Ketoprofen Lysine Salt Versus Corticosteroids in Early Outpatient Management of Mild and Moderate COVID-19: A Retrospective Study
Abstract
1. Introduction
2. Methods
3. Statistical Analysis
4. Results
4.1. Patient Characteristics
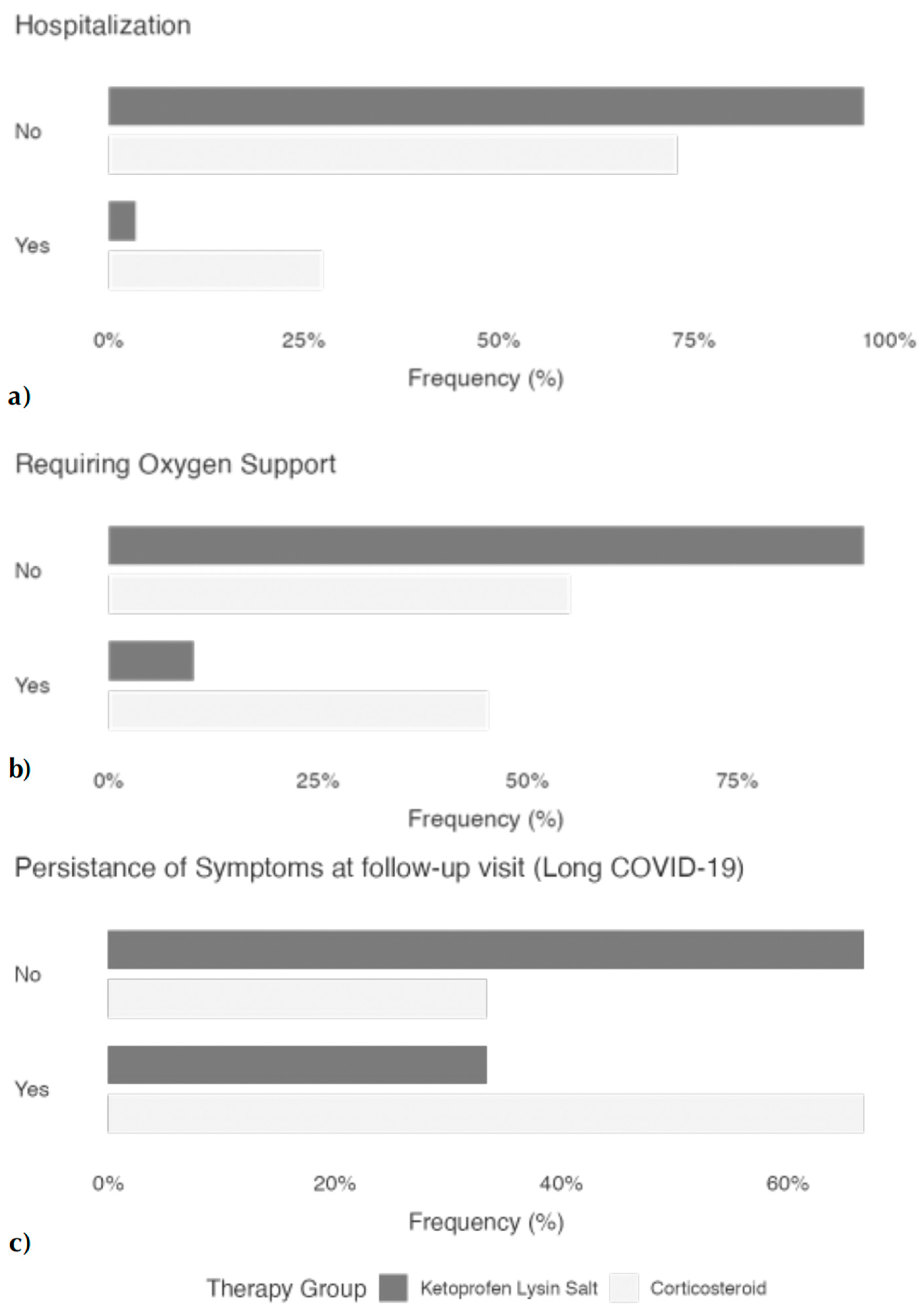
4.2. Main Clinical Outcomes
5. Discussion
Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, J. SARS-CoV-2: An Emerging Coronavirus that Causes a Global Threat. Int. J. Biol. Sci. 2020, 16, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Prado, E.; Simbaña-Rivera, K.; Barreno, L.G.; Rubio-Neira, M.; Guaman, L.P.; Kyriakidis, N.C.; Muslin, C.; Jaramillo, A.M.G.; Barba-Ostria, C.; Cevallos-Robalino, D.; et al. Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a comprehensive literature review. Diagn. Microbiol. Infect. Dis. 2020, 98, 115094. [Google Scholar] [CrossRef]
- Young, B.E.; Ong, S.W.X.; Kalimuddin, S.; Low, J.G.; Tan, S.Y.; Loh, J.; Ng, O.T.; Marimuthu, K.; Ang, L.W.; Mak, T.M.; et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA 2020, 323, 1488–1494. [Google Scholar] [CrossRef]
- Bianco, A.; Valente, T.; Perrotta, F.; Stellato, E.; Brunese, L.; Wood, B.J.; Carrafiello, G.; Parrella, R.; Aronne, L.; Boccia, M.; et al. Remarkable vessel enlargement within lung consolidation in COVID-19 compared to AH1N1 pneumonia: A retrospective study in Italy. Heliyon 2021, 7, e07112. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Ho, Y.-C. SARS-CoV-2: A storm is raging. J. Clin. Investig. 2020, 130, 2202–2205. [Google Scholar] [CrossRef]
- Bianco, A.; Conte, S.; Mariniello, D.F.; Allocca, V.; Matera, M.G.; D’agnano, V.; Lanata, L.; Cazzola, M.; Perrotta, F. Mucolytic and Antioxidant Properties of Carbocysteine as a Strategy in COVID-19 Therapy. Life 2022, 12, 1824. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Moore, N.; Bosco-Levy, P.; Thurin, N.; Blin, P.; Droz-Perroteau, C. NSAIDs and COVID-19: A Systematic Review and Meta-analysis. Drug Saf. 2021, 44, 929–938. [Google Scholar] [CrossRef]
- Vosu, J.; Britton, P.; Howard-Jones, A.; Isaacs, D.; Kesson, A.; Khatami, A.; Marais, B.; Nayda, C.; Outhred, A. Is the risk of ibuprofen or other non-steroidal anti-inflammatory drugs increased in COVID-19? J. Paediatr. Child. Health 2020, 56, 1645–1646. [Google Scholar] [CrossRef]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Abu Esba, L.C.; Alqahtani, R.A.; Thomas, A.; Shamas, N.; Alswaidan, L.; Mardawi, G. Ibuprofen and NSAID Use in COVID-19 Infected Patients Is Not Associated with Worse Outcomes: A Prospective Cohort Study. Infect. Dis. Ther. 2021, 10, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.Y.; MacKenna, B.; E Morton, C.; Schultze, A.; Walker, A.J.; Bhaskaran, K.; Brown, J.P.; Rentsch, C.T.; Williamson, E.; Drysdale, H.; et al. Use of non-steroidal anti-inflammatory drugs and risk of death from COVID-19: An OpenSAFELY cohort analysis based on two cohorts. Ann. Rheum. Dis. 2021, 80, 943–951. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, S.; Gan, L.; Wang, Z.; Peng, S.; Li, Q.; Liu, H.; Liu, X.; Wang, Z.; Shi, Q.; et al. Use of non-steroidal anti-inflammatory drugs and adverse outcomes during the COVID-19 pandemic: A systematic review and meta-analysis. eClinicalMedicine 2022, 46, 101373. [Google Scholar] [CrossRef] [PubMed]
