Association of Average Daily Morphine Milligram Equivalents and Falls in Older Adult Chronic Opioid Users
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Data Collection
2.3. Primary Exposure
2.4. Primary Outcome
2.5. Data Analysis
2.6. IRB and Protection of Human Subjects
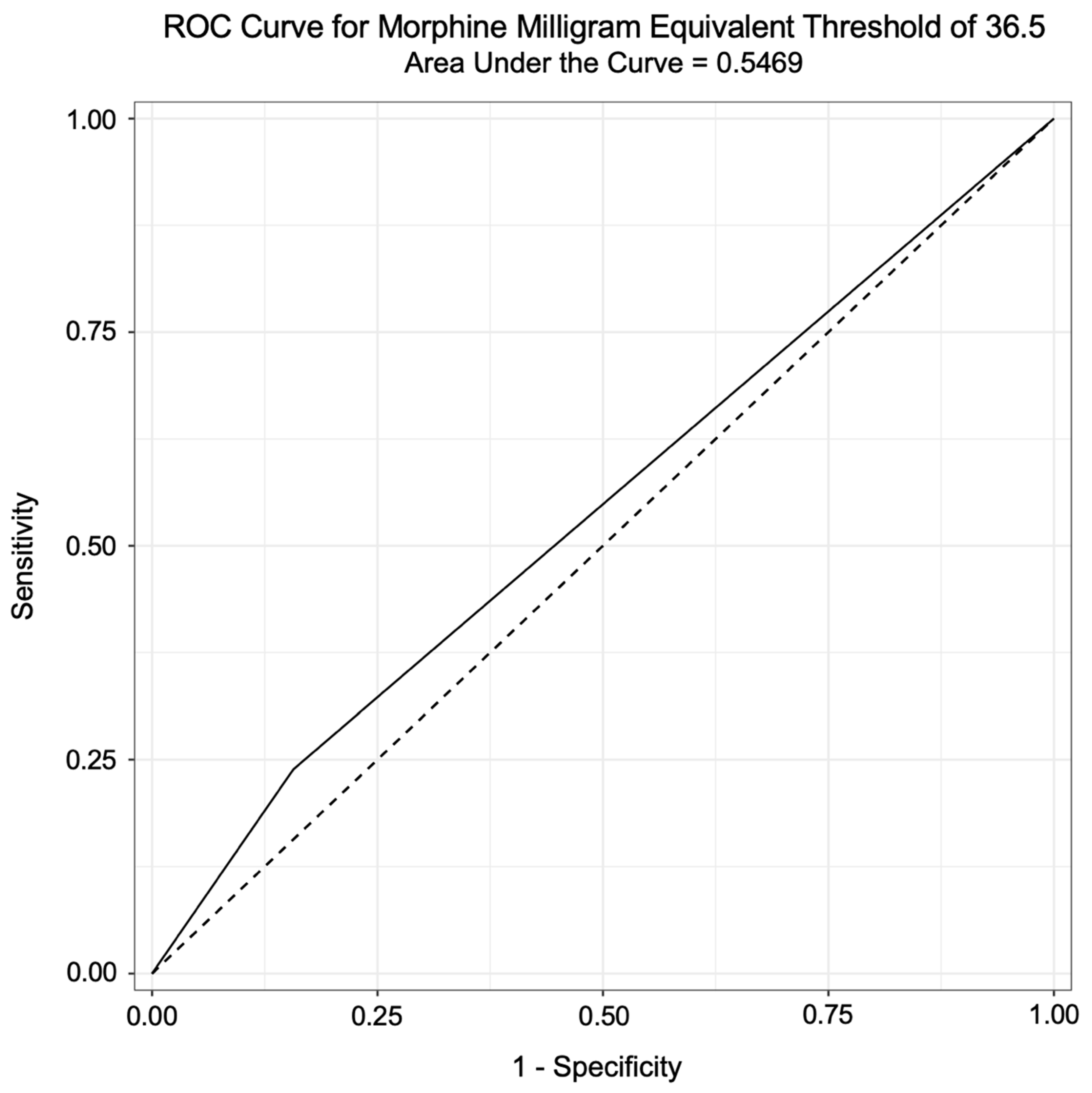
3. Results
3.1. Participants
3.2. Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef]
- Maher, R.L.; Hanlon, J.T.; Hajjar, E.R. Clinical Consequences of Polypharmacy in Elderly. Expert Opin. Drug Saf. 2014, 13, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Trisan, R. Balance Problems and Fall Risks in the Elderly. Clin. Geriatr. Med. 2019, 35, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Falls Prevention Facts. NCOA. Available online: https://www.ncoa.org/news/resources-for-reporters/get-the-facts/falls-prevention-facts/ (accessed on 4 June 2020).
