Discrepancy between Subjective and Objective Measurements for the Evaluation of Medication Adherence—A Cross-Sectional Study in Patients with Cardiovascular Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Patient Characteristics
2.3. Evaluation of Medication Adherence Using a Subjective Measurement
2.4. Assessment of the Medication Adherence Rate Using Pill Counting
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Evaluation of Medication Adherence Using the Ueno Scale
3.3. Factors Associated with Poor Adherence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Annual Report on the Aging Society [Summary] FY2022. Available online: https://www8.cao.go.jp/kourei/english/annualreport/2022/pdf/2022.pdf (accessed on 28 December 2023).
- MHLW (Ministry of Health, Labour, and Welfare). Overview of the 2023 Vital Statistics (Confirmed Figures). Available online: https://www.mhlw.go.jp/toukei/saikin/hw/jinkou/kakutei23/index.html (accessed on 30 September 2024).
- Gehi, A.K.; Ali, S.; Na, B.; Whooley, M.A. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: The heart and soul study. Arch. Intern. Med. 2007, 167, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ockene, I.S.; Rosal, M.C.; Merriam, P.A.; Ockene, J.K.; Gandhi, P.J. Randomized trial of a pharmacist-delivered intervention for improving lipid-lowering medication adherence among patients with coronary heart disease. Cholesterol 2010, 2010, 383281. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.R.; Williams, S.L.; Haskard, K.B.; Dimatteo, M.R. The challenge of patient adherence. Ther. Clin. Risk Manag. 2005, 1, 189–199. [Google Scholar] [PubMed]
- Iuga, A.O.; McGuire, M.J. Adherence and health care costs. Risk Manag. Healthc. Policy 2014, 7, 35–44. [Google Scholar]
- World Health Organization. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003; Available online: https://apps.who.int/iris/handle/10665/42682 (accessed on 28 December 2023).
- Butt, M.D.; Ong, S.C.; Butt, F.Z.; Sajjad, A.; Rasool, M.F.; Imran, I.; Ahmad, T.; Alqahtani, F.; Babar, Z.U. Assessment of health-related quality of life, medication adherence, and prevalence of depression in kidney failure patients. Int. J. Environ. Res. Public Health 2022, 19, 15266. [Google Scholar] [CrossRef] [PubMed]
- Ladova, K.; Matoulkova, P.; Zadak, Z.; Macek, K.; Vyroubal, P.; Vlcek, J.; Morisky, D.E. Self-reported adherence by MARS-CZ reflects LDL cholesterol goal achievement among statin users: Validation study in the Czech Republic. J. Eval. Clin. Pract. 2014, 20, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Zeller, A.; Ramseier, E.; Teagtmeyer, A.; Battegay, E. Patients’ self-reported adherence to cardiovascular medication using electronic monitors as comparators. Hypertens. Res. 2008, 31, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.Y.; Fresco, P. Medication adherence measures: An overview. BioMed Res. Int. 2015, 2015, 217047. [Google Scholar] [CrossRef]