- Kushner, P.; McCarberg, B.H.; Grange, L.; Kolosov, A.; Haveric, A.L.; Zucal, V.; Petruschke, R.; Bissonnette, S. The use of non-steroidal anti-inflammatory drugs (NSAIDs) in COVID-19. npj Prim. Care Respir. Med. 2022, 32, 35. [Google Scholar] [CrossRef]
- de Bruin, N.; Schneider, A.-K.; Reus, P.; Talmon, S.; Ciesek, S.; Bojkova, D.; Cinatl, J.; Lodhi, I.; Charlesworth, B.; Sinclair, S.; et al. Ibuprofen, Flurbiprofen, Etoricoxib or Paracetamol Do Not Influence ACE2 Expression and Activity In Vitro or in Mice and Do Not Exacerbate In-Vitro SARS-CoV-2 Infection. Int. J. Mol. Sci. 2022, 23, 1049. [Google Scholar] [CrossRef]
- Jamali, F.; Brocks, D.R. Clinical Pharmacokinetics of Ketoprofen and Its Enantiomers. Clin. Pharmacokinet. 1990, 19, 197–217. [Google Scholar] [CrossRef]
- Bassetti, M.; Andreoni, M.; Santus, P.; Scaglione, F. NSAIDs for early management of acute respiratory infections. Curr. Opin. Infect. Dis. 2024, 37, 304–311. [Google Scholar] [CrossRef]
- Levoin, N.; Blondeau, C.; Guillaume, C.; Grandcolas, L.; Chretien, F.; Jouzeau, J.-Y.; Benoit, E.; Chapleur, Y.; Netter, P.; Lapicque, F. Elucidation of the mechanism of inhibition of cyclooxygenases by acyl-coenzyme A and acylglucuronic conjugates of ketoprofen. Biochem. Pharmacol. 2004, 68, 1957–1969. [Google Scholar] [CrossRef]
- Marseglia, G.L.; Ciprandi, G. Clinical use of ketoprofen lysine salt: A reappraisal in adolescents with acute respiratory infections. Allergol. Immunopathol. 2023, 51, 76–82. [Google Scholar] [CrossRef]
- Kuczyńska, J.; Nieradko-Iwanicka, B. Future prospects of ketoprofen in improving the safety of the gastric mucosa. Biomed. Pharmacother. 2021, 139, 111608. [Google Scholar] [CrossRef] [PubMed]
- Novelli, R.; Aramini, A.; Boccella, S.; Bagnasco, M.; Cattani, F.; Ferrari, M.P.; Goisis, G.; Minnella, E.M.; Allegretti, M.; Pace, V. Ketoprofen lysine salt has a better gastrointestinal and renal tolerability than ketoprofen acid: A comparative tolerability study in the Beagle dog. Biomed. Pharmacother. 2022, 153, 113336. [Google Scholar] [CrossRef]
- Lapicque, F.; Jankowski, R.; Netter, P.; Bannwarth, B.; Guillemin, C.; Bene, M.-C.; Monot, C.; Wayoff, M. Drug Assay in Ground Tissues: Example of Ketoprofen Diffusion into Tonsillar Tissue. J. Pharm. Sci. 1990, 79, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Mariniello, D.F.; Allocca, V.; D’agnano, V.; Villaro, R.; Lanata, L.; Bagnasco, M.; Aronne, L.; Bianco, A.; Perrotta, F. Strategies Tackling Viral Replication and Inflammatory Pathways as Early Pharmacological Treatment for SARS-CoV-2 Infection: Any Potential Role for Ketoprofen Lysine Salt? Molecules 2022, 27, 8919. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Scialò, F.; Mariniello, D.F.; Nigro, E.; Komici, K.; Allocca, V.; Bianco, A.; Perrotta, F.; D’agnano, V. Effects of Different Corticosteroid Doses in Elderly Unvaccinated Patients with Severe to Critical COVID-19. Life 2022, 12, 1924. [Google Scholar] [CrossRef] [PubMed]