- Moreland, B.; Kakara, R.; Henry, A. Trends in Nonfatal Falls and Fall-Related Injuries Among Adults Aged ≥ 65 Years—United States, 2012–2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 875–881. [Google Scholar] [CrossRef]
- Stevens, J.A.; Phelan, E.A. Development of STEADI: A fall prevention resource for health care providers. Health Promot. Pract. 2013, 14, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.A.; Loomis, J. The STEADI toolkit: Incorporating a fall prevention guideline into the primary care setting. Nurse Pract. 2017, 42, 50–55. [Google Scholar] [CrossRef]
- Stevens, J.A.; Smith, M.L.; Parker, E.M.; Jiang, L.; Floyd, F.D. Implementing a Clinically Based Fall Prevention Program. Am. J. Lifestyle Med. 2020, 14, 71–77. [Google Scholar] [CrossRef]
- Karani, M.V.; Haddad, Y.; Lee, R. The Role of Pharmacists in Preventing Falls among America’s Older Adults. Front. Public Health 2016, 4, 250. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.R.; Mallet, L.; Rochefort, C.M.; Eguale, T.; Buckeridge, D.L.; Tamblyn, R. Medication-related falls in the elderly: Causative factors and preventive strategies. Drugs Aging 2012, 29, 359–376. [Google Scholar] [CrossRef]
- McClure, F.L.; Niles, J.K.; Kaufman, H.W.; Gudin, J. Concurrent Use of Opioids and Benzodiazepines: Evaluation of Prescription Drug Monitoring by a United States Laboratory. J. Addict. Med. 2017, 11, 420–426. [Google Scholar] [CrossRef]
- Bohnert, A.S.B.; Logan, J.E.; Ganoczy, D.; Dowell, D. A Detailed Exploration Into the Association of Prescribed Opioid Dosage and Overdose Deaths Among Patients With Chronic Pain. Med. Care 2016, 54, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.R.; Martimianakis, M.A.; Nasirzadeh, Y.; Northup, E.; Gold, K.; Friesen, F.; Bhatia, A.; Ng, S.L. Compassionate Care in the Age of Evidence-Based Practice: A Critical Discourse Analysis in the Context of Chronic Pain Care. Acad. Med. 2018, 93, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm. Rep. 2016, 65, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Gazelka, H.M.; Leal, J.C.; Lapid, M.I.; Rummans, T.A. Opioids in Older Adults: Indications, Prescribing, Complications, and Alternative Therapies for Primary Care. Mayo Clin. Proc. 2020, 95, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, A.; Ramirez, G.; Smith, M.L.; Foster, M.; Nabil, A.K.; Jani, S.N.; Ory, M.G. Opioid Use and the Risk of Falls, Fall Injuries and Fractures among Older Adults: A Systematic Review and Meta-Analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1989–1995. [Google Scholar] [CrossRef] [PubMed]
- Niznik, J.; Ferreri, S.P.; Armistead, L.; Urick, B.; Vest, M.-H.; Zhao, L.; Hughes, T.; McBride, J.M.; Busby-Whitehead, J. A deprescribing medication program to evaluate falls in older adults: Methods for a randomized pragmatic clinical trial. Trials 2022, 23, 256. [Google Scholar] [CrossRef] [PubMed]
- Schieber, L.Z.; Guy, G.P.; Seth, P.; Losby, J.L. Variation in Adult Outpatient Opioid Prescription Dispensing by Age and Sex—United States, 2008–2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 298–302. [Google Scholar] [CrossRef] [PubMed]
- CDC. Keep on Your Feet. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/injury/features/older-adult-falls/index.html (accessed on 10 May 2021).
