Decoding Clozapine-Induced Agranulocytosis: Unraveling Interactions and Mitigation Strategies
Abstract
1. Introduction
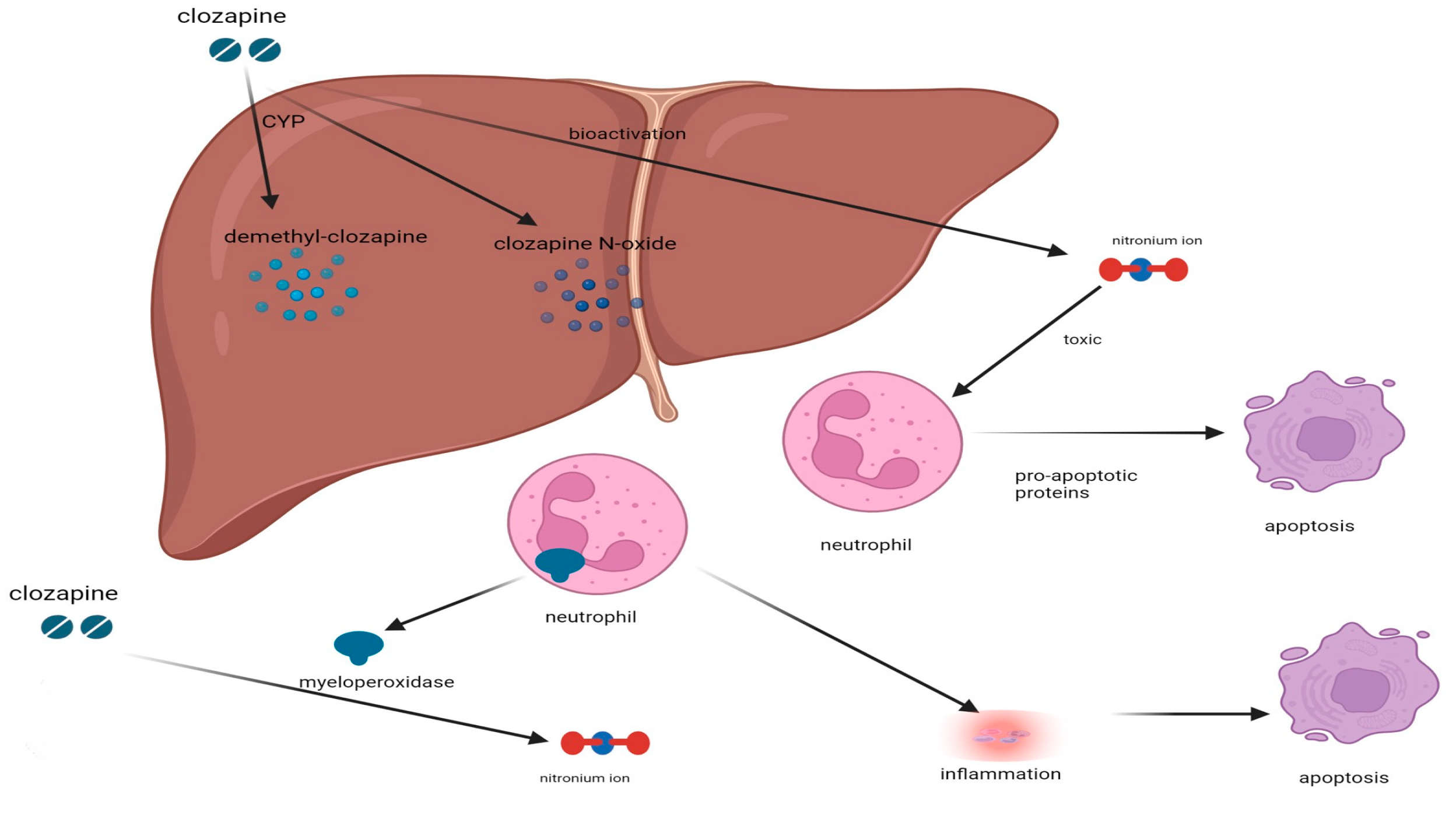
2. Potential Mechanism Underlying CIA
3. Impact of Concurrent Medications on the Risk of Agranulocytosis in Clozapine-Treated Patients
4. Impact of Comorbid Conditions on the Risk of Agranulocytosis in Clozapine-Treated Patients
5. Effect of Therapeutic Duration and Drug Dosage on the Induction of Clozapine-Mediated Agranulocytosis
6. Management and Prevention of Agranulocytosis during Clozapine Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.-H.; Zhong, X.-M.; Lu, L.; Zheng, W.; Wang, S.-B.; Rao, W.-W.; Wang, S.; Ng, C.H.; Ungvari, G.S.; Wang, G.; et al. The prevalence of agranulocytosis and related death in clozapine-treated patients: A comprehensive meta-analysis of observational studies. Psychol. Med. 2020, 50, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Myles, N.; Myles, H.; Xia, S.; Large, M.; Kisely, S.; Galletly, C.; Bird, R.; Siskind, D. Meta-analysis examining the epidemiology of clozapine-associated neutropenia. Acta Psychiatr. Scand. 2018, 138, 101–109. [Google Scholar] [CrossRef]
- Taylor, D.; Vallianatou, K.; Whiskey, E.; Dzahini, O.; MacCabe, J. Distinctive pattern of neutrophil count change in clozapine-associated, life-threatening agranulocytosis. Schizophrenia 2022, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Stübner, S.; Grohmann, R.; Engel, R.; Bandelow, B.; Ludwig, W.-D.; Wagner, G.; Müller-Oerlinghausen, B.; Möller, H.-J.; Hippius, H.; Rüther, E. Blood Dyscrasias Induced by Psychotropic Drugs. Pharmacopsychiatry 2004, 37 (Suppl. 1), 70–78. [Google Scholar] [CrossRef]
- Nielsen, J.; Correll, C.U.; Manu, P.; Kane, J.M. Termination of clozapine treatment due to medical reasons: When is it warranted and how can it be avoided? J. Clin. Psychiatry 2013, 74, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, C.-F.; Petersen, T.S.; Nielsen, J.; Jørgensen, A.; Jimenez-Solem, E.; Fink-Jensen, A. Clozapine- and non-clozapine-associated neutropenia in patients with schizophrenia: A retrospective cohort study. Ther. Adv. Psychopharmacol. 2022, 12, 20451253211072341. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, J.M.; Van Rooijen, G.; Van De Kerkhof, M.P.J.; Sutterland, A.L.; Correll, C.U.; De Haan, L. Clozapine and long-Term mortality risk in patients with schizophrenia: A systematic review and meta-analysis of studies lasting 1.1–12.5 years. Schizophr. Bull. 2019, 45, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Bishara, D.; Taylor, D. Adverse Effects of Clozapine in Older Patients: Epidemiology, Prevention and Management. Drugs Aging 2014, 31, 11–20. [Google Scholar] [CrossRef]