- Parrella, R.; Marra, A.; Scarano, F.; Manzillo, E.; Esposito, V.; Punzi, R.; Fragranza, F.; D’agnano, V.; Cazzola, M.; Bianco, A. Corticosteroids and Delayed Conversion of SARS-CoV-2 RNA Nasopharyngeal Swabs in Hospitalized Patients with COVID-19 Pneumonia. Arch. Bronconeumol. 2022, 58, 55–58. [Google Scholar] [CrossRef]
- Mariniello, D.F.; Aronne, L.; Vitale, M.; Schiattarella, A.; Pagliaro, R.; Komici, K. Current challenges and perspectives in lung cancer care during COVID-19 waves. Curr. Opin. Pulm. Med. 2023, 29, 239–247. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Management of COVID-19: Interim Guidance, 27 May 2020; Document Number: WHO/2019-nCoV/clinical/2020.5; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 323, 1824–1836. [Google Scholar] [CrossRef]
- Bassetti, M.; Giacobbe, D.R.; Bruzzi, P.; Barisione, E.; Centanni, S.; Castaldo, N.; Corcione, S.; De Rosa, F.G.; Di Marco, F.; Gori, A.; et al. Clinical Management of Adult Patients with COVID-19 Outside Intensive Care Units: Guidelines from the Italian Society of Anti-Infective Therapy (SITA) and the Italian Society of Pulmonology (SIP). Infect. Dis. Ther. 2021, 10, 1837–1885. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Thaweethai, T.; Jolley, S.E.; Karlson, E.W.; Levitan, E.B.; Levy, B.; McComsey, G.A.; McCorkell, L.; Nadkarni, G.N.; Parthasarathy, S.; Singh, U.; et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA 2023, 329, 1934–1946. [Google Scholar] [CrossRef] [PubMed]
- ANalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Brooks, P.M.; Day, R.O. Nonsteroidal antiinflammatory drugs--differences and similarities. N. Engl. J. Med. 1991, 324, 1716–1725. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. FitzGerald, Prostaglandins and inflammation. Arter. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Archambault, A.-S.; Zaid, Y.; Rakotoarivelo, V.; Turcotte, C.; Doré, É.; Dubuc, I.; Martin, C.; Flamand, O.; Amar, Y.; Cheikh, A.; et al. High levels of eicosanoids and docosanoids in the lungs of intubated COVID-19 patients. FASEB J. 2021, 35, e21666. [Google Scholar] [CrossRef] [PubMed]
- Kehrïbar, D.Y.; Cïhangïroğlu, M.; Sehmen, E.; Avci, B.; Çapraz, M.; Boran, M.; Günaydin, C.; Özgen, M. The assessment of the serum levels of TWEAK and prostaglandin F2α in COVID-19. Turk. J. Med. Sci. 2020, 50, 1786–1791. [Google Scholar] [CrossRef]
- Kazancioglu, S.; Yilmaz, F.M.; Bastug, A.; Ozbay, B.O.; Aydos, O.; Yücel, Ç.; Bodur, H.; Yilmaz, G. Assessment of Galectin-1, Galectin-3, and Prostaglandin E2 Levels in Patients with COVID-19. Jpn. J. Infect. Dis. 2021, 74, 530–536. [Google Scholar] [CrossRef]
- Hong, W.; Chen, Y.; You, K.; Tan, S.; Wu, F.; Tao, J.; Chen, X.; Zhang, J.; Xiong, Y.; Yuan, F.; et al. Celebrex Adjuvant Therapy on Coronavirus Disease 2019: An Experimental Study. Front. Pharmacol. 2020, 11, 561674. [Google Scholar] [CrossRef]
