Methadone and Buprenorphine as Medication for Addiction Treatment Diversely Affect Inflammation and Craving Depending on Their Doses
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Drug Administration
2.4. Craving Assessment
2.5. Biochemical Measurements in Blood
2.6. Ethical Approval
2.7. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Craving
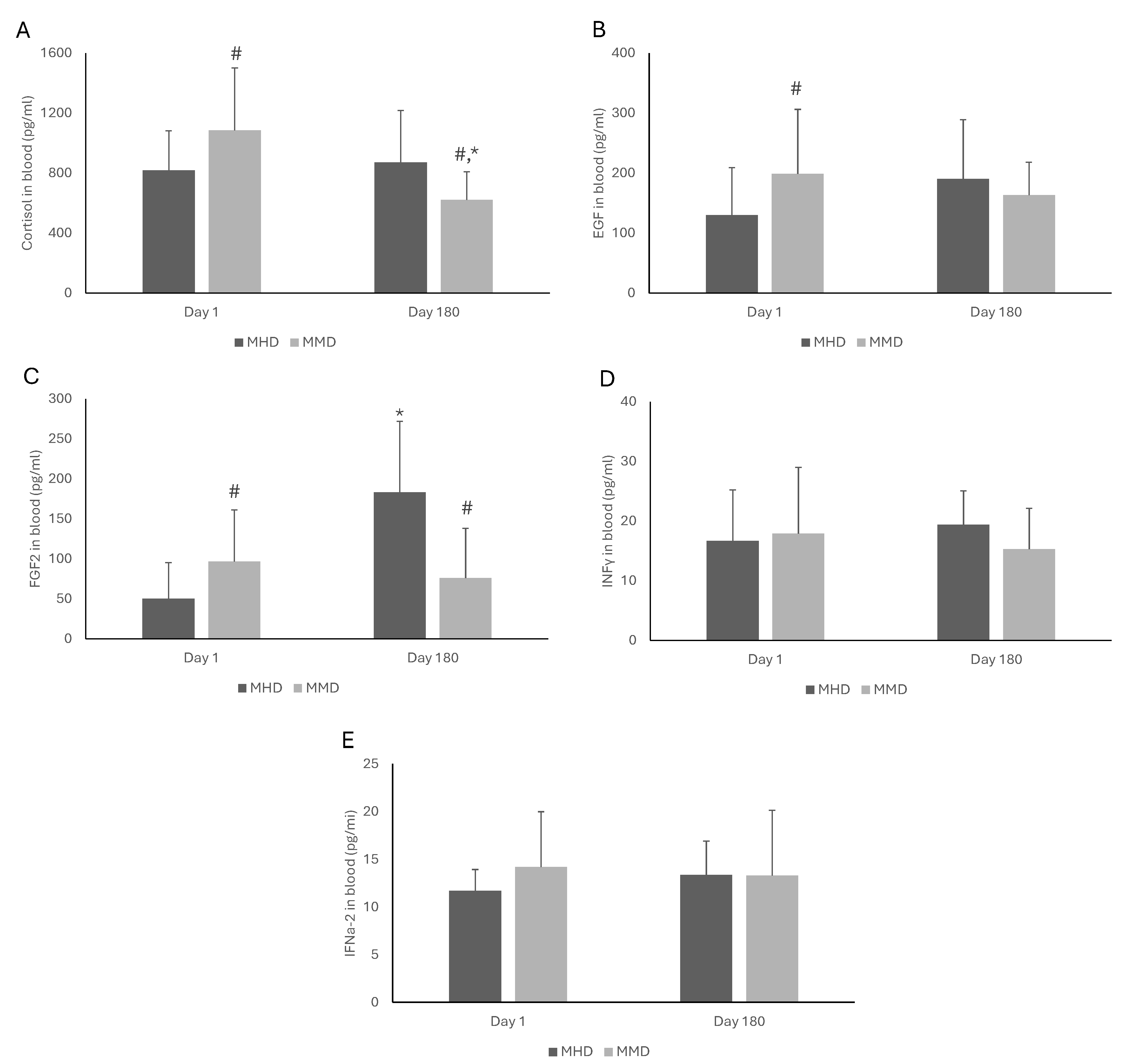
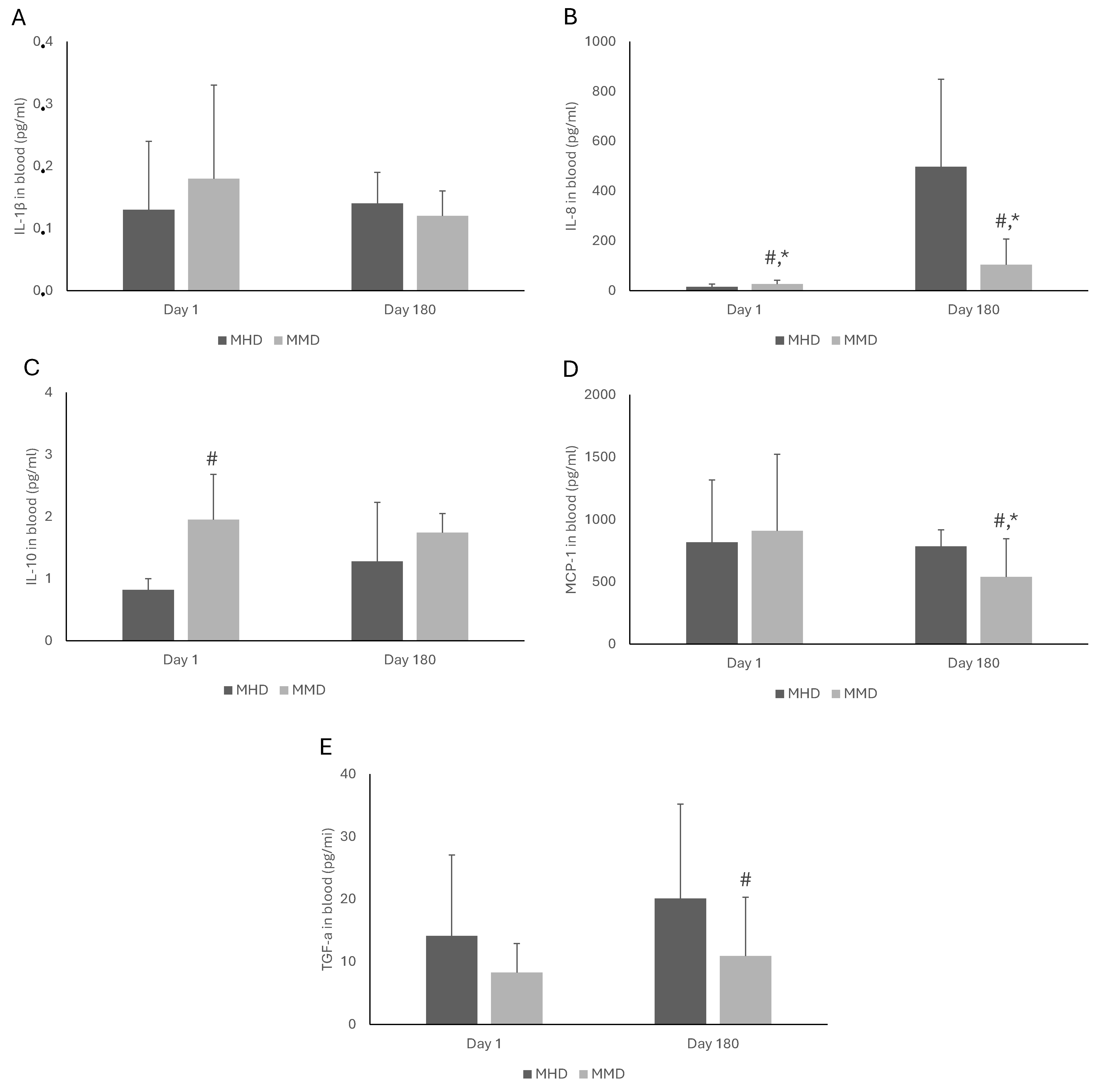
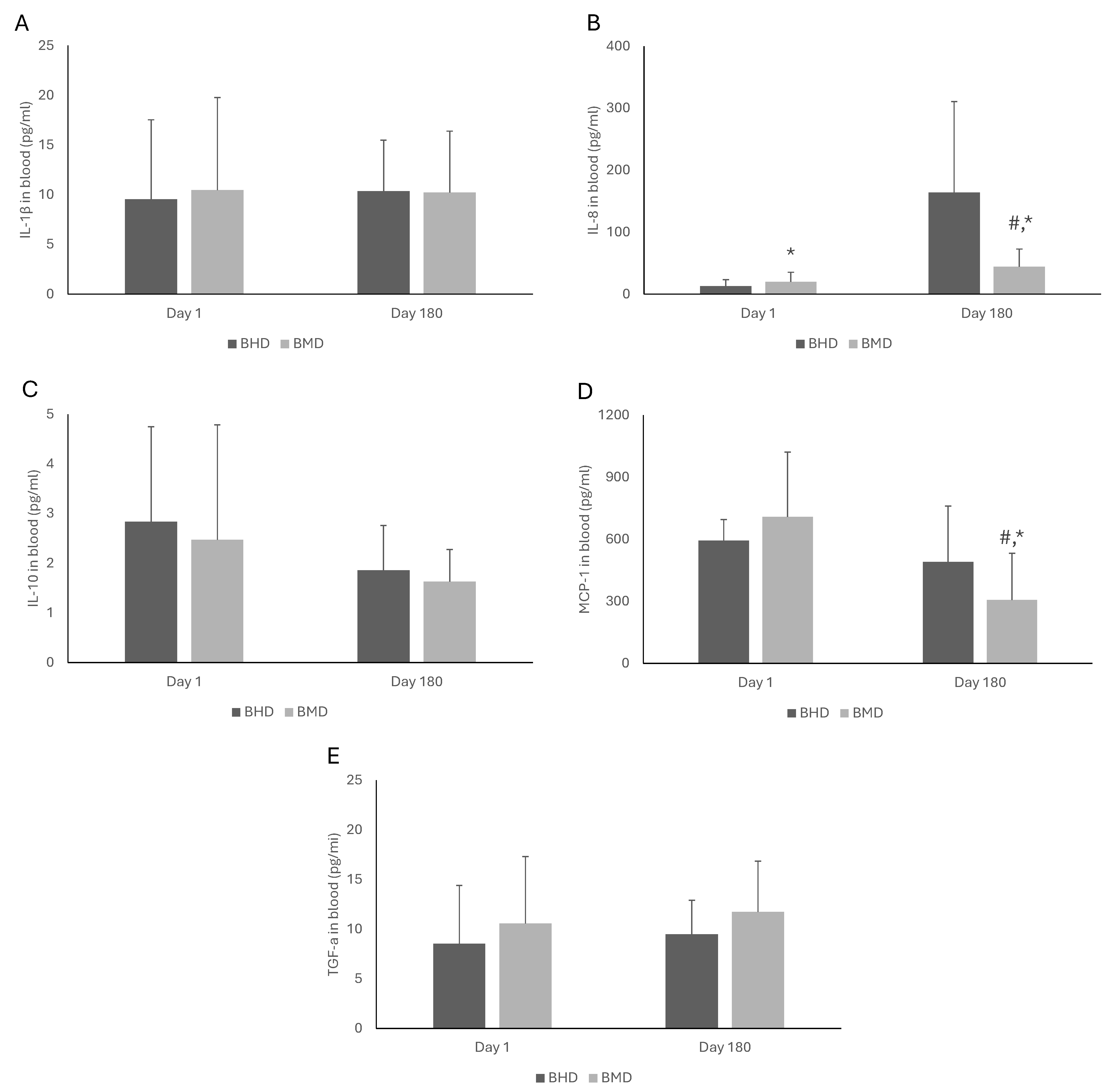
3.3. Blood Biomarkers
3.4. Correlation Between Craving and Blood Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tlali, M.; Scheibe, A.; Ruffieux, Y.; Cornell, M.; Wettstein, A.E.; Egger, M.; Davies, M.A.; Maartens, G.; Johnson, L.F.; Haas, A.D. Diagnosis and treatment of opioid-related disorders in a South African private sector medical insurance scheme: A cohort study. Int. J. Drug Policy 2022, 109, 103853. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Haghighi, S.; Chavoshinezhad, S.; Mozafari, R.; Noorbakhsh, F.; Borhani-Haghighi, A.; Haghparast, A. Neuroinflammatory Response in Reward-Associated Psychostimulants and Opioids: A Review. Cell. Mol. Neurobiol. 2023, 43, 649–682. [Google Scholar] [CrossRef] [PubMed]
- Langlois, L.D.; Nugent, F.S. Opiates and Plasticity in the Ventral Tegmental Area. ACS Chem. Neurosci. 2017, 8, 1830–1838. [Google Scholar] [CrossRef]
- Kreutzwiser, D.; Tawfic, Q.A. Methadone for Pain Management: A Pharmacotherapeutic Review. CNS Drugs 2020, 34, 827–839. [Google Scholar] [CrossRef]
- Leventelis, C.; Goutzourelas, N.; Kortsinidou, A.; Spanidis, Y.; Toulia, G.; Kampitsi, A.; Tsitsimpikou, C.; Stagos, D.; Veskoukis, A.S.; Kouretas, D. Buprenorphine and Methadone as Opioid Maintenance Treatments for Heroin-Addicted Patients Induce Oxidative Stress in Blood. Oxidative Med. Cell. Longev. 2019, 2019, 9417048. [Google Scholar] [CrossRef]
- Leventelis, C.; Tasoulis, S.; Kouretas, D.; Metsios, S.G.; Veskoukis, S.A. Pomegranate juice consumption by patients under medication for addiction treatment as regulator of craving and blood redox status: The study protocol of a randomized control trial (The NUTRIDOPE Study). Int. J. Clin. Ski. 2023, 17, 133–143. [Google Scholar] [CrossRef]
- Bobb, R.; Malayala, S.V.; Ajayi, E.; Wimbush, A.; Bobb, A. Hepatitis C Treatment in Persons Who Inject Drugs in a Medication Assisted Treatment Program: A Retrospective Review of an Integrated Model. J. Prim. Care Community Health 2023, 14, 21501319231164884. [Google Scholar] [CrossRef]
- Zeziulin, O.; Mollan, K.R.; Shook-Sa, B.E.; Hanscom, B.; Lancaster, K.E.; Dumchev, K.; Go, V.F.; Chu, V.A.; Kiriazova, T.; Syarif, Z.; et al. Depressive symptoms and use of HIV care and medication-assisted treatment among people with HIV who inject drugs. AIDS 2021, 35, 495–501. [Google Scholar] [CrossRef]
- Carlsen, S.L.; Lunde, L.H.; Torsheim, T. Opioid and Polydrug Use Among Patients in Opioid Maintenance Treatment. Subst. Abus. Rehabil. 2020, 11, 9–18. [Google Scholar] [CrossRef]