- Herst, L.; Powell, G. Is clorapine safe in the elderly? Aust. N. Z. J. Psychiatry 1997, 31, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Alvir, J.M.J.; Lieberman, J.A.; Safferman, A.Z.; Schwimmer, J.L.; Schaaf, J.A. Clozapine-Induced Agranulocytosis--Incidence and Risk Factors in the United States. N. Engl. J. Med. 1993, 329, 162–167. [Google Scholar] [CrossRef]
- Munro, J.; O’Sullivan, D.; Andrews, C.; Arana, A.; Mortimer, A.; Kerwin, R. Active monitoring of 12760 clozapine recipients in the UK and Ireland: Beyond pharmacovigilance. Br. J. Psychiatry 1999, 175, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Wenthur, C.J.; Lindsley, C.W. Classics in Chemical Neuroscience: Clozapine. ACS Chem. Neurosci. 2013, 4, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y.; Okayli, G. Reduction of suicidality during clozapine treatment of neuroleptic- resistant schizophrenia: Impact on risk-benefit assessment. Am. J. Psychiatry 1995, 152, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, V.; Kosidou, K.; Widman, L.; Orsini, N.; Hodsoll, J.; Dalman, C.; MacCabe, J.H. Clozapine Treatment and Offending: A Within-Subject Study of Patients with Psychotic Disorders in Sweden. Schizophr. Bull. 2020, 46, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Hayhurst, K.P.; Brown, P.; Lewis, S.W. The cost-effectiveness of clozapine: A controlled, population-based, mirror-image study. J. Psychopharmacol. 2002, 16, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Samara, M.T.; Dold, M.; Gianatsi, M.; Nikolakopoulou, A.; Helfer, B.; Salanti, G.; Leucht, S. Efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia: A network meta-analysis. JAMA Psychiatry 2016, 73, 199–210. [Google Scholar] [CrossRef]
- Meltzer, H.Y. Update on Typical and Atypical Antipsychotic Drugs. Annu. Rev. Med. 2013, 64, 393–406. [Google Scholar] [CrossRef]
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23, 2087. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.D.; Barr, A.M.; Honer, W.G.; Procyshyn, R.M. Reversal of Dopamine Supersensitivity as a Mechanism of Action of Clozapine. Psychother. Psychosom. 2018, 87, 306–307. [Google Scholar] [CrossRef]
- Pirmohamed, M.; Park, K. Mechanism of clozapine-induced agranulocytosis current status of research and implications for drug Development. CNS Drugs 1997, 7, 139–158. [Google Scholar] [CrossRef]
- Whiskey, E.; Taylor, D. Restarting clozapine after neutropenia evaluating the possibilities and practicalities. CNS Drugs 2007, 21, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Sernoskie, S.C.; Jee, A.; Uetrecht, J. The Role of Myeloperoxidase in Clozapine-Induced Inflammation: A Mechanistic Update for Idiosyncratic Drug-Induced Agranulocytosis. Int. J. Mol. Sci. 2023, 24, 1243. [Google Scholar] [CrossRef] [PubMed]