- Yan, Q.; Li, P.; Ye, X.; Huang, X.; Feng, B.; Ji, T.; Chen, Z.; Li, F.; Zhang, Y.; Luo, K.; et al. Longitudinal Peripheral Blood Transcriptional Analysis Reveals Molecular Signatures of Disease Progression in COVID-19 Patients. J. Immunol. 2021, 206, 2146–2159. [Google Scholar] [CrossRef]
- Theken, K.N.; FitzGerald, G.A. Bioactive lipids in antiviral immunity. Science 2021, 371, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Alfajaro, M.M.; Chow, R.D.; Wei, J.; Filler, R.B.; Eisenbarth, S.C.; Wilen, C.B. Non-steroidal anti-inflammatory drugs dampen the cytokine and antibody response to SARS-CoV-2 infection. J. Virol. 2021, 95, e00014-21. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, A.; Mestres-Truyol, J.; Ojeda-Montes, M.J.; Macip, G.; Saldivar-Espinoza, B.; Cereto-Massagué, A.; Pujadas, G.; Garcia-Vallvé, S. Prediction of Novel Inhibitors of the Main Protease (M-pro) of SARS-CoV-2 through Consensus Docking and Drug Reposition. Int. J. Mol. Sci. 2020, 21, 3793. [Google Scholar] [CrossRef]
- Xu, T.; Gao, X.; Wu, Z.; Selinger, D.W.; Zhou, Z. Indomethacin has a potent antiviral activity against SARS CoV-2 in vitro and canine coronavirus in vivo. bioRxiv 2020. [Google Scholar] [CrossRef]
- Sisakht, M.; Solhjoo, A.; Mahmoodzadeh, A.; Fathalipour, M.; Kabiri, M.; Sakhteman, A. Potential inhibitors of the main protease of SARS-CoV-2 and modulators of arachidonic acid pathway: Non-steroidal anti-inflammatory drugs against COVID-19. Comput. Biol. Med. 2021, 136, 104686. [Google Scholar] [CrossRef]
- Perico, N.; Cortinovis, M.; Suter, F.; Remuzzi, G. Home as the new frontier for the treatment of COVID-19: The case for anti-inflammatory agents. Lancet Infect. Dis. 2023, 23, e22–e33. [Google Scholar] [CrossRef] [PubMed]
- Carey, M.A.; Bradbury, J.A.; Seubert, J.M.; Langenbach, R.; Zeldin, D.C.; Germolec, D.R. Contrasting effects of cyclooxygenase-1 (COX-1) and COX-2 deficiency on the host response to influenza A viral infection. J. Immunol. 2005, 175, 6878–6884. [Google Scholar] [CrossRef]
- Suter, F.; Consolaro, E.; Pedroni, S.; Moroni, C.; Pastò, E.; Paganini, M.V.; Pravettoni, G.; Cantarelli, U.; Rubis, N.; Perico, N.; et al. A simple, home-therapy algorithm to prevent hospitalisation for COVID-19 patients: A retrospective observational matched-cohort study. eClinicalMedicine 2021, 37, 100941. [Google Scholar] [CrossRef]
- Schnitzer, T.J.; Burmester, G.R.; Mysler, E.; Hochberg, M.C.; Doherty, M.; Ehrsam, E.; Gitton, X.; Krammer, G.; Mellein, B.; Matchaba, P.; et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: Randomised controlled trial. Lancet 2004, 364, 665–674. [Google Scholar] [CrossRef]
- Bresalier, R.S.; Sandler, R.S.; Quan, H.; Bolognese, J.A.; Oxenius, B.; Horgan, K.; Lines, C.; Riddell, R.; Morton, D.; Lanas, A.; et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N. Engl. J. Med. 2005, 352, 1092–1102. [Google Scholar] [CrossRef]
