Preferences of Hospital Pharmacists for the Different Attributes of Intravitreal Treatments for Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema in Spain: The SEEKING Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Variables
- Selection of attributes and levels (Supplementary Table S1) [25]: Five treatment attributes, including best corrected visual acuity (BCVA), ocular adverse events (AE), annual drug cost, available presentation, and mechanism of action (MoA), were selected from those used in clinical trials and other studies, extracted from a literature review, and after consensus of an expert panel of HPs, and also determined by a consensus of the most relevant levels for each attribute.
- Application of an orthogonal design with Statistical Package for the Social Sciences (SPSS): The five attributes with two levels each (nAMD) and the five attributes, four of them with two levels and one with three levels (DME) defined in step 1 (Supplementary Table S1), were used. The maximum number of possible scenarios was 32 for nAMD and 48 for DME. Since it is impossible to establish a ranking among all these possibilities at a cognitive level, eight scenarios (cards from A to H) were selected by applying an orthogonal design with SPSS. HPs’ preferences for the final eight scenarios were evaluated with a ranking system (Supplementary Table S2).
2.3. Analysis
- Some experience (≤5 years): Years of experience in managing intravitreal treatments for nAMD/DME equal to or less than the median time obtained in the study sample.
- Broad experience (>5 years): Years of experience in managing intravitreal treatments for nAMD/DME higher than the median time obtained in the study sample.
2.4. Sample Size and Statistical Analysis
3. Results
3.1. HPs’ Preferences for the Different Attributes of Intravitreal Treatments
3.2. Utility Scores and Importance
3.3. HPs’ Preferences According to Clinical Experience
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse events |
| BCVA | Best corrected visual acuity |
| CA | Conjoint analysis |
| DME | Diabetic macular edema |
| HP | Hospital pharmacist |
| MoA | Mechanism of action |
| nAMD | Neovascular age-related macular degeneration |
| SD | Standard deviation |
References
- Das, U.N. Diabetic macular edema, retinopathy and age-related macular degeneration as inflammatory conditions. Arch. Med. Sci. 2016, 12, 1142–1157. [Google Scholar] [CrossRef] [PubMed]
- Soriguer, F.; Goday, A.; Bosch-Comas, A.; Bordiú, E.; Calle-Pascual, A.; Carmena, R.; Casamitjana, R.; Castaño, L.; Castell, C.; Catalá, M.; et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: The Di@bet.es Study. Diabetologia 2012, 55, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Letow, J.; Wolpers, C.; Pascual-Camps, I.; Holz, F.G.; Finger, R.P. Prevalence, incidence and future projection of diabetic eye disease in Europe: A systematic review and meta-analysis. Eur. J. Epidemiol. 2020, 35, 11–23. [Google Scholar] [CrossRef]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Mauschitz, M.M.; Holz, F.G.; Finger, R.P. Prevalence and incidence of age-related macular degeneration in Europe: A systematic review and meta-analysis. Br. J. Ophthalmol. 2020, 104, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, J.M.; Arias, L.; Abraldes, M.J.; Montero, J.; Udaondo, P. Economic burden of age-related macular degeneration in routine clinical practice: The RAMDEBURS study. Int. Ophthalmol. 2021, 41, 3427–3436. [Google Scholar] [CrossRef]
- Arias, L.; Armadá, F.; Donate, J.; García-Arumí, J.; Giralt, J.; Pazos, B.; Piñero, A.; Martínez, F.; Mondéjar, J.J.; Ortega, I.; et al. Delay in treating age-related macular degeneration in Spain is associated with progressive vision loss. Eye 2009, 23, 326–333. [Google Scholar] [CrossRef]
- Rose, M.A.; Vukicevic, M.; Koklanis, K.; Rees, G.; Sandhu, S.; Itsiopoulos, C. Experiences and perceptions of patients undergoing treatment and quality of life impact of diabetic macular edema: A systematic review. Psychol. Health Med. 2019, 24, 383–401. [Google Scholar] [CrossRef]
- García-Layana, A.; García-Arumí, J.; Figueroa, M.S.; Arias Barquet, L.; Ruíz-Moreno, J.M.; Monclús-Arbona, L.; The Spanish Amd Multicenter Group. Management of Wet Age-Related Macular Degeneration in Spain: Challenges for Treat and Extend Implementation in Routine Clinical Practice. J. Ophthalmol. 2019, 2019, 9821509. [Google Scholar] [CrossRef]
- Mondéjar, J.; Pellico, G.; Sallén, T.; Núñez, P.; Puigcerver, M.; Pallàs, I. Management optimization of neovascular age-related macular degeneration in Spain: Evolution towards proactive treatment models. J. Healthc. Qual. Res. 2023, 38, 284–293. [Google Scholar] [CrossRef]
- World Health Organization (WHO). World Report on Vision. 2019. Available online: https://www.who.int/publications/i/item/world-report-on-vision (accessed on 26 June 2024).