- Leventelis, C.; Makri, S.; Ververi, A.; Papageorgiou, K.; Tentolouri, A.; Mountzouridi, E.; Tekos, F.; Barmpas, P.T.; Tasoulis, S.; Metsios, G.S.; et al. Pomegranate juice ameliorates craving and oxidative stress on patients under medication for opioid addiction treatment with methadone and buprenorphine: A ranzomised controlled trial. Clin. Nutr. ESPEN 2025, 66, 34–45. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Yang, S.N.; Lin, J.C.; Chang, J.L.; Lin, J.G.; Lo, W.Y. Inflammatory response in heroin addicts undergoing methadone maintenance treatment. Psychiatry Res. 2015, 226, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Lee, S.Y.; Tao, P.L.; Chang, Y.H.; Chen, S.H.; Chu, C.H.; Chen, P.S.; Lee, I.H.; Yeh, T.L.; Yang, Y.K.; et al. Dextromethorphan attenuated inflammation and combined opioid use in humans undergoing methadone maintenance treatment. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2012, 7, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Kleykamp, B.A.; De Santis, M.; Dworkin, R.H.; Huhn, A.S.; Kampman, K.M.; Montoya, I.D.; Preston, K.L.; Ramey, T.; Smith, S.M.; Turk, D.C.; et al. Craving and opioid use disorder: A scoping review. Drug Alcohol Depend. 2019, 205, 107639. [Google Scholar] [CrossRef] [PubMed]
- Moeini, M.; Esmaeil, N.; Mokhtari, H.R.; Eskandari, N.; Banafshe, H.R. Neuro-Immuno-Endocrine Interactions in Early Life Stress and Heroin Withdrawal Timeline. Eur. Addict. Res. 2020, 26, 28–39. [Google Scholar] [CrossRef]
- Morcuende, A.; Navarrete, F.; Nieto, E.; Manzanares, J.; Femenía, T. Inflammatory Biomarkers in Addictive Disorders. Biomolecules 2021, 11, 1824. [Google Scholar] [CrossRef]
- Butelman, E.R.; Goldstein, R.Z.; Nwaneshiudu, C.A.; Girdhar, K.; Roussos, P.; Russo, S.J.; Alia-Klein, N. Neuroimmune Mechanisms of Opioid Use Disorder and Recovery: Translatability to Human Studies, and Future Research Directions. Neuroscience 2023, 528, 102–116. [Google Scholar] [CrossRef]
- Franchi, S.; Moschetti, G.; Amodeo, G.; Sacerdote, P. Do All Opioid Drugs Share the Same Immunomodulatory Properties? A Review From Animal and Human Studies. Front. Immunol. 2019, 10, 2914. [Google Scholar] [CrossRef]
- Filipczak-Bryniarska, I.; Nowak, B.; Sikora, E.; Nazimek, K.; Woroń, J.; Wordliczek, J.; Bryniarski, K. The influence of opioids on the humoral and cell-mediated immune responses in mice. The role of macrophages. Pharmacol. Rep. PR 2012, 64, 1200–1215. [Google Scholar] [CrossRef]
- Toce, M.S.; Chai, P.R.; Burns, M.M.; Boyer, E.W. Pharmacologic Treatment of Opioid Use Disorder: A Review of Pharmacotherapy, Adjuncts, and Toxicity. J. Med. Toxicol. Off. J. Am. Coll. Med. Toxicol. 2018, 14, 306–322. [Google Scholar] [CrossRef]
- Boland, J.W.; Foulds, G.A.; Ahmedzai, S.H.; Pockley, A.G. A preliminary evaluation of the effects of opioids on innate and adaptive human in vitro immune function. BMJ Support. Palliat. Care 2014, 4, 357–367. [Google Scholar] [CrossRef]