- Donati, M.; Conforti, A.; Lenti, M.C.; Capuano, A.; Bortolami, O.; Motola, D.; Moretti, U.; Vannacci, A.; Rafaniello, C.; Vaccheri, A.; et al. Risk of acute and serious liver injury associated to nimesulide and other NSAIDs: Data from drug-induced liver injury case-control study in Italy. Br. J. Clin. Pharmacol. 2016, 82, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Julou, L.; Guyonnet, J.C.; Ducrot, R.; Fournel, J.; Pasquet, J. Ketoprofen (19.583 R.P.) (2-(3-benzoylphenyl)-propionic acid). Main pharmacological properties--outline of toxicological and pharmacokinetic data. Scand. J. Rheumatol. Suppl. 1976, 1976, 33–44. [Google Scholar]
- Scaglione, F. Utilizzo dei FANS nelle infezioni virali respiratorie, incluso COVID 19. Focus su ketoprofene sale di lisina. Clin. Pract. 2022. [Google Scholar] [CrossRef]
- Dawson, W.; Boot, J.R.; Harvey, J.; Walker, J.R. The pharmacology of benoxaprofen with particular to effects on lipoxygenase product formation. Eur. J. Rheumatol. Inflamm. 1982, 5, 61–68. [Google Scholar]
- Graziosi, A.; Senatore, M.; Gazzaniga, G.; Agliardi, S.; Pani, A.; Scaglione, F. Ketoprofen Lysine Salt vs. Ketoprofen Acid: Assessing the Evidence for Enhanced Safety and Efficacy. Life 2025, 15, 659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brandolini, L.; D’Angelo, M.; Antonosante, A.; Villa, S.; Cristiano, L.; Castelli, V.; Benedetti, E.; Catanesi, M.; Aramini, A.; Luini, A.; et al. Differential protein modulation by ketoprofen and ibuprofen underlines different cellular response by gastric epithelium. J. Cell. Physiol. 2018, 233, 2304–2312. [Google Scholar] [CrossRef]
- Mannila, A.; Kokki, H.; Heikkinen, M.; Laisalmi, M.; Lehtonen, M.; Louhisto, H.L.; Järvinen, T.; Savolainen, J. Cerebrospinal fluid distribution of ketoprofen after intravenous administration in young children. Clin. Pharmacokinet. 2006, 45, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Marmo, E.; Ottavo, R.; Giordano, L.; Paone, G.; Falcone, O.; Spaziante, G.; Visone, C.; Campidonico, U. Experimental assessment of some pharmacodynamic features of ketoprofen lysine. Pain relief activity, antipyretic effects, anti-inflammatory activity, anti-platelet aggregation activity and interference with the biosynthesis of prostaglandins. Arch. Sci. Med. 1980, 137, 387–404. [Google Scholar]
- Nigro, E.; Perrotta, F.; Polito, R.; D’agnano, V.; Scialò, F.; Bianco, A.; Daniele, A. Metabolic Perturbations and Severe COVID-19 Disease: Implication of Molecular Pathways. Int. J. Endocrinol. 2020, 2020, 8896536. [Google Scholar] [CrossRef]
- Colleluori, G.; Graciotti, L.; Pesaresi, M.; Di Vincenzo, A.; Perugini, J.; Di Mercurio, E.; Caucci, S.; Bagnarelli, P.; Zingaretti, C.M.; Nisoli, E.; et al. Visceral fat inflammation and fat embolism are associated with lung’s lipidic hyaline membranes in subjects with COVID-19. Int. J. Obes. 2022, 46, 1009–1017. [Google Scholar] [CrossRef]
- Perrotta, F.; Scialò, F.; Mallardo, M.; Signoriello, G.; D’agnano, V.; Bianco, A.; Daniele, A.; Nigro, E. Adiponectin, Leptin, and Resistin Are Dysregulated in Patients Infected by SARS-CoV-2. Int. J. Mol. Sci. 2023, 24, 1131. [Google Scholar] [CrossRef] [PubMed]
