed on 25 March 2025).
- Hill, S.L.; Davies, A. Subcutaneous Rituximab with Recombinant Human Hyaluronidase in the Treatment of Non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia. Future Oncol. 2018, 14, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Assouline, S.; Buccheri, V.; Delmer, A.; Gaidano, G.; Trneny, M.; Berthillon, N.; Brewster, M.; Catalani, O.; Li, S.; McIntyre, C.; et al. Pharmacokinetics, safety, and efficacy of subcutaneous versus intravenous rituximab plus chemotherapy as treatment for chronic lymphocytic leukaemia (SAWYER): A phase 1b, open-label, randomised controlled non-inferiority trial. Lancet Haematol. 2016, 3, e128–e138. Available online: https://www.thelancet.com/action/showFullText?pii=S2352302616000041 (accessed on 25 March 2025). [CrossRef] [PubMed]
- Davies, A.; Berge, C.; Boehnke, A.; Dadabhoy, A.; Lugtenburg, P.; Rule, S.; Rummel, M.; McIntyre, C.; Smith, R.; Badoux, X. Subcutaneous Rituximab for the Treatment of B-Cell Hematologic Malignancies: A Review of the Scientific Rationale and Clinical Development. Adv. Ther. 2017, 34, 2210. [Google Scholar] [CrossRef] [PubMed]
- Si, T.; Ma, X.; Zhu, W.; Zhou, Y. Clinical efficacy and safety of subcutaneous rituximab in non-Hodgkin lymphoma: A systematic literature review and meta-analysis. Hematology 2023, 28, 2284047. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Abu Esba, L.C.; Yousef, C.C.; Alharbi, M.; Modaimegh, H.; Metwali, H.; Abdulkarim, H.; Alshamrani, M.; Aseeri, M.; Almansour, M.; et al. Practical challenges and considerations in adopting biosimilars in oncology clinical practice within a large healthcare system. Expert Rev. Clin. Pharmacol. 2025, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Islami, M.M.; Khan, M.A.; Aseeri, M.A.; Alshamrani, M.A.; Alnatsheh, A.; Alamoudi, S.; Alzahrani, A.A. Comparison of Biosimilar Filgrastim with Innovator Fligrastim for Peripheral Blood Stem Cells Mobilization, Collection of CD34+ Stem Cells, and Engraftment in Patients Undergoing Autologous and Allogeneic Stem Cell Transplantation: A Single-Center Experience. Ann. Transpl. 2023, 28, e938585. [Google Scholar] [CrossRef]
- Product Information: RITUXAN HYCELATM Subcutaneous Injection, Rituximab, Hyaluronidase Human Subcutaneous Injection; Genentech USA, Inc (per FDA): South San Francisco, CA, USA, 2017.
- Biosimilar Product Regulatory Review and Approval. 2025. Available online: https://www.fda.gov/files/drugs/published/Biosimilar-Product-Regulatory-Review-and-Approval.pdf (accessed on 25 March 2025).
- Ismail, S.; Abu Esba, L.; Khan, M.; Al-Abdulkarim, H.; Modimagh, H.; Yousef, C. An Institutional Guide for Formulary Decisions of Biosimilars. Hosp. Pharm. 2022, 58, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) | Protocol Development | CTEP. 2025. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 25 March 2025).