- Farmer, K.C. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin. Ther. 1999, 21, 1074–1090, discussion 1073. [Google Scholar] [CrossRef]
- Choudhry, N.K.; Kronish, I.M.; Vongpatanasin, W.; Ferdinand, K.C.; Pavlik, V.N.; Egan, B.M.; Schoenthaler, A.; Houston Miller, N.; Hyman, D.J.; American Heart Association Council on Hypertension; et al. Medication adherence and blood pressure control: A scientific statement from the American Heart Association. Hypertension 2022, 79, e1–e14. [Google Scholar] [CrossRef]
- Ueno, H.; Yamazaki, Y.; Yonekura, Y.; Park, M.J.; Ishikawa, H.; Kiuchi, T. Reliability and validity of a 12-item medication adherence scale for patients with chronic disease in Japan. BMC Health Serv. Res. 2018, 18, 592. [Google Scholar] [CrossRef]
- Ueno, H.; Ishikawa, H.; Kato, M.; Okuhara, T.; Okada, H.; Kiuchi, T. Factors related to self-care drug treatment and medication adherence of elderly people in Japan. Public Health Pract. 2021, 2, 100106. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Matsumoto, A.; Momosaki, R. Pharmacotherapy and the role of pharmacists in rehabilitation medicine. Prog. Rehabil. Med. 2022, 7, 20220025. [Google Scholar] [CrossRef] [PubMed]
- Bahit, M.C.; Korjian, S.; Daaboul, Y.; Baron, S.; Bhatt, D.L.; Kalayci, A.; Chi, G.; Nara, P.; Shaunik, A.; Gibson, C.M. Patient adherence to secondary prevention therapies after an acute coronary syndrome: A scoping review. Clin. Ther. 2023, 45, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- De Geest, S.; Zullig, L.L.; Dunbar-Jacob, J.; Helmy, R.; Hughes, D.A.; Wilson, I.B.; Vrijens, B. ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Ann. Intern. Med. 2018, 169, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Osula, D.; Wu, B.; Schesing, K.; Das, S.R.; Moss, E.; Alvarez, K.; Clark, C.; Halm, E.A.; Brown, N.J.; Vongpatanasin, W. Comparison of pharmacy refill data with chemical adherence testing in assessing medication nonadherence in a safety net hospital setting. J. Am. Heart Assoc. 2022, 11, e027099. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Khan, H.; Heydon, E.; Shroufi, A.; Fahimi, S.; Moore, C.; Stricker, B.; Mendis, S.; Hofman, A.; Mant, J.; et al. Adherence to cardiovascular therapy: A meta-analysis of prevalence and clinical consequences. Eur. Heart J. 2013, 34, 2940–2948. [Google Scholar] [CrossRef] [PubMed]
- Faridi, K.F.; Peterson, E.D.; McCoy, L.A.; Thomas, L.; Enriquez, J.; Wang, T.Y. Timing of first postdischarge follow-up and medication adherence after acute myocardial infarction. JAMA Cardiol. 