- Spanish Eyes Epidemiological (SEE) Study Group. Prevalence of age-related macular degeneration in Spain. Br. J. Ophthalmol. 2011, 95, 931–936. [Google Scholar] [CrossRef]
- Abreu-Gonzalez, R.; Gallego-Pinazo, R.; Abraldes, M.; Pinilla, I.; Lopez-Galvez, M.I. Management of diabetic macular edema patients in clinical practice in Spain. Eur. J. Ophthalmol. 2019, 29, 664–672. [Google Scholar] [CrossRef]
- Fernández-Vigo, J.I.; Contreras, I.; Crespo, M.J.; Beckford, C.; Flores-Moreno, I.; Cobo-Soriano, R.; Pareja, J.; Martín, M.D.; Moreno, L.; Arrevola-Velasco, L. Expert Panel Consensus for Addressing Anti-VEGF Treatment Challenges of Diabetic Macular Edema in Spain. Clin. Ophthalmol. 2022, 16, 3097–3106. [Google Scholar] [CrossRef] [PubMed]
- Sociedad Española de Retina y Vítreo (SERV). Guías de Práctica Clínica y Monografías. Guía 3: Manejo de las Complicaciones Oculares de la Diabetes. Retinopatía Diabética y Edema Macular. 2021. Available online: https://serv.es/ (accessed on 6 March 2024).
- Sociedad Española de Retina y Vítreo (SERV). Guías de Práctica Clínica y Monografías. Guía 1: Tratamiento de la DMAE Exudativa. 2023. Available online: https://serv.es/ (accessed on 6 March 2024).
- Mantovani, L.G.; Monzini, M.S.; Mannucci, P.M.; Scalone, L.; Villa, M.; Gringeri, A.; Conan Study Group. Differences between patients’, physicians’ and pharmacists’ preferences for treatment products in haemophilia: A discrete choice experiment. Haemophilia 2005, 11, 589–597. [Google Scholar] [CrossRef] [PubMed]
- de Andrés-Nogales, F.; Casado, M.Á.; Trillo, J.L.; Ruiz-Moreno, J.M.; Martínez-Sesmero, J.M.; Peralta, G.; Poveda, J.L.; Ortiz, P.; Ignacio, E.; Zarranz-Ventura, J.; et al. A Multiple Stakeholder Multicriteria Decision Analysis in Diabetic Macular Edema Management: The MULTIDEX-EMD Study. Pharmacoecon. Open 2020, 4, 615–624. [Google Scholar] [CrossRef]