- D’Elia, M.; Patenaude, J.; Hamelin, C.; Garrel, D.R.; Bernier, J. No detrimental effect from chronic exposure to buprenorphine on corticosteroid-binding globulin and corticosensitive immune parameters. Clin. Immunol. 2003, 109, 179–187. [Google Scholar] [CrossRef]
- Gomez-Flores, R.; Weber, R.J. Differential effects of buprenorphine and morphine on immune and neuroendocrine functions following acute administration in the rat mesencephalon periaqueductal gray. Immunopharmacology 2000, 48, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Franchi, S.; Gerra, G.; Leccese, V.; Panerai, A.E.; Somaini, L. Buprenorphine and methadone maintenance treatment of heroin addicts preserves immune function. Brain Behav. Immun. 2008, 22, 606–613. [Google Scholar] [CrossRef]
- Martínez-Cuevas, F.L.; Cruz, S.L.; González-Espinosa, C. Methadone Requires the Co-Activation of μ-Opioid and Toll-Like-4 Receptors to Produce Extracellular DNA Traps in Bone-Marrow-Derived Mast Cells. Int. J. Mol. Sci. 2024, 25, 2137. [Google Scholar] [CrossRef] [PubMed]
- Meijerink, H.; Indrati, A.; Utami, F.; Soedarmo, S.; Alisjahbana, B.; Netea, M.G.; van Crevel, R.; Wisaksana, R.; van der Ven, A.J. Heroin use is associated with suppressed pro-inflammatory cytokine response after LPS exposure in HIV-infected individuals. PLoS ONE 2015, 10, e0122822. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.; Dias Lima, V.; Fairbairn, N.; Kerr, T.; Montaner, J.; Grebely, J.; Wood, E. The impact of methadone maintenance therapy on hepatitis C incidence among illicit drug users. Addiction 2014, 109, 2053–2059. [Google Scholar] [CrossRef]
- Akbari, A.; Mosayebi, G.; Samiei, A.R.; Ghazavi, A. Methadone therapy modulate the dendritic cells of heroin addicts. Int. Immunopharmacol. 2019, 66, 330–335. [Google Scholar] [CrossRef]
- Jaureguiberry-Bravo, M.; Lopez, L.; Berman, J.W. Frontline Science: Buprenorphine decreases CCL2-mediated migration of CD14+ CD16+ monocytes. J. Leukoc. Biol. 2018, 104, 1049–1059. [Google Scholar] [CrossRef]
- Velasquez, S.; Rappaport, J. Buprenorphine: Therapeutic potential beyond substance abuse. J. Leukoc. Biol. 2018, 104, 1047–1048. [Google Scholar] [CrossRef]
- Lu, R.B.; Wang, T.Y.; Lee, S.Y.; Chen, S.L.; Chang, Y.H.; See Chen, P.; Lin, S.H.; Chu, C.H.; Huang, S.Y.; Tzeng, N.S.; et al. Correlation between interleukin-6 levels and methadone maintenance therapy outcomes. Drug Alcohol Depend. 2019, 204, 107516. [Google Scholar] [CrossRef]
- Fatseas, M.; Denis, C.; Massida, Z.; Verger, M.; Franques-Rénéric, P.; Auriacombe, M. Cue-induced reactivity, cortisol response and substance use outcome in treated heroin dependent individuals. Biol. Psychiatry 2011, 70, 720–727. [Google Scholar] [CrossRef] [PubMed]
- NIDA. How Effective Are Medications to Treat Opioid Use Disorder? 3 December 2021. Available online: https://nida.nih.gov/publications/research-reports/medications-to-treat-opioid-addiction/efficacy-medications-opioid-use-disorder (accessed on 20 July 2024).