2016, 1, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Mathews, R.; Wang, W.; Kaltenbach, L.A.; Thomas, L.; Shah, R.U.; Ali, M.; Peterson, E.D.; Wang, T.Y. Hospital variation in adherence rates to secondary prevention medications and the implications on quality. Circulation 2018, 137, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Michou, M.; Panagiotakos, D.B.; Costarelli, V. Low health literacy and excess body weight: A systematic review. Cent. Eur. J. Public Health 2018, 26, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.W.; Adams, C.E.; Cano, M.A.; Correa-Fernández, V.; Li, Y.; Waters, A.J.; Wetter, D.W.; Vidrine, J.I. Associations between health literacy and established predictors of smoking cessation. Am. J. Public Health 2013, 103, e43–e49. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Nakashima, A.; Nakamura, Y.; Sakamoto, Y.; Matsuo, K.; Goto, M.; Uchiyama, M.; Okamura, K.; Mitsutake, R.; Urata, H.; et al. Association between medication adherence and illness perceptions in atrial fibrillation patients treated with direct oral anticoagulants: An observational cross-sectional pilot study. PLoS ONE 2018, 13, e0204814. [Google Scholar] [CrossRef]
- Giardini, A.; Martin, M.T.; Cahir, C.; Lehane, E.; Menditto, E.; Strano, M.; Pecorelli, S.; Monaco, A.; Marengoni, A. Toward appropriate criteria in medication adherence assessment in older persons: Position Paper. Aging Clin. Exp. Res. 2016, 28, 371–381. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n (%) |
|---|---|
| Age (years) a | 74 (67–81) |
| Male/Female gender | 52 (55.3)/42 (44.7) |
| Obesity | 34 (36.2) |
| Smoking history | 40 (42.6) |
| Alcohol consumption habits | 43 (45.7) |
| Good sleep | 64 (68.1) |
| Exercise habits | 64 (68.1) |
| Good ADLs | 50 (53.2) |
| History of side effects or allergies | 17 (18.1) |
| Hospitalization history | 60 (63.8) |
| Prescription from another hospital | 54 (57.4) |
| Living alone | 13 (13.8) |
| Employment | 35 (37.2) |
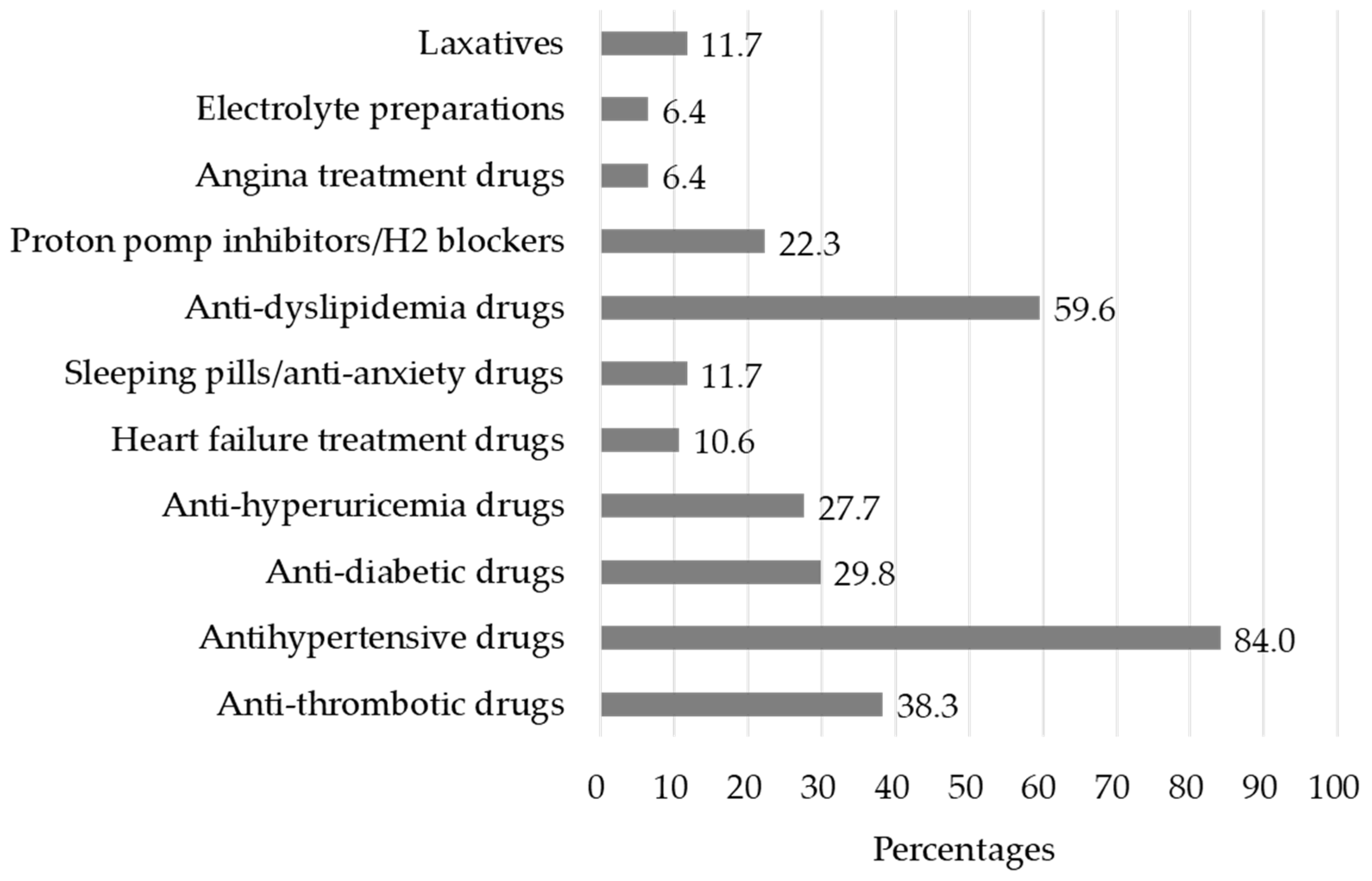
| Number of medications a | 4 (2–7) |
| ODP | 16 (17.0) |
| Medication adherence rate = 100% (Good Adherence) | 49 (52.1) |
| Ueno Scale | Good Adherence (n = 49) | Poor Adherence (n = 45) | p Value |
|---|---|---|---|
| Subjective compliance | 15 (15–15) | 15 (14.5–15) | 0.083 |
| Collaboration | 12 (10–15) | 12 (9.5–14) | 0.250 |
| Willingness | 10 (7–12.5) | 10 (7–12) | 0.918 |
| Acceptance | 13 (12–15) | 13 (12–14) | 0.459 |
| Total | 51 (44–56) | 50 (45–54) | 0.426 |
| Characteristics | Good Adherence (n = 49) | Poor Adherence (n = 45) | p Value |
|---|---|---|---|
| Age (years) a | 75 (64–81) | 74 (67–78) | 0.8143 |
| Gender | 0.3812 | ||
| Male | 25 (51.0) | 27 (60.0) | |
| Female | 24 (49.0) | 18 (40.0) | |
| Obesity | 12 (24.5) | 22 (48.9) | 0.014 |
| Smoking history | 16 (32.7) | 24 (53.3) | 0.043 |
| Alcohol consumption habits | 22 (44.9) | 21 (46.7) | 0.864 |
| Good sleep | 34 (69.4) | 30 (66.7) | 0.777 |
| Exercise habits | 32 (65.3) | 32 (71.1) | 0.546 |
| Good ADLs | 22 (44.9) | 28 (62.2) | 0.093 |
| History of side effects or allergies | 9 (18.4) | 8 (17.8) | 0.941 |
| Hospitalization history | 31 (63.3) | 29 (64.4) | 0.905 |
| Prescription from another hospital | 28 (57.1) | 26 (57.8) | 0.950 |