- Ogura, M.; Sancho, J.M.; Cho, S.G.; Nakazawa, H.; Suzumiya, J.; Tumyan, G.; Kim, J.S.; Lennard, A.; Mariz, J.; Ilyin, N.; et al. Efficacy, pharmacokinetics, and safety of the biosimilar CT-P10 in comparison with rituximab in patients with previously untreated low-tumour-burden follicular lymphoma: A randomised, double-blind, parallel-group, phase 3 trial. Lancet Haematol. 2018, 5, e543–e553. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.J. Management and preparedness for infusion and hypersensitivity reactions. Oncologist 2007, 12, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Kasi, P.M.; Tawbi, H.A.; Oddis, C.V.; Kulkarni, H.S. Clinical review: Serious adverse events associated with the use of rituximab—A critical care perspective. Crit. Care 2012, 16, 231. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall (N = 35) |
|---|---|
| Gender | |
| Male | 22 (63%) |
| Female | 13 (37%) |
| Weight (kilograms) | 73 ± 18 |
| Age (years) | 55 ± 18 |
| Laboratory values | |
| Serum creatinine (µmol/L) | 70 ± 31 |
| Alanine aminotransferase (U/L) | 18 ± 13 |
| Aspartate aminotransferase (U/L) | 28 ± 36 |
| Platelet (×109 g/L) | 273 ± 126 |
| Hemoglobin (g/dL) | 12 ± 2 |
| Hematocrit (%) | 36 ± 6 |
| White blood cells (×109/L) | 6 ± 3 |
| Absolute neutrophils count (×109/L) | 4 ± 3 |
| Potassium (mmol/L) | 4 ± 1 |
| Sodium (mmol/L) | 138 ± 2 |
| Calcium (mmol/L) | 2 ± 0.1 |
| Phosphate (mmol/L) | 1 ± 0.2 |
| Comorbidities | |
| Medically free | 16 (45%) |
| Hypertension | 15 (43%) |
| Diabetes mellitus | 11 (31%) |
| Cardiovascular disease | 7 (20%) |
| Hypothyroidism | 4 (11%) |
| Benign prostatic hyperplasia | 2 (6%) |
| Chronic obstructive pulmonary disease | 2 (6%) |
| Diagnosis | |
| Diffuse large B-cell lymphoma | 22 (63%) |
| Follicular lymphoma | 7 (20%) |
| Lymphocyte-predominant Hodgkin lymphoma | 3 (8%) |
| Primary mediastinal large B-cell lymphoma | 2 (6%) |
| Splenic marginal zone lymphoma | 1 (3%) |
| Protocol | |
| R-CHOP | 22 (63%) |
| R-B | 7 (20%) |
| R-GDP | 3 (8%) |
| DA-R-EPOCH | 2 (6%) |
| BR-Pola | 1 (3%) |
| Response | Overall (n = 33) |
|---|---|
| Complete response | 26 (79%) |
| Progressive disease (PD) | 5 (15%) |
| Partial response | 2 (6%) |
| Overall response rate | 28 (85%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almandeel, T.; Khan, M.A.; Algethami, A.; Alaboud, M.S.; Alkathiri, M.A.; Aseeri, M.; Absi, A.; Almansour, M.; Alotaibi, A. Real-World Evidence Assessing the Safety of Administering Intravenous Rituximab Biosimilar in the First Cycle and Subcutaneous Rituximab in Subsequent Cycles in B-Cell Lymphoma Patients. Pharmacy 2025, 13, 83. https://doi.org/10.3390/pharmacy13030083
Almandeel T, Khan MA, Algethami A, Alaboud MS, Alkathiri MA, Aseeri M, Absi A, Almansour M, Alotaibi A. Real-World Evidence Assessing the Safety of Administering Intravenous Rituximab Biosimilar in the First Cycle and Subcutaneous Rituximab in Subsequent Cycles in B-Cell Lymphoma Patients. Pharmacy. 2025; 13(3):83. https://doi.org/10.3390/pharmacy13030083
Chicago/Turabian StyleAlmandeel, Tamather, Mansoor Ahmed Khan, Ashwag Algethami, Mashael S. Alaboud, Munirah A. Alkathiri, Mohammed Aseeri, Ahmed Absi, Mubarak Almansour, and Abdullah Alotaibi. 2025. "Real-World Evidence Assessing the Safety of Administering Intravenous Rituximab Biosimilar in the First Cycle and Subcutaneous Rituximab in Subsequent Cycles in B-Cell Lymphoma Patients" Pharmacy 13, no. 3: 83. https://doi.org/10.3390/pharmacy13030083
APA StyleAlmandeel, T., Khan, M. A., Algethami, A., Alaboud, M. S., Alkathiri, M. A., Aseeri, M., Absi, A., Almansour, M., & Alotaibi, A. (2025). Real-World Evidence Assessing the Safety of Administering Intravenous Rituximab Biosimilar in the First Cycle and Subcutaneous Rituximab in Subsequent Cycles in B-Cell Lymphoma Patients. Pharmacy, 13(3), 83. https://doi.org/10.3390/pharmacy13030083