- National Cancer Institute. Clinical Trial Randomization Tool; National Cancer Institute: Rockville, MD, USA, 2023. Available online: https://ctrandomization.cancer.gov (accessed on 12 October 2023).
- Strain, E.C.; Bigelow, G.E.; Liebson, I.A.; Stitzer, M.L. Moderate- vs high-dose methadone in the treatment of opioid dependence: A randomized trial. JAMA 1999, 281, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.; Ang, A.; Hillhouse, M.P.; Saxon, A.J.; Nielsen, S.; Wakim, P.G.; Mai, B.E.; Mooney, L.J.; Potter, J.S.; Blaine, J.D. Treatment outcomes in opioid dependent patients with different buprenorphine/naloxone induction dosing patterns and trajectories. Am. J. Addict. 2015, 24, 667–675. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Guidelines for the Psychosocially Assisted Pharmacological Treatment of Opioid Dependence; Prescribing Guidelines, Annex 12; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Leventelis, C.; Veskoukis, S.A.; Malliori, M.; Koutsilieris, M.; Zyga, S.; Rojas Gil, A.P.; Kampitsi, A.; Goutzourelas, N.; Tsironi, M. Validation of heroin craving questionnaire in Greek patients under substitution treatment with methadone and buprenorphine: How to prevent a relapse. J. Addict. Behav. Ther. Rehabil. 2020, 9, 10-37532. [Google Scholar]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Bankers-Fulbright, J.L.; Kalli, K.R.; McKean, D.J. Interleukin-1 signal transduction. Life Sci. 1996, 59, 61–83. [Google Scholar] [CrossRef]
- Dixit, N.; Simon, S.I. Chemokines, selectins and intracellular calcium flux: Temporal and spatial cues for leukocyte arrest. Front. Immunol. 2012, 3, 188. [Google Scholar] [CrossRef]
- Callewaere, C.; Banisadr, G.; Rostène, W.; Parsadaniantz, S.M. Chemokines and chemokine receptors in the brain: Implication in neuroendocrine regulation. J. Mol. Endocrinol. 2007, 38, 355–363. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Takatsu, K. Interleukin-5 and IL-5 receptor in health and diseases. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 463–485. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.J.; Bruce, C.; Chioni, A.M.; Kocher, H.M.; Grose, R.P. The ins and outs of fibroblast growth factor receptor signalling. Clin. Sci. 2014, 127, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Chung, E.; Coffey, R.J. EGF receptor ligands. Exp. Cell Res. 2003, 284, 2–13. [Google Scholar] [CrossRef]
- Schluger, J.H.; Bart, G.; Green, M.; Ho, A.; Kreek, M.J. Corticotropin-releasing factor testing reveals a dose-dependent difference in methadone maintained vs control subjects. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2003, 28, 985–994. [Google Scholar] [CrossRef]
- Khosravi, M.; Kasaeiyan, R. Reasons for Increasing Daily Methadone Maintenance Dosage among Deceptive Patients: A Qualitative Study. J. Med. Life 2020, 13, 572–579. [Google Scholar] [CrossRef]
- Tsai, H.J.; Wang, S.C.; Liu, S.W.; Ho, I.K.; Chang, Y.S.; Tsai, Y.T.; Lin, K.M.; Liu, Y.L. Assessment of CYP450 genetic variability effect on methadone dose and tolerance. Pharmacogenomics 2014, 15, 977–986. [Google Scholar] [CrossRef]
- Aloisi, A.M.; Aurilio, C.; Bachiocco, V.; Biasi, G.; Fiorenzani, P.; Pace, M.C.; Paci, V.; Pari, G.; Passavanti, G.; Ravaioli, L.; et al. Endocrine consequences of opioid therapy. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S162–S168. [Google Scholar] [CrossRef]
- Chou, Y.C.; Shih, S.F.; Tsai, W.D.; Li, C.S.; Xu, K.; Lee, T.S. Improvement of quality of life in methadone treatment patients in northern Taiwan: A follow-up study. BMC Psychiatry 2013, 13, 190. [Google Scholar] [CrossRef]
- Walter, M.; Wiesbeck, G.A.; Degen, B.; Albrich, J.; Oppel, M.; Schulz, A.; Schächinger, H.; Dürsteler-MacFarland, K.M. Heroin reduces startle and cortisol response in opioid-maintained heroin-dependent patients. Addict. Biol. 2011, 16, 145–151. [Google Scholar] [CrossRef]
- Inan, S.; Eisenstein, T.K.; Watson, M.N.; Doura, M.; Meissler, J.J.; Tallarida, C.S.; Chen, X.; Geller, E.B.; Rawls, S.M.; Cowan, A.; et al. Coadministration of Chemokine Receptor Antagonists with Morphine Potentiates Morphine’s Analgesic Effect on Incisional Pain in Rats. J. Pharmacol. Exp. Ther. 2018, 367, 433–441. [Google Scholar] [CrossRef]
- Mazahery, C.; Benson, B.L.; Cruz-Lebrón, A.; Levine, A.D. Chronic Methadone Use Alters the CD8+ T Cell Phenotype In Vivo and Modulates Its Responsiveness Ex Vivo to Opioid Receptor and TCR Stimuli. J. Immunol. 2020, 204, 1188–1200. [Google Scholar] [CrossRef] [PubMed]