| Living alone | 10 (20.4) | 3 (6.7) | 0.074 |
| Employment | 18 (36.7) | 17 (37.8) | 0.917 |
| Number of medications a | 4 (2–6) | 4 (3–7) | 0.506 |
| Polypharmacy | 16 (32.7) | 15 (33.3) | 0.944 |
| ODP | 9 (18.4) | 7 (15.6) | 0.717 |
| Types of medications | |||
| Anti-thrombotic drugs | 17 (34.7) | 19 (42.2) | 0.453 |
| Antihypertensive drugs | 44 (89.8) | 35 (77.8) | 0.112 |
| Anti-diabetic drugs | 11 (22.4) | 17 (37.8) | 0.105 |
| Diuretic drugs | 4 (8.2) | 2 (4.4) | 0.461 |
| Anti-hyperuricemia drugs | 15 (30.6) | 11 (24.4) | 0.504 |
| Heart failure treatment drugs | 6 (12.2) | 4 (8.9) | 0.598 |
| Sleeping pills/anti-anxiety drugs | 7 (14.2) | 4 (8.9) | 0.416 |
| Anti-dyslipidemia drugs | 28 (57.1) | 28 (62.2) | 0.616 |
| Proton pomp inhibitors/H2 blockers | 10 (20.4) | 11 (24.4) | 0.639 |
| Angina treatment drugs | 1 (2.0) | 5 (11.1) | 0.072 |
| Electrolyte preparations | 2 (4.1) | 4 (8.9) | 0.341 |
| Laxatives | 5 (10.2) | 6 (13.3) | 0.637 |
| Characteristics | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Obesity | 3.527 | 1.387–9.423 | 0.008 |
| Smoking history | 2.442 | 0.985–6.277 | 0.054 |
| Good ADLs | 1.341 | 0.501–3.625 | 0.559 |
| Living alone | 0.336 | 0.062–1.430 | 0.144 |
| Angina treatment drug usage | 7.393 | 0.982–154.3 | 0.052 |
| Subjective compliance (Ueno scale) a | 0.867 | 0.476–1.468 | 0.597 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazaki, M.; Hirata, H.; Takaki, S.; Misaki, M.; Mori, Y.; Tokura, K.; Sato, N.; Nakashima, A.; Yanagida, A.; Okajima, I.; et al. Discrepancy between Subjective and Objective Measurements for the Evaluation of Medication Adherence—A Cross-Sectional Study in Patients with Cardiovascular Diseases. Pharmacy 2024, 12, 153. https://doi.org/10.3390/pharmacy12050153
Miyazaki M, Hirata H, Takaki S, Misaki M, Mori Y, Tokura K, Sato N, Nakashima A, Yanagida A, Okajima I, et al. Discrepancy between Subjective and Objective Measurements for the Evaluation of Medication Adherence—A Cross-Sectional Study in Patients with Cardiovascular Diseases. Pharmacy. 2024; 12(5):153. https://doi.org/10.3390/pharmacy12050153
Chicago/Turabian StyleMiyazaki, Motoyasu, Hitomi Hirata, Satoko Takaki, Momoko Misaki, Yukako Mori, Kaoko Tokura, Natsuki Sato, Akio Nakashima, Atsuko Yanagida, Isa Okajima, and et al. 2024. "Discrepancy between Subjective and Objective Measurements for the Evaluation of Medication Adherence—A Cross-Sectional Study in Patients with Cardiovascular Diseases" Pharmacy 12, no. 5: 153. https://doi.org/10.3390/pharmacy12050153
APA StyleMiyazaki, M., Hirata, H., Takaki, S., Misaki, M., Mori, Y., Tokura, K., Sato, N., Nakashima, A., Yanagida, A., Okajima, I., Urata, H., & Imakyure, O. (2024). Discrepancy between Subjective and Objective Measurements for the Evaluation of Medication Adherence—A Cross-Sectional Study in Patients with Cardiovascular Diseases. Pharmacy, 12(5), 153. https://doi.org/10.3390/pharmacy12050153