- Legge, S.E.; Walters, J.T.R. Genetics of clozapine-associated neutropenia: Recent advances, challenges and future perspective. Pharmacogenomics 2019, 20, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Konte, B.; Walters, J.T.R.; Rujescu, D.; Legge, S.E.; Pardiñas, A.F.; Cohen, D.; Pirmohamed, M.; Tiihonen, J.; Hartmann, A.M.; Bogers, J.P.; et al. HLA-DQB1 6672G>C (rs113332494) is associated with clozapine-induced neutropenia and agranulocytosis in individuals of European ancestry. Transl. Psychiatry 2021, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Legge, S.E.; Hamshere, M.L.; Ripke, S.; Pardinas, A.F.; Goldstein, J.I.; Rees, E.; Richards, A.L.; Leonenko, G.; Jorskog, L.F.; Clozapine-Induced Agranulocytosis Consortium; et al. Genome-wide common and rare variant analysis provides novel insights into clozapine-associated neutropenia. Mol. Psychiatry 2017, 22, 1502–1508. [Google Scholar] [CrossRef]
- Siskind, D.; Siskind, V.; Kisely, S. Clozapine Response Rates among People with Treatment-Resistant Schizophrenia: Data from a Systematic Review and Meta-Analysis. Can. J. Psychiatry 2017, 62, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.; Löhrs, L.; Siskind, D.; Honer, W.G.; Falkai, P.; Hasan, A. Clozapine augmentation strategies—A systematic meta-review of available evidence. Treatment options for clozapine resistance. J. Psychopharmacol. 2019, 33, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, J.R.; Gajwani, P. Lamotrigine and Clozapine for Bipolar Disorder. Am. J. Psychiatry 2000, 157, 1523. [Google Scholar] [CrossRef]
- Siskind, D.J.; Lee, M.; Ravindran, A.; Zhang, Q.; Ma, E.; Motamarri, B.; Kisely, S. Augmentation strategies for clozapine refractory schizophrenia: A systematic review and meta-analysis. Aust. N. Z. J. Psychiatry 2018, 52, 751–767. [Google Scholar] [CrossRef]
- Flanagan, R.J.; Dunk, L. Haematological toxicity of drugs used in psychiatry. Hum. Psychopharmacol. Clin. Exp. 2008, 23 (Suppl. 1), S27–S41. [Google Scholar] [CrossRef]
- Demler, T.L.; Trigoboff, E. Are clozapine blood dyscrasias associated with concomitant medications? Innov. Clin. Neurosci. 2011, 8, 35–41. [Google Scholar] [PubMed]
- Chetty, M.; Murray, M. CYP-mediated clozapine interactions: How predictable are they? Curr. Drug Metab. 2007, 8, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Lally, J.; Ajnakina, O.; Pritchard, M.; Krivoy, A.; Gaughran, F.; Shetty, H.; Flanagan, R.J.; MacCabe, J.H. Sodium valproate and clozapine induced neutropenia: A case control study using register data. Schizophr. Res. 2018, 195, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Imbarlina, M.J.; Sarkar, S.; Marwah, S.; Parepally, H.; Johnston, P.R.; Brar, J.S.; Chengappa, K.R. Leukopenia in clozapine treated patients may be induced by other drugs: A case series. Eur. Psychiatry 2004, 19, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Wang, X.