- Gamble, M.C.; Williams, B.R.; Singh, N.; Posa, L.; Freyberg, Z.; Logan, R.W.; Puig, S. Mu-opioid receptor and receptor tyrosine kinase crosstalk: Implications in mechanisms of opioid tolerance, reduced analgesia to neuropathic pain, dependence, and reward. Front. Syst. Neurosci. 2022, 16, 1059089. [Google Scholar] [CrossRef]
- Doi, S.; Mori, T.; Uzawa, N.; Arima, T.; Takahashi, T.; Uchida, M.; Yawata, A.; Narita, M.; Uezono, Y.; Suzuki, T.; et al. Characterization of methadone as a β-arrestin-biased μ-opioid receptor agonist. Mol. Pain 2016, 12, 1744806916654146. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Hu, D.; Wang, J.; Wu, W.; Zhao, H.H.; Wang, L.; Gleeson, J.; Haddad, G.G. Buprenorphine and methadone differentially alter early brain development in human cortical organoids. Neuropharmacology 2023, 239, 109683. [Google Scholar] [CrossRef] [PubMed]
- Alzu’bi, A.; Baker, W.B.; Al-Trad, B.; Zoubi, M.S.A.; AbuAlArjah, M.I.; Abu-El-Rub, E.; Tahat, L.; Helaly, A.M.; Ghorab, D.S.; El-Huneidi, W.; et al. The impact of chronic fentanyl administration on the cerebral cortex in mice: Molecular and histological effects. Brain Res. Bull. 2024, 209, 110917. [Google Scholar] [CrossRef]
- Terwisscha van Scheltinga, A.F.; Bakker, S.C.; Kahn, R.S.; Kas, M.J. Fibroblast growth factors in neurodevelopment and psychopathology. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2013, 19, 479–494. [Google Scholar] [CrossRef]
- Koido, K.; Innos, J.; Haring, L.; Zilmer, M.; Ottas, A.; Vasar, E. Taurine and Epidermal Growth Factor Belong to the Signature of First-Episode Psychosis. Front. Neurosci. 2016, 10, 331. [Google Scholar] [CrossRef]
- Sotoyama, H.; Namba, H.; Chiken, S.; Nambu, A.; Nawa, H. Exposure to the cytokine EGF leads to abnormal hyperactivity of pallidal GABA neurons: Implications for schizophrenia and its modeling. J. Neurochem. 2013, 126, 518–528. [Google Scholar] [CrossRef]
- Turner, C.A.; Watson, S.J.; Akil, H. The fibroblast growth factor family: Neuromodulation of affective behavior. Neuron 2012, 76, 160–174. [Google Scholar] [CrossRef]
- Eisenstein, T.K. The Role of Opioid Receptors in Immune System Function. Front. Immunol. 2019, 10, 2904. [Google Scholar] [CrossRef]
- June, H.L.; Liu, J.; Warnock, K.T.; Bell, K.A.; Balan, I.; Bollino, D.; Puche, A.; Aurelian, L. CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2015, 40, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Wakida, N.; Kiguchi, N.; Saika, F.; Nishiue, H.; Kobayashi, Y.; Kishioka, S. CC-chemokine ligand 2 facilitates conditioned place preference to methamphetamine through the activation of dopamine systems. J. Pharmacol. Sci. 2014, 125, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Lu, R.B.; Lee, S.Y.; Chang, Y.H.; Chen, S.L.; Tsai, T.Y.; Tseng, H.H.; Chen, P.S.; Chen, K.C.; Yang, Y.K.; et al. Association Between Inflammatory Cytokines, Executive Function, and Substance Use in Patients With Opioid Use Disorder and Amphetamine-Type Stimulants Use Disorder. Int. J. Neuropsychopharmacol. 2023, 26, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.X.; Chen, L.; Zhang, J.J.; Cheng, Y. Aberrations in peripheral inflammatory cytokine levels in substance use disorders: A meta-analysis of 74 studies. Addiction 2020, 115, 2257–2267. [Google Scholar] [CrossRef]
- Cuitavi, J.; Andrés-Herrera, P.; Meseguer, D.; Campos-Jurado, Y.; Lorente, J.D.; Caruana, H.; Hipólito, L. Focal mu-opioid receptor activation promotes neuroinflammation and microglial activation in the mesocorticolimbic system: Alterations induced by inflammatory pain. Glia 2023, 71, 1906–1920. [Google Scholar] [CrossRef]
- Hutchinson, M.R.; Coats, B.D.; Lewis, S.S.; Zhang, Y.; Sprunger, D.B.; Rezvani, N.; Baker, E.M.; Jekich, B.M.; Wieseler, J.L.; Somogyi, A.A.; et al. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav. Immun. 2008, 22, 1178–1189. [Google Scholar] [CrossRef]
- Murlanova, K.; Jouroukhin, Y.; Novototskaya-Vlasova, K.; Huseynov, S.; Pletnikova, O.; Morales, M.J.; Guan, Y.; Kamiya, A.; Bergles, D.E.; Dietz, D.M.; et al. Loss of Astrocytic µ Opioid Receptors Exacerbates Aversion Associated with Morphine Withdrawal in Mice: Role of Mitochondrial Respiration. Cells 2023, 12, 1412. [Google Scholar] [CrossRef]
- Peterson, P.K.; Gekker, G.; Hu, S.; Anderson, W.R.; Kravitz, F.; Portoghese, P.S.; Balfour, H.H., Jr.; Chao, C.C. Morphine amplifies HIV-1 expression in chronically infected promonocytes cocultured with human brain cells. J. Neuroimmunol. 1994, 50, 167–175. [Google Scholar] [CrossRef]
- Chocyk, A.; Czyrak, A.; Wedzony, K. Acute and repeated cocaine induces alterations in FosB/DeltaFosB expression in the paraventricular nucleus of the hypothalamus. Brain Res. 2006, 1090, 58–68. [Google Scholar] [CrossRef]