- Martínez-López, I.; Maurino, J.; Sanmartín-Fenollera, P.; Ontañon-Nasarre, A.; Santiago-Pérez, A.; Moya-Carmona, I.; García-Collado, C.G.; Fernández-Del Olmo, R.; García-Arcelay, E.; Sarmiento, M.; et al. Assessing Pharmacists’ Preferences towards Efficacy Attributes of Disease-Modifying Therapies in Relapsing-Remitting Multiple Sclerosis. Pharmacy 2020, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Luce, R.D.; Tukey, J.W. Simultaneous conjoint measurement: A new type of fundamental measurement. J. Math. Psychol. 1964, 1, 1–27. [Google Scholar] [CrossRef]
- McFadden, D. The choice theory approach to market research. Mark. Sci. 1986, 5, 275–297. [Google Scholar] [CrossRef]
- Farley, K.; Thompson, C.; Hanbury, A.; Chambers, D. Exploring the feasibility of Conjoint Analysis as a tool for prioritizing innovations for implementation. Implement. Sci. 2013, 8, 56. [Google Scholar] [CrossRef]
- Webb, E.J.D.; Meads, D.; Eskyte, I.; King, N.; Dracup, N.; Chataway, J.; Ford, H.L.; Marti, J.; Pavitt, S.H.; Schmierer, K.; et al. A Systematic Review of Discrete-Choice Experiments and Conjoint Analysis Studies in People with Multiple Sclerosis. Patient. 2018, 11, 391–402. [Google Scholar] [CrossRef]
- Al-Omari, B.; Farhat, J.; Ershaid, M. Conjoint Analysis: A Research Method to Study Patients’ Preferences and Personalize Care. J. Pers. Med. 2022, 12, 274. [Google Scholar] [CrossRef]
- Del Río-Muñoz, B.; Azanza-Munarriz, C.; Becerril-Ríos, N.; Goicochea-Briceño, H.; Horno, R.; Lendínez-Mesa, A.; Sánchez-Franco, C.; Sarmiento, M.; Bueno-Gil, G.; Medrano, N.; et al. Preferences Toward Attributes of Disease-Modifying Therapies: The Role of Nurses in Multiple Sclerosis Care. J. Neurosci. Nurs. 2022, 54, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castillo, R.; González-Gallardo, C.; Muñoz-Ávila, J.I.; Font, P.; Villalba-González, M.; Stoikow, I.; Fernández-Choquet de Isla, I.; Pugliese, F.; Anaya-Alaminos, R.; García-Serrano, J.L.; et al. Treatment of neovascular age-related macular degeneration within 48 h from diagnosis improves long-term functional outcome. Biomed. Pharmacother. 