-Y.; Chou, P.-H.; Lin, C.-H. Valproate-related neutropenia and lithium-related leukocytosis in patients treated with clozapine: A retrospective cohort study. BMC Psychiatry 2023, 23, 170. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Mican, L.M.; Fischer, C.; Campbell, A.H. Evaluating the Incidence of Leukopenia and Neutropenia with Valproate, Quetiapine, or the Combination in Children and Adolescents. Ann. Pharmacother. 2009, 43, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Mijovic, A.; Maccabe, J.H. Clozapine-induced agranulocytosis. Ann. Hematol. 2020, 99, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, J.; Noguchi, S.; Murakami, T.; Murakami, N. Antithyroid drug–induced agranulocytosis: The usefulness of routine white blood cell count monitoring. Arch. Intern. Med. 1990, 150, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Węclewicz, M.M.; Miętkiewicz, M.; Malinowski, B.; Grześk, E.; Klonowska, J. Potential Mechanisms of Hematological Adverse Drug Reactions in Patients Receiving Clozapine in Combination with Proton Pump Inhibitors. J. Psychiatr. Pract. 2017, 23, 114–120. [Google Scholar] [CrossRef]
- Shalbafan, M.; Bechard, L.; Ifteni, P.; Chen, C.-H. Neutropenia after the coadministration of clozapine and nirmatrelvir-ritonavir in a patient with SARS-CoV-2 infection: A case report with a literature review. Front. Psychiatry 2022, 13, 1096006. [Google Scholar]
- Pick, A.M.; Nystrom, K.K. Nonchemotherapy drug-induced neutropenia and agranulocytosis: Could medications be the culprit? J. Pharm. Pract. 2014, 27, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Okunaka, M.; Kano, D.; Matsui, R.; Kawasaki, T.; Uesawa, Y. Comprehensive Analysis of Chemotherapeutic Agents That Induce Infectious Neutropenia. Pharmaceuticals 2021, 14, 681. [Google Scholar] [CrossRef]
- Van Der Klauw, M.M.; Goudsmit, R.; Halie, M.R.; Van’t Veer, M.B.; Herings, R.M.; Wilson, J.P.; Stricker, B.H.C. A population-based case-cohort study of drug-associated agranulocytosis. Arch. Intern. Med. 1999, 159, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Coates, T.D. Drug-Induced Neutropenia and Agranulocytosis. 2018. Available online: https://teksmedik.com/uptodate20/d/topic.htm?path=drug-induced-neutropenia-and-agranulocytosis (accessed on 26 December 2023).
- Sedhai, Y.R.; Lamichhane, A.; Gupta, V. Agranulocytosis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559275/ (accessed on 26 December 2023).