- Dorris, S.L.; Peebles, R.S.J. PGI2 as a regulator of inflammatory diseases. Mediat. Inflamm. 2012, 2012, 926968. [Google Scholar] [CrossRef] [PubMed]
- Van Solingen, R.M.; Rosenstein, E.D.; Mihailescu, G.; Drejka, M.L.; Kalia, A.; Cohen, A.J.; Kramer, N. Comparison of the effects of ketoprofen on platelet function in the presence and absence of aspirin. Am. J. Med. 2001, 111, 285–289. [Google Scholar] [CrossRef]
- Boccia, M.; Aronne, L.; Celia, B.; Mazzeo, G.; Ceparano, M.; D’Agnano, V.; Parrella, R.; Valente, T.; Bianco, A.; Perrotta, F. COVID-19 and coagulative axis: Review of emerging aspects in a novel disease. Monaldi. Arch. Chest. Dis. 2020, 90. [Google Scholar] [CrossRef]
- Canzano, P.; Brambilla, M.; Porro, B.; Cosentino, N.; Tortorici, E.; Vicini, S.; Poggio, P.; Cascella, A.; Pengo, M.F.; Veglia, F.; et al. Platelet and Endothelial Activation as Potential Mechanisms Behind the Thrombotic Complications of COVID-19 Patients. JACC Basic. Transl. Sci. 2021, 6, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Komici, K.; Bianco, A.; Perrotta, F.; Iacono, A.D.; Bencivenga, L.; D’Agnano, V.; Rocca, A.; Bianco, A.; Rengo, G.; Guerra, G. Clinical Characteristics, Exercise Capacity and Pulmonary Function in Post-COVID-19 Competitive Athletes. J. Clin. Med. 2021, 10, 3053. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH). Therapeutic Management of Hospitalized Adults with COVID-19. Available online: https://adsp.nm.org/uploads/1/4/3/0/143064172/nih_hosp_adult_guideline.pdf (accessed on 2 November 2024).
- Matthay, M.A.; Wick, K.D. Corticosteroids, COVID-19 pneumonia, and acute respiratory distress syndrome. J. Clin. Investig. 2020, 130, 6218–6221. [Google Scholar] [CrossRef]
- Shuto, H.; Komiya, K.; Yamasue, M.; Uchida, S.; Ogura, T.; Mukae, H.; Tateda, K.; Hiramatsu, K.; Kadota, J.-I. A systematic review of corticosteroid treatment for noncritically ill patients with COVID-19. Sci. Rep. 2020, 10, 20935. [Google Scholar] [CrossRef]
- Li, X.; Yuan, X.; Xu, Z.; Huang, L.; Shi, L.; Lu, X.; Wang, F.-S.; Fu, J. Effect of methylprednisolone therapy on hospital stay and viral clearance in patients with moderate COVID-19. Infect. Med. 2022, 1, 236–244. [Google Scholar] [CrossRef]
- Cazzola, M.; Ora, J.; Bianco, A.; Rogliani, P.; Matera, M.G. Matera, Guidance on nebulization during the current COVID-19 pandemic. Respir. Med. 2021, 176, 106236. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Nicolau, D.V.; Langford, B.; Mahdi, M.; Jeffers, H.; Mwasuku, C.; Krassowska, K.; Fox, R.; Binnian, I.; Glover, V.; et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): A phase 2, open-label, randomised controlled trial. Lancet Respir. Med. 2021, 9, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-M.; Bafadhel, M.; Dorward, J.; Hayward, G.; Saville, B.R.; Gbinigie, O.; Ogburn, E.; Evans, P.H.; Thomas, N.P.B.; Patel, M.G.; et al. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): A randomised, controlled, open-label, adaptive platform trial. Lancet 2021, 398, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Ezer, N.; Belga, S.; Daneman, N.; Chan, A.; Smith, B.M.; Daniels, S.-A.; Moran, K.; Besson, C.; Smyth, L.Y.; Bartlett, S.J.; et al. Inhaled and intranasal ciclesonide for the treatment of COVID-19 in adult outpatients: CONTAIN phase II randomised controlled trial. BMJ 2021, 375, e068060. [Google Scholar] [CrossRef] [PubMed]