- Ruffle, J.K. Molecular neurobiology of addiction: What’s all the (Δ)FosB about? Am. J. Drug Alcohol Abus. 2014, 40, 428–437. [Google Scholar] [CrossRef]
- Garcia-Perez, D.; Laorden, M.L.; Milanes, M.V.; Nunez, C. Glucorticoids regulation of FosB/ΔFosB expression induced by chronic opiate exposure in the brain stress system. PLoS ONE 2012, 7, e50264. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, C.; Gallardo, J.; Berríos-Cárcamo, P.; Handy, Á.; Santapau, D.; González-Madrid, A.; Ezquer, M.; Morales, P.; Luarte, A.; Corvalán, D.; et al. Methadone directly impairs central nervous system cells in vitro. Sci. Rep. 2024, 14, 16978. [Google Scholar] [CrossRef]
- Franchi, S.; Moretti, S.; Castelli, M.; Lattuada, D.; Scavullo, C.; Panerai, A.E.; Sacredote, P. Mu opioid receptor activation modulates Toll like receptor 4 in murine macrophages. Brain Behav. Immun. 2012, 26, 480–488. [Google Scholar] [CrossRef]
- Colby, C.R.; Whisler, K.; Steffen, E.J.; Self, D.W. Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 2488–2493. [Google Scholar] [CrossRef] [PubMed]
- Hemshekhar, M.; Anaparti, V.; Hitchon, C.; Mookherjee, N. Buprenorphine Alters Inflammatory and Oxidative Stress Molecular Markers in Arthritis. Mediat. Inflamm. 2017, 2017, 2515408. [Google Scholar] [CrossRef]
- Chen, S.L.; Tao, P.L.; Chu, C.H.; Chen, S.H.; Wu, H.E.; Tseng, L.F.; Hong, J.S.; Lu, R.B. Low-dose memantine attenuated morphine addictive behavior through its anti-inflammation and neurotrophic effects in rats. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2012, 7, 444–453. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Li, Q.; Yang, W.; Zhu, J.; Wang, W. White matter impairment in heroin addicts undergoing methadone maintenance treatment and prolonged abstinence: A preliminary DTI study. Neurosci. Lett. 2011, 494, 49–53. [Google Scholar] [CrossRef]
- Huang, P.; Kehner, G.B.; Cowan, A.; Liu-Chen, L.Y. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: Norbuprenorphine is a potent opioid agonist. J. Pharmacol. Exp. Ther. 2001, 297, 688–695. [Google Scholar] [CrossRef]
- Athanasos, P.; Ling, W.; Bochner, F.; White, J.M.; Somogyi, A.A. Buprenorphine Maintenance Subjects Are Hyperalgesic and Have No Antinociceptive Response to a Very High Morphine Dose. Pain Med. 2019, 20, 119–128. [Google Scholar] [CrossRef]
- Brown, S.M.; Campbell, S.D.; Crafford, A.; Regina, K.J.; Holtzman, M.J.; Kharasch, E.D. P-glycoprotein is a major determinant of norbuprenorphine brain exposure and antinociception. J. Pharmacol. Exp. Ther. 2012, 343, 53–61. [Google Scholar] [CrossRef]
- Jain, L.; Meeks, T.W.; Blazes, C.K. Reconsidering the usefulness of long-term high-dose buprenorphine. Front. Psychiatry 2024, 15, 1401676. [Google Scholar] [CrossRef] [PubMed]
- Re, G.F.; Jia, J.; Xu, Y.; Zhang, Z.; Xie, Z.R.; Kong, D.; Lu, D.; Li, Y.; Peng, Q.Y.; Yu, J.; et al. Dynamics and correlations in multiplex immune profiling reveal persistent immune inflammation in male drug users after withdrawal. Int. Immunopharmacol. 2022, 107, 108696. [Google Scholar] [CrossRef]
- Wang, T.Y.; Lee, S.Y.; Chang, Y.H.; Chen, S.L.; Chen, P.S.; Chu, C.H.; Huang, S.Y.; Tzeng, N.S.; Lee, I.H.; Chen, K.C.; et al. Correlation of cytokines, BDNF levels, and memory function in patients with opioid use disorder undergoing methadone maintenance treatment. Drug Alcohol Depend. 2018, 191, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front. Behav. Neurosci. 2021, 14, 601939. [Google Scholar] [CrossRef] [PubMed]
- Gregus, A.M.; Levine, I.S.; Eddinger, K.A.; Yaksh, T.L.; Buczynski, M.W. Sex differences in neuroimmune and glial mechanisms of pain. Pain 2021, 162, 2186–2200. [Google Scholar] [CrossRef]
- Ajonijebu, D.; Abboussi, O.; Russell, V.A.; Mabandla, M.V.; Daniels, W. Epigenetics: A link between addiction and social environment. Cell. Mol. Life Sci. CMLS 2017, 74, 2735–2747. [Google Scholar] [CrossRef]
- Fattore, L.; Melis, M. Sex differences in impulsive and compulsive behaviors: A focus on drug addiction. Addict. Biol. 2016, 21, 1043–1051. [Google Scholar] [CrossRef]
- Araujo, N.P.; Camarini, R.; Souza-Formigoni, M.L.; Carvalho, R.C.; Abilio, V.C.; Silva, R.H.; Ricardo, V.P.; Ribeiro, R.; Frussa-Filho, R. The importance of housing conditions on behavioral sensitization and tolerance to ethanol. Pharmacol. Biochem. Behav. 2005, 82, 40–45. [Google Scholar] [CrossRef]
- Matsuda, T.; Sakaue, M.; Ago, Y.; Samoto, Y.; Koyama, Y.; Baba, A. Functional alteration of brain dopaminergic system in isolated aggressive mice. Nihon Shinkey Seishin Yakurigaku Zasshi Jpn. J. Psychopharmacol. 2001, 21, 71–76. [Google Scholar]