- Ojong, M.; Allen, S.N. Management and prevention of agranulocytosis in patients receiving clozapine. Ment. Health Clin. 2013, 3, 139–143. [Google Scholar] [CrossRef]
- Paribello, P.; Manchia, M.; Zedda, M.; Pinna, F.; Carpiniello, B. Leukocytosis Associated with Clozapine Treatment: A Case Series and Systematic Review of the Literature. Medicina 2021, 57, 816. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, P.; Zhang, Q.; Chen, R.; Wang, P.; Liu, B.; Sun, W.; Jian, X.; Xiang, S.; Zhou, J.; et al. Genetic risk of clozapine-induced leukopenia and neutropenia: A genome-wide association study. Transl. Psychiatry 2021, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Raveendranathan, D.; Sharma, E.; Venkatasubramanian, G.; Rao, M.G.; Varambally, S.; Gangadhar, B.N. Late-onset clozapine-induced agranulocytosis in a patient with comorbid multiple sclerosis. Gen. Hosp. Psychiatry 2013, 35, 574.e5–574.e6. [Google Scholar] [CrossRef] [PubMed]
- Gee, S.; Gaughran, F.; MacCabe, J.; Shergill, S.; Whiskey, E.; Taylor, D. Management of clozapine treatment during the COVID-19 pandemic. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320928167. [Google Scholar] [CrossRef] [PubMed]
- Gee, S.; Taylor, D. COVID-19 infection causes a reduction in neutrophil counts in patients taking clozapine. J. Psychiatry Neurosci. 2021, 46, 232–237. [Google Scholar] [CrossRef]
- Islam, F.; Hain, D.; Lewis, D.; Law, R.; Brown, L.C.; Tanner, J.-A.; Müller, D.J. Pharmacogenomics of Clozapine-induced agranulocytosis: A systematic review and meta-analysis. Pharmacogenom. J. 2022, 22, 230–240. [Google Scholar] [CrossRef]
- Andersohn, F.; Konzen, C.; Garbe, E. Systematic Review: Agranulocytosis Induced by Nonchemotherapy Drugs. Ann. Intern. Med. 2007, 146, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Dunk, L.R.; Annan, L.J.; Andrews, C.D. Rechallenge with clozapine following leucopenia or neutropenia during previous therapy. Br. J. Psychiatry 2006, 188, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.M.; Barnes, T.R.; Young, A.H. The Maudsley Prescribing Guidelines in Psychiatry, 13th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2018. [Google Scholar]
- US Food and Drug Administration. Clozaril (Clozapine) Prescribing Information. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/019758s088lbl.pdf (accessed on 26 December 2023).
- European Agency for the Evaluation of Medicinal Products. SmPC Leponex (Clozapine). 2002. Available online: https://www.ema.europa.eu/en/documents/referral/summary-information-referral-opinion-following-arbitration-pursuant-article-30-council-directive/83/ec-leponex-associated-names-international-non-proprietary-name-inn-clozapine-background-inform_en.pdf (accessed on 27 December 2023).
- Shad, M.U. Pharmacogenomic screening for agranulocytosis and efficacy with clozapine. J. Transl. Genet. Genom. 2023, 7, 141–165. [Google Scholar] [CrossRef]
- Yunis, J.J.; Corzo, D.; Salazar, M.; Lieberman, J.A.; Howard, A.; Yunis, E.J. HLA associations in clozapine-induced agranulocytosis. Blood 1995, 86, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, M.C.; Dettling, M.; Cascorbi, I.; Mosyagin, I.; Salisbury, B.A.; Pierz, K.A.; Zou, W.; Whalen, H.; Malhotra, A.K.; Lencz, T.; et al. Candidate Gene Analysis Identifies a Polymorphism in HLA-DQB1 Associated with Clozapine-Induced Agranulocytosis. J. Clin. Psychiatry 2011, 72, 458–463. [Google Scholar] [CrossRef]
- Pandarakalam, J.P. Combination therapy for treatment resistant schizophrenia. Br. J. Med. Pract. 2019, 12, a015. [Google Scholar]