2023, 160, 114368. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.R. Conjoint analysis. In Wiley International Encyclopedia of Marketing; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Coast, J.; Flynn, T.N.; Salisbury, C.; Louviere, J.; Peters, T.J. Maximising responses to discrete choice experiments: A randomised trial. Appl. Health Econ. Health Policy 2006, 5, 249–260. [Google Scholar] [CrossRef]
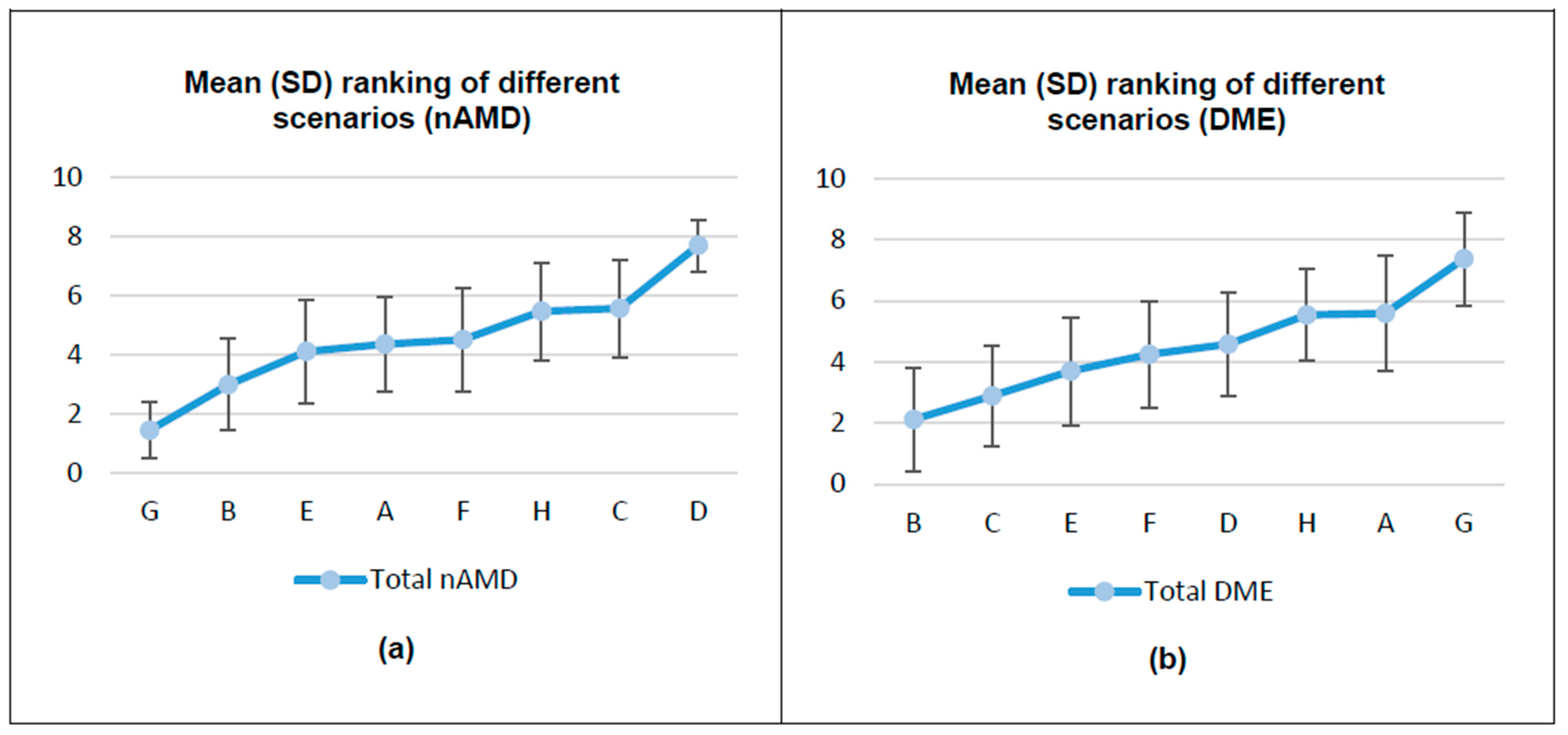
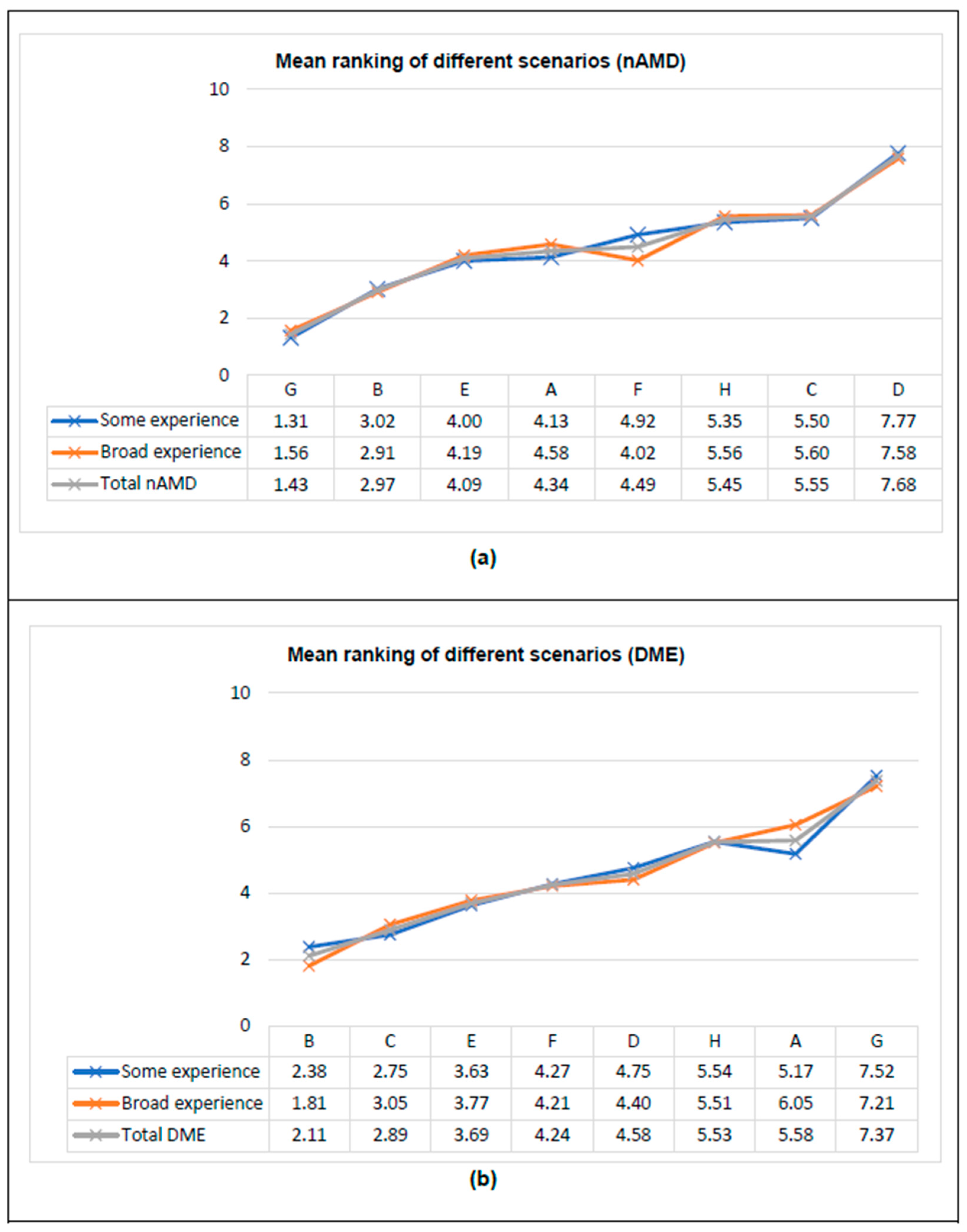
| Characteristics | Total Sample (n = 91) * |
|---|---|
| Age, years, mean (SD) | 39.2 (10.2) |
| Sex, female, n (%) | 55 (60.4%) |
| Years as a HP, mean (SD) (valid n = 89) | 12.6 (8.3) |
| Years of managing patients with intravitreal treatments for nAMD/DME, n (%) | |
| <1 year | 8 (8.8%) |
| ≥1 year | 83 (91.2%) |
| Number of years of experience managing intravitreal treatments, mean (SD) (valid n = 83) | 7.4 (5.0) |
| Level of clinical experience according to years of experience managing intravitreal treatments (quartile definition) | |
| Some experience (≤5 years) | 48 (52.7%) |
| Broad experience (>5 years) | 43 (47.3%) |
| Participation in nAMD/DME clinical trials, n (%) | 12 (13.2%) |
| Author or co-author of scientific publications, n (%) | 44 (48.4%) |
| Participation in a pharmacy commission for the evaluation of medicines, n (%) | 73 (80.2%) |
| Current position | |
| Head of service | 16 (17.6%) |
| Specialist pharmacist | 63 (69.2%) |
| Pharmacy resident | 12 (13.2%) |
| Pharmacist profile, n (%) | |
| Manufacturer/sterile area | 44 (48.4%) |
| Logistic area | 47 (51.6%) |
| Type of hospital, n (%) | |
| Regional hospital | 25 (27.5%) |
| University hospital | 66 (72.5%) |
| Number of treatments prepared for intravitreal administration in a month, mean (SD) | |
| For nAMD (valid n = 87) | 215.7 (232.8) |
| For DME (valid n = 86) | 76.0 (94.2) |
| Variable | Utility Estimation | nAMD (n = 91) | DME (n = 91) | ||||
|---|---|---|---|---|---|---|---|