- Najjar, S.; Pearlman, D.M.; Alper, K.; Najjar, A.; Devinsky, O. Neuroinflammation and psychiatric illness. J. Neuroinflamm. 2013, 10, 43. [Google Scholar] [CrossRef]
- Chang, H.B.; Munroe, S.; Gray, K.; Porta, G.; Bouaihy, A.; Marsland, A.; Brent, D.; Melhem, N.M. The role of substance use, smoking, and inflammation in risk for suicidal behavior. J. Affect. Disord. 2019, 243, 33–41. [Google Scholar] [CrossRef]
- Tolliver, B.K.; Anton, R.F. Assessment and treatment of mood disorders in the context of substance abuse. Dialogues Clin. Neurosci. 2015, 17, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Daudhters, S.B.; Reynolds, E.K.; MacPherson, L.; Kahler, C.W.; Danielson, C.K.; Zvolensky, M.; Lejuez, C.W. Distress tolerance and early adolescent externalizing and internalizing symptoms: The moderating role of gender and ethnicity. Behav. Res. Ther. 2009, 47, 198–205. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Groups | ||||
|---|---|---|---|---|---|
| Total (n = 66) | MHD | BHD | MMD | BMD | |
| Sex, n (%) | |||||
| Males | 47 (71.2) | 11 (64.7) | 11 (64.7) | 12 (70.58) | 13 (86.6) |
| Females | 19 (28.8) | 6 (35.3) | 6 (35.3) | 5 (29.42) | 2 (13.4) |
| Age (years), n (%) | |||||
| 18–24 | 4 (6.1) | 0 (0.0) | 2 (11.8) | 2 (11.8) | 0 (0.0) |
| 25–30 | 9 (13.6) | 2 (11.8) | 3 (17.6) | 2 (11.7) | 2 (13.3) |
| 31–40 | 23 (34.8) | 7 (41.2) | 4 (23.5) | 6 (35.3) | 6 (40.0) |
| 41–50 | 25 (37.9) | 6 (35.2) | 7 (41.2) | 6 (35.3) | 6 (40.0) |
| 51–60 | 5 (7.6) | 2 (11.8) | 1 (5.9) | 1 (5.9) | 1 (6.7) |
| Educational status, n (%) | |||||
| Primary school | 16 (24.2) | 6 (35.3) | 3 (17.6) | 4 (23.5) | 3 (20.0) |
| Middle/high school | 38 (57.6) | 8 (47.1) | 8 (47.1) | 10 (58.9) | 12 (80.0) |
| University/postgraduate | 12 (18.2) | 3 (17.6) | 6 (35.3) | 3 (17.6) | 0 (0.0) |
| Family status, n (%) | |||||
| Married | 12 (18.1) | 4 (23.5) | 3 (17.6) | 2 (11.8) | 3 (20.0) |
| Unmarried | 35 (53.0) | 9 (53.0) | 8 (47.1) | 10 (58.8) | 8 (53.4) |
| Widowed | 4 (6.1) | 3 (17.6) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Divorced | 11 (16.7) | 1 (5.9) | 4 (23.5) | 2 (11.8) | 4 (26.6) |
| Separated | 4 (6.1) | 0 (0.0) | 1 (5.9) | 3 (17.6) | 0 (0.0) |
| Place of residence, n (%) | |||||
| Urban | 50 (75.8) | 15 (88.2) | 13 (76.5) | 11 (64.7) | 11 (73.3) |
| Rural | 16 (24.2) | 2 (11.8) | 4 (23.5) | 6 (35.3) | 4 (26.7) |
| Age at first use, mean (SD) | 18.4 (5.1) | 17.6 (4.7) | 18.5 (4.1) | 17.5 (4.5) | 20.3 (6.4) |
| Years of substance abuse, mean (SD) | 16.9 (8.5) | 15.4 (9.3) | 20.4 (8.6) | 14.1 (6.8) | 19.6 (7.9) |
| Day 1 | Day 180 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCQ Dimensions | MHD | MMD | BHD | BMD | MHD | MMD | BHD | BMD | MHD 1 vs. 180 | MMD 1 vs. 180 | BHD 1 vs. 180 | BMD 1 vs. 180 |
| Mean Score ± SD | p | |||||||||||
| D.U. | 21.7 ± 9.7 | 27.8 ± 7.6 | 20.3 ± 5.7 | 25.8 ± 9.3 | 20.6 ± 6.4 | 16.3 ± 5.6 | 19.2 ± 5.3 | 15.3 ± 4.1 | 0.69 | <0.001 | 0.56 | <0.001 |
| p | 0.04 | 0.04 | 0.04 | 0.02 | ||||||||
| I.P.U. | 22.9 ± 7.1 | 27.5 ± 5.8 | 20.1 ± 5.8 | 25.6 ± 8.1 | 22.1 ± 7.3 | 17.6 ± 5.2 | 18.8 ± 8.5 | 14.1 ± 4.1 | 0.74 | <0.001 | 0.60 | <0.001 |
| p | 0.04 | 0.03 | 0.04 | 0.04 | ||||||||
| A.P.O. | 21.4 ± 10.5 | 25.7 ± 11.9 | 19.6 ± 9.1 | 25.9 ± 7.2 | 22.9 ± 12.6 | 16.4 ± 11.2 | 18.5 ± 12.5 | 15.4 ± 9.9 | 0.70 | 0.02 | 0.77 | 0.002 |
| p | 0.27 | 0.03 | 0.12 | 0.40 | ||||||||
| R.W.D. | 29.0 ± 13.0 | 29.8 ± 12.2 | 24.9 ± 7.2 | 29.9 ± 5.8 | 27.9 ± 9.3 | 24.5 ± 11.5 | 24.8 ± 12 | 25.5 ± 10.9 | 0.77 | 0.20 | 0.97 | 0.17 |
| p | 0.85 | 0.04 | 0.35 | 0.86 | ||||||||
| L.C.U. | 26.6 ± 9.9 | 26.9 ± 8.6 | 22.8 ± 7.1 | 30.5 ± 9.6 | 27.8 ± 8.7 | 21.0 ± 6.7 | 24.4 ± 7.9 | 19.1 ± 6.1 | 0.70 | 0.03 | 0.53 | <0.001 |
| p | 0.92 | 0.01 | 0.01 | 0.04 | ||||||||
| Biomarkers | Desire to Use Heroin | Intention and Planning to Use | Anticipation of Positive Outcome | Relief from Withdrawal or Dysphoria | Lack of Control over Use | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MHD | MMD | MHD | MMD | MHD | MMD | MHD | MMD | MHD | MMD | ||
| Cortisol | r | 0.75 | 0.50 | 0.75 | 0.52 | −0.47 | −0.25 | −0.23 | −0.15 | −0.13 | −0.18 |
| p | 0.032 | 0.017 | 0.034 | 0.015 | 0.207 | 0.303 | 0.626 | 0.527 | 0.742 | 0.457 | |
| EGF | r | 0.03 | 0.01 | 0.31 | 0.03 | 0.55 | 0.27 | 0.36 | −0.57 | 0.32 | −0.11 |
| p | 0.948 | 0.983 | 0.458 | 0.904 | 0.127 | 0.256 | 0.422 | 0.004 | 0.396 | 0.648 | |
| FGF-2 | r | −0.49 | 0.24 | −0.27 | 0.20 | −0.01 | 0.13 | 0.25 | −0.56 | −0.12 | −0.02 |
| p | 0.215 | 0.298 | 0.511 | 0.397 | 0.982 | 0.591 | 0.596 | 0.013 | 0.751 | 0.934 | |
| IFNγ | r | −0.05 | 0.49 | 0.05 | 0.27 | −0.16 | 0.17 | −0.23 | −0.62 | −0.70 | 0.04 |