- Lally, J.; Malik, S.; Whiskey, E.; Taylor, D.M.; Gaughran, F.P.; Krivoy, A.; Flanagan, R.J.; Mijovic, A.; MacCabe, J.H. Clozapine-Associated Agranulocytosis Treatment with Granulocyte Colony-Stimulating Factor/Granulocyte-Macrophage Colony-Stimulating Factor. J. Clin. Psychopharmacol. 2017, 37, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Kanaan, R.A.; Kerwin, R.W. Lithium and clozapine rechallenge: A retrospective case analysis. Br. J. Psychol. 2006, 67, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Azzarà, A.; Kast, R.E.; Carulli, G.; Petrini, M. Lithium and hematology: Established and proposed uses. J. Leukoc. Biol. 2009, 85, 20–28. [Google Scholar] [CrossRef]
- Boazak, M.; Goldsmith, D.R.; Cotes, R.O. Mask off? Lithium augmentation for clozapine rechallenge after neutropenia or agranulocytosis: Discontinuation might be risky. Prim. Care Companion CNS Disord. 2018, 20, 25337. [Google Scholar] [CrossRef]
| Medications | Interaction Mechanism | Incidence of Agranulocytosis | Incidence of Neutropenia | Mitigation Strategy | References |
|---|---|---|---|---|---|
| Carbamazepine | 0.14% | 0.5% | Monitor blood levels; consider alternative AEDs | [4,30] | |
| Clozapine | 0.4% | 2.9% | Monitor blood levels | [1,35] | |
| Valproate with clozapine | Sodium valproate may potentiate CIN by inhibiting its metabolism, enhancing oxidative stress, and suppressing bone marrow function. | 8.8% | Monitor blood levels; consider alternative AEDs | [33,35] | |
| Antithyroid Medications (e.g., propylthiouracil, methimazole) | Propylthiouracil: 0.55%; Methimazole: 0.31% | Monitor blood levels; consider alternative Antithyroid Medications | [38] | ||
| Proton Pump Inhibitors Medications (e.g., omeprazole) with clozapine | PPIs induce CYP1A2, which increases N-desmethylclozapine levels and possibly influences flavin-containing monooxygenase (FM-O3), increasing nitrenium ion production. | Significantly higher when combined with PPIs. | Monitor blood levels; consider using PPIs with lower interaction potential, like rabeprazole; prefer alternative gastroesophageal reflux disease treatments over PPIs when possible, like misoprostol | [39] | |
| Nirmatrelvir-Ritonavir (Paxlovid) with clozapine | Interaction likely involves inhibition of CYP1A2 by the ritonavir component, potentially increasing clozapine levels; concomitant SARS-CoV-2 infection may exacerbate neutropenia. | Increased risk of neutropenia in the context of SARS-CoV-2 infection and multiple drug interactions. | Monitor blood levels; consider adjusting the clozapine dosage | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alalawi, A.; Albalawi, E.; Aljohani, A.; Almutairi, A.; Alrehili, A.; Albalawi, A.; Aldhafiri, A. Decoding Clozapine-Induced Agranulocytosis: Unraveling Interactions and Mitigation Strategies. Pharmacy 2024, 12, 92. https://doi.org/10.3390/pharmacy12030092
Alalawi A, Albalawi E, Aljohani A, Almutairi A, Alrehili A, Albalawi A, Aldhafiri A. Decoding Clozapine-Induced Agranulocytosis: Unraveling Interactions and Mitigation Strategies. Pharmacy. 2024; 12(3):92. https://doi.org/10.3390/pharmacy12030092
Chicago/Turabian StyleAlalawi, Ali, Enas Albalawi, Abdullah Aljohani, Abdullah Almutairi, Abdulraouf Alrehili, Areej Albalawi, and Ahmed Aldhafiri. 2024. "Decoding Clozapine-Induced Agranulocytosis: Unraveling Interactions and Mitigation Strategies" Pharmacy 12, no. 3: 92. https://doi.org/10.3390/pharmacy12030092
APA StyleAlalawi, A., Albalawi, E., Aljohani, A., Almutairi, A., Alrehili, A., Albalawi, A., & Aldhafiri, A. (2024). Decoding Clozapine-Induced Agranulocytosis: Unraveling Interactions and Mitigation Strategies. Pharmacy, 12(3), 92. https://doi.org/10.3390/pharmacy12030092