| nAMD (n = 91) | DME (n = 91) | Importance (Relative) | Importance (Averaged) | Importance (Relative) | Importance (Averaged) | ||
| BCVA | ≥15 letters | 1.294 | 1.266 | 38.6% | 36.5% | 44.6% | 34.5% |
| <15 letters | −1.294 | −1.266 | |||||
| Ocular AE | Severe but less frequent AE | −0.915 | −0.723 | 27.3% | 27.7% | 25.5% | 25.1% |
| Mild but more frequent AE | 0.915 | 0.723 | |||||
| Annual drug cost | Increase | −0.547 | −0.508 | 16.3% | 15.3% | 17.9% | 15.4% |
| No effect/decrease | 0.547 | 0.508 | |||||
| Available presentation | Prefilled syringe | 0.371 | 0.234 | 11.1% | 13.3% | 7.3% | 15.5% |
| Vial | −0.371 | −0.183 | |||||
| Implant | NA | −0.051 | |||||
| MoA | MoA with more spacing between doses | 0.225 | 0.135 | 6.7% | 7.3% | 4.8% | 9.5% |
| MoA with less spacing between doses | −0.225 | −0.135 | |||||
| Variable | Utility Estimation | Importance (Relative) | Importance (Averaged) | |||||
|---|---|---|---|---|---|---|---|---|
| Some Experience (n = 48) | Broad Experience (n = 43) | Some Experience (n = 48) | Broad Experience (n = 43) | Some Experience (n = 48) | Broad Experience (n = 43) | p-Value | ||
| nAMD | ||||||||
| BCVA | ≥15 letters | 1.385 | 1.192 | 41.1% | 35.8% | 38.5% | 34.3% | 0.2826 |
| <15 letters | −1.385 | −1.192 | ||||||
| Ocular AE | Severe but less frequent AE | −0.849 | −0.988 | 25.2% | 29.7% | 25.8% | 29.8% | 0.3132 |
| Mild but more frequent AE | 0.849 | 0.988 | ||||||
| Annual drug cost | Increase | −0.536 | −0.558 | 15.9% | 16.8% | 14.7% | 15.9% | 0.6368 |
| No effect/decrease | 0.536 | 0.558 | ||||||
| Available presentation | Prefilled syringe | 0.458 | 0.273 | 13.6% | 8.2% | 15.5% | 10.8% | 0.1169 |
| Vial | −0.458 | −0.273 | ||||||
| MoA | MoA with more spacing between doses | 0.141 | 0.320 | 4.2% | 9.6% | 5.5% | 9.3% | 0.0253 |
| MoA with less spacing between doses | −0.141 | −0.320 | ||||||
| DME | ||||||||
| BCVA | ≥15 letters | 1.245 | 1.291 | 42.5% | 46.0% | 33.0% | 36.2% | 0.4000 |
| <15 letters | −1.245 | −1.291 | ||||||