| KLS Group (n = 120) | Corticosteroids Group (n = 165) | p | |
|---|---|---|---|
| Males, n (%) | 65 (54) | 85 (51.5) | 0.658 |
| Age, years (mean ± SD) | 67.8 [49.8–71.3] | 66.0 [56.0–72.0] | 0.402 |
| Pack years(mean ± SD) | 30.0 [20–52] | 25.0 [22.5–30] | 0.160 |
| Smoker Status | 0.025 | ||
| Current/Former, n (%) | 85/120 (70.8) | 100/165 (60.6) | |
| Never, n (%) | 35/120 (29.2) | 65/165 (39.4) | |
| Unknown | 5/120 (4.2) | 0/165 (0) | |
| Comorbidities | |||
| COPD | 22/120 (18.3) | 26/165 (15.8) | 0.57 |
| Systemic Hypertension | 51/120 (42.5) | 80/165 (48.5) | 0.32 |
| Coronary Artery Disease or Chronic heath failure | 11/120 (9.2) | 30/165 (18.2) | 0.03 |
| CKD | 2/120 (1.7) | 8/165 (4.8) | 0.15 |
| Malignant Neoplasms | 5/120 (4.2) | 2/165 (1.2) | 0.11 |
| Type II Diabetes | 36/120 (30.0) | 25/165 (15.1) | <0.01 |
| Hypercolesterolemia | 43/120 (35.8) | 45/165 (27.3) | 0.12 |
| Stroke or Dementia | 4/120 (3.3) | 3/165 (1.8) | 0.41 |
| Hematological Disorders | 15/120 (12.5) | 10/165 (6.1) | 0.06 |
| Charlson Comorbidity Index(mean ± SD) | 3.0 [2–5] | 2.0 [1–4] | 0.177 |
| SARS-CoV-2 Vaccine Status, n (%) | 0.897 | ||
| Fully Vaccinated | 60/120 (50) | 85/165 (51.5) | |
| Unvaccinated/Partially Vaccinated | 60/120 (50) | 80/165 (48.5) | |
| Early COVID-19 Symptoms, n (%) | |||
| Fever | 65/120 (54.2) | 90/165 (54.5) | 0.95 |
| Sore throat | 56/120 (46.7) | 85/165 (51.5) | 0.42 |
| Dyspnea/Chest Tightness | 48/120 (40.0) | 113/165 (68.5) | <0.001 |
| Muscle or body aches | 69/120 (57.5) | 96/165 (58.2) | 0.91 |
| Loss of smell/Taste | 22/120 (18.3) | 38/165 (23.0) | 0.34 |
| Diarrhea | 13/120 (10.8) | 18/165 (10.9) | 0.98 |
| Fatigue | 28/120 (23.3) | 44/165 (26.6) | 0.52 |
| Patients with long COVID Symptoms (Yes), n (%) | 40/120 (33) | 110/165 (67) | <0.001 |
| Long COVID Symtpoms, n (%) | |||
| Dyspnea/Chest Tightness | 35/40 (87.5) | 95/110 (86.4) | 0.848 |
| Cough | 20/40 (50.0) | 58/110 (52.7) | 0.776 |
| Tachycardia | 5/40 (12.5) | 20/110 (18.2) | 0.591 |
| Astenia | 19/40 (47.5) | 61/110 (55.4) | 0.399 |
| Amnesia/neurological symptoms | 4/40 (10) | 13/110 (11.8) | 0.763 |
| Time to negative swab, days | 16.0 [8.0–25.0] | 23.0 [15.0–30] | 0.006 |
| Odds Ratio | 95% IC Value | p-Value | |
|---|---|---|---|
| Hospitalization risk | 0.140 | 0.0587–0.3421 | <0.001 |
| Need for oxygen supplementation | 0.150 | 0.076–0.294 | <0.001 |
| Presence of long COVID symptoms | 0.250 | 0.152–0.412 | <0.001 |
| Multivariate analysis for hospitalization risk | |||||
| 95% Confidence Interval | |||||
| Predictor | Standard Error | Odds-Ratio | Lower | Upper | p |
| Smoking Status (never versus current/former) | 0.432 | 0.4688 | 0.2011 | 1.093 | 0.079 |