| p | 0.914 | 0.028 | 0.900 | 0.257 | 0.673 | 0.484 | 0.622 | 0.004 | 0.043 | 0.854 | |
| IFNa-2 | r | −0.80 | −0.07 | −0.45 | −0.15 | −0.08 | 0.12 | 0.17 | 0.17 | −0.05 | −0.23 |
| p | 0.017 | 0.755 | 0.263 | 0.526 | 0.841 | 0.639 | 0.709 | 0.495 | 0.890 | 0.333 | |
| IL-1β | r | −0.02 | −0.05 | −0.03 | −0.08 | −0.12 | 0.08 | −0.13 | 0.11 | −0.09 | 0.53 |
| p | 0.962 | 0.835 | 0.951 | 0.750 | 0.760 | 0.736 | 0.780 | 0.645 | 0.812 | 0.018 | |
| IL-8 | r | 0.02 | 0.25 | −0.01 | 0.29 | 0.12 | 0.31 | −0.68 | −0.47 | −0.12 | −0.25 |
| p | 0.961 | 0.280 | 0.989 | 0.215 | 0.750 | 0.203 | 0.093 | 0.043 | 0.751 | 0.297 | |
| IL-10 | r | −0.01 | 0.20 | −0.04 | 0.15 | 0.10 | 0.08 | −0.68 | 0.10 | −0.16 | −0.04 |
| p | 0.985 | 0.397 | 0.926 | 0.536 | 0.800 | 0.734 | 0.094 | 0.694 | 0.674 | 0.862 | |
| MCP-1 | r | −0.37 | 0.18 | −0.29 | 0.27 | −0.09 | −0.56 | −0.71 | −0.16 | 0.06 | −0.14 |
| p | 0.367 | 0.435 | 0.486 | 0.246 | 0.827 | 0.013 | 0.042 | 0.511 | 0.882 | 0.556 | |
| TGF-a | r | −0.21 | −0.14 | −0.18 | −0.22 | 0.10 | −0.16 | −0.35 | −0.11 | 0.06 | 0.02 |
| p | 0.620 | 0.746 | 0.673 | 0.600 | 0.800 | 0.767 | 0.446 | 0.801 | 0.887 | 0.969 | |
| Biomarkers | Desire to Use Heroin | Intention and Planning to Use | Anticipation of Positive Outcome | Relief from Withdrawal or Dysphoria | Lack of Control over Use | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BHD | BMD | BHD | BMD | BHD | BMD | BHD | BMD | BHD | BMD | ||
| Cortisol | r | 0.61 | 0.74 | 0.66 | 0.76 | −0.29 | −0.05 | 0.29 | −0.07 | 0.01 | 0.04 |
| p | 0.037 | 0.041 | 0.041 | 0.045 | 0.380 | 0.918 | 0.415 | 0.869 | 0.981 | 0.930 | |
| EGF | r | −0.14 | −0.16 | −0.04 | 0.911 | −0.05 | −0.01 | 0.36 | 0.764 | 0.09 | 0.656 |
| p | 0.681 | 0.699 | 0.918 | −0.02 | 0.873 | 0.758 | 0.305 | −0.42 | 0.779 | 0.15 | |
| FGF-2 | r | −0.05 | −0.19 | −0.21 | 0.955 | −0.19 | −0.13 | 0.08 | 0.305 | 0.15 | 0.731 |
| p | 0.882 | 0.656 | 0.533 | −0.14 | 0.568 | 0.983 | 0.828 | −0.29 | 0.632 | −0.05 | |
| IFNγ | r | −0.22 | 0.82 | −0.15 | 0.748 | −0.22 | 0.91 | 0.01 | 0.491 | −0.26 | 0.910 |
| p | 0.508 | 0.013 | 0.668 | 0.53 | 0.520 | 0.804 | 0.984 | 0.69 | 0.420 | 0.57 | |
| IFNa-2 | r | −0.18 | −0.29 | 0.02 | 0.174 | 0.03 | −0.34 | −0.65 | −0.20 | 0.15 | 0.143 |
| p | 0.588 | 0.492 | 0.955 | −0.38 | 0.926 | 0.012 | 0.037 | 0.049 | 0.652 | −0.16 | |
| IL-1β | r | −0.12 | −0.30 | 0.06 | 0.30 | −0.62 | 0.17 | 0.14 | 0.19 | −0.13 | 0.18 |
| p | 0.729 | 0.465 | 0.869 | 0.466 | 0.045 | 0.752 | 0.708 | 0.652 | 0.691 | 0.674 | |
| IL-8 | r | 0.60 | 0.55 | −0.35 | 0.10 | −0.68 | 0.57 | −0.13 | 0.63 | −0.22 | 0.06 |
| p | 0.045 | 0.158 | 0.297 | 0.822 | 0.011 | 0.235 | 0.711 | 0.094 | 0.495 | 0.890 | |
| IL-10 | r | 0.18 | 0.41 | 0.25 | 0.29 | −0.02 | 0.35 | −0.53 | 0.61 | 0.09 | 0.45 |
| p | 0.596 | 0.204 | 0.459 | 0.488 | 0.952 | 0.464 | 0.114 | 0.105 | 0.770 | 0.262 | |
| MCP-1 | r | 0.82 | 0.11 | −0.57 | 0.89 | −0.68 | 0.28 | −0.44 | −0.03 | −0.47 | −0.18 |
| p | 0.002 | 0.793 | 0.070 | 0.005 | 0.022 | 0.587 | 0.201 | 0.948 | 0.119 | 0.667 | |
| TGF-a | r | −0.17 | 0.12 | −0.02 | 0.25 | 0.02 | 0.21 | 0.07 | 0.14 | −0.23 | 0.11 |
| p | 0.620 | 0.610 | 0.945 | 0.296 | 0.953 | 0.386 | 0.840 | 0.555 | 0.463 | 0.632 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leventelis, C.; Veskoukis, A.S.; Rojas Gil, A.P.; Papadopoulos, P.; Garderi, M.; Angeli, A.; Kampitsi, A.; Tsironi, M. Methadone and Buprenorphine as Medication for Addiction Treatment Diversely Affect Inflammation and Craving Depending on Their Doses. Pharmacy 2025, 13, 40. https://doi.org/10.3390/pharmacy13020040
Leventelis C, Veskoukis AS, Rojas Gil AP, Papadopoulos P, Garderi M, Angeli A, Kampitsi A, Tsironi M. Methadone and Buprenorphine as Medication for Addiction Treatment Diversely Affect Inflammation and Craving Depending on Their Doses. Pharmacy. 2025; 13(2):40. https://doi.org/10.3390/pharmacy13020040
Chicago/Turabian StyleLeventelis, Christonikos, Aristidis S. Veskoukis, Andrea Paola Rojas Gil, Panagiotis Papadopoulos, Maria Garderi, Asimina Angeli, Antzouletta Kampitsi, and Maria Tsironi. 2025. "Methadone and Buprenorphine as Medication for Addiction Treatment Diversely Affect Inflammation and Craving Depending on Their Doses" Pharmacy 13, no. 2: 40. https://doi.org/10.3390/pharmacy13020040
APA StyleLeventelis, C., Veskoukis, A. S., Rojas Gil, A. P., Papadopoulos, P., Garderi, M., Angeli, A., Kampitsi, A., & Tsironi, M. (2025). Methadone and Buprenorphine as Medication for Addiction Treatment Diversely Affect Inflammation and Craving Depending on Their Doses. Pharmacy, 13(2), 40. https://doi.org/10.3390/pharmacy13020040