| Ocular AE | Severe but less frequent AE | −0.646 | −0.808 | 22.1% | 28.8% | 24.3% | 26.0% | 0.6592 |
| Mild but more frequent AE | 0.646 | 0.808 | ||||||
| Annual drug cost | Increase | −0.521 | −0.494 | 17.8% | 17.6% | 16.5% | 14.2% | 0.3891 |
| No effect/decrease | 0.521 | 0.494 | ||||||
| Available presentation | Prefilled syringe | 0.354 | 0.101 | 12.2% | 3.8% | 16.4% | 14.5% | 0.5565 |
| Vial | −0.359 | 0.014 | ||||||
| Implant | 0.005 | −0.114 | ||||||
| MoA | MoA with more spacing between doses | 0.161 | 0.105 | 5.5% | 3.7% | 9.8% | 9.2% | 0.7549 |
| MoA with less spacing between doses | −0.161 | −0.105 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poveda, J.L.; Arnáiz, P.; López, S.; Muñoz, B.; Fernández-Ferreiro, A. Preferences of Hospital Pharmacists for the Different Attributes of Intravitreal Treatments for Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema in Spain: The SEEKING Study. Pharmacy 2025, 13, 68. https://doi.org/10.3390/pharmacy13030068
Poveda JL, Arnáiz P, López S, Muñoz B, Fernández-Ferreiro A. Preferences of Hospital Pharmacists for the Different Attributes of Intravitreal Treatments for Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema in Spain: The SEEKING Study. Pharmacy. 2025; 13(3):68. https://doi.org/10.3390/pharmacy13030068
Chicago/Turabian StylePoveda, José Luis, Pablo Arnáiz, Silvia López, Belén Muñoz, and Anxo Fernández-Ferreiro. 2025. "Preferences of Hospital Pharmacists for the Different Attributes of Intravitreal Treatments for Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema in Spain: The SEEKING Study" Pharmacy 13, no. 3: 68. https://doi.org/10.3390/pharmacy13030068
APA StylePoveda, J. L., Arnáiz, P., López, S., Muñoz, B., & Fernández-Ferreiro, A. (2025). Preferences of Hospital Pharmacists for the Different Attributes of Intravitreal Treatments for Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema in Spain: The SEEKING Study. Pharmacy, 13(3), 68. https://doi.org/10.3390/pharmacy13030068