| CAD or CHF (yes versus no) | 1453.530 | 1.67 × 10−8 | 0.0000 | Inf | 0.990 |
| Type II Diabetes (yes versus no) | 0.841 | 0.4126 | 0.0794 | 2.145 | 0.293 |
| Haematological Disorders (yes versus no) | 1771.930 | 3.63 × 10−8 | 0.0000 | Inf | 0.992 |
| Dyspnea/Chest tightness Yes versus No | 0.516 | 2.3344 | 0.8495 | 6.415 | 0.100 |
| Therapy Group: Ketoprofen Lysin Salt—Corticosteroid | 0.567 | 0.0796 | 0.0262 | 0.242 | <0.001 |
| Multivariate analysis for oxygen support supplementation | |||||
| 95% Confidence Interval | |||||
| Predictor | Standard Error | Odds-Ratio | Lower | Upper | p |
| Smoking Status (never versus current/former) | 0.395 | 1.711 | 0.7883 | 3.713 | 0.174 |
| CAD or CHF (yes versus no) | 1383.976 | 5.23 × 10−9 | 0.0000 | Inf | 0.989 |
| Type II Diabetes (yes versus no) | 0.628 | 0.340 | 0.0994 | 1.166 | 0.086 |
| Haematological Disorders (yes versus no) | 1586.235 | 1.15 × 10−8 | 0.0000 | Inf | 0.991 |
| Dyspnea/Chest tightness Yes versus No | 0.584 | 15.155 | 4.8214 | 47.638 | <0.001 |
| Therapy Group: Ketoprofen Lysin Salt—Corticosteroid | 0.384 | 0.127 | 0.0598 | 0.269 | <0.001 |
| Multivariate analysis for post-COVID 19 symptoms | |||||
| 95% Confidence Interval | |||||
| Predictor | Standard Error | Odds-Ratio | Lower | Upper | p |
| Smoking Status (never versus current/former) | 0.290 | 0.847 | 0.480 | 1.495 | 0.567 |
| CAD or CHF (yes versus no) | 0.474 | 3.280 | 1.296 | 8.297 | 0.012 |
| Type II Diabetes (yes versus no) | 0.404 | 0.252 | 0.114 | 0.557 | <0.001 |
| Haematological Disorders (yes versus no) | 0.566 | 3.182 | 1.049 | 9.655 | 0.041 |
| Dyspnea/Chest tightness Yes versus No | 0.318 | 1.810 | 0.970 | 3.377 | 0.062 |
| Therapy Group: Ketoprofen Lysin Salt—Corticosteroid | 0.280 | 0.324 | 0.187 | 0.562 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariniello, D.F.; Pagliaro, R.; D’Agnano, V.; Schiattarella, A.; Perrotta, F.; Bianco, A. Ketoprofen Lysine Salt Versus Corticosteroids in Early Outpatient Management of Mild and Moderate COVID-19: A Retrospective Study. Pharmacy 2025, 13, 65. https://doi.org/10.3390/pharmacy13030065
Mariniello DF, Pagliaro R, D’Agnano V, Schiattarella A, Perrotta F, Bianco A. Ketoprofen Lysine Salt Versus Corticosteroids in Early Outpatient Management of Mild and Moderate COVID-19: A Retrospective Study. Pharmacy. 2025; 13(3):65. https://doi.org/10.3390/pharmacy13030065
Chicago/Turabian StyleMariniello, Domenica Francesca, Raffaella Pagliaro, Vito D’Agnano, Angela Schiattarella, Fabio Perrotta, and Andrea Bianco. 2025. "Ketoprofen Lysine Salt Versus Corticosteroids in Early Outpatient Management of Mild and Moderate COVID-19: A Retrospective Study" Pharmacy 13, no. 3: 65. https://doi.org/10.3390/pharmacy13030065
APA StyleMariniello, D. F., Pagliaro, R., D’Agnano, V., Schiattarella, A., Perrotta, F., & Bianco, A. (2025). Ketoprofen Lysine Salt Versus Corticosteroids in Early Outpatient Management of Mild and Moderate COVID-19: A Retrospective Study. Pharmacy, 13(3), 65. https://doi.org/10.3390/pharmacy13030065