- Jones, C.M.; McAninch, J.K. Emergency Department Visits and Overdose Deaths From Combined Use of Opioids and Benzodiazepines. Am. J. Prev. Med. 2015, 49, 493–501. [Google Scholar] [CrossRef]
- Lembke, A. Tapering Long-Term Opioid Therapy. Am. Fam. Physician 2020, 101, 49–52. [Google Scholar]
- O’Brien, M.D.C.; Wand, A.P.F. A systematic review of the evidence for the efficacy of opioids for chronic non-cancer pain in community-dwelling older adults. Age Ageing 2020, 49, 175–183. [Google Scholar] [CrossRef]
- Hastings, J.S.; Howison, M.; Inman, S.E. Predicting high-risk opioid prescriptions before they are given. Proc. Natl. Acad. Sci. USA 2020, 117, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Santosa, K.B.; Hu, H.-M.; Brummett, C.M.; Olsen, M.A.; Englesbe, M.J.; Williams, E.A.; Waljee, J.F. New persistent opioid use among older patients following surgery: A Medicare claims analysis. Surgery 2020, 167, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Gangal, A.; Stoff, B.; Blalock, T. The 2022 CDC opioid prescription guideline update: Relevant recommendations and future considerations. JAAD Int. 2023, 13, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Baruth, J.M.; Gentry, M.T.; Rummans, T.A.; Miller, D.M.; Burton, M.C. Polypharmacy in older adults: The role of the multidisciplinary team. Hosp. Pract. 2020, 48, 56–62. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Had a Fall (%) n = 207 (27.1) | Did Not Fall (%) n = 383 (50.3) | Unknown (%) n = 172 (22.6) |
|---|---|---|---|
| DEMOGRAPHIC | |||
| Age (mean (SD)) | 75.8 (7.6) | 74.6 (7.1) | 74.6 (7.9) |
| Male sex | 28.5 | 39.1 | 29.8 |
| Race | |||
| American Indian or Alaska Native | 0.0 | 1.0 | 0.0 |
| Asian | 0.5 | 0.5 | 0.0 |
| Black or African American | 12.1 | 13.5 | 17.0 |
| Other Race | 0.0 | 0.3 | 1.2 |
| Patient Refused | 0.0 | 0.3 | 0.0 |
| Unknown | 0.0 | 0.0 | 0.6 |
| White | 87.4 | 84.4 | 81.3 |
| DIAGNOSES | |||
| Acute Pain | 98.1 | 97.7 | 93.6 |
| COPD | 25.6 | 14.8 | 19.3 |
| CVD | 98.1 | 94.0 | 94.2 |
| Sleep Apnea | 22.7 | 16.9 | 18.7 |
| Cancer | 33.3 | 24.2 | 21.1 |
| Psychiatric | 67.1 | 52.6 | 52.6 |
| MEDICATION USE | |||
| MME (mean (SD)) | 30.1 (67.2) | 24.2 (41.9) | 31.4 (58.1) |
| 95% CIs | ||||
|---|---|---|---|---|
| Predictor Variable | Odds Ratio * | Lower | Upper | p-Value |
| DEMOGRAPHIC | ||||
| Age | 1.10 | 0.02 | 0.18 | 0.016 |
| Sex | 1.01 | 0.00 | 0.01 | 0.022 |
| DIAGNOSES | ||||
| Acute Pain | 0.95 | −0.32 | 0.21 | 0.694 |
| COPD | 1.13 | 0.03 | 0.22 | 0.014 |
| CVD | 1.16 | −0.04 | 0.33 | 0.120 |
| Sleep Apnea | 1.12 | 0.01 | 0.21 | 0.029 |
| Cancer | 1.11 | 0.02 | 0.19 | 0.014 |
| Psychiatric | 1.13 | 0.05 | 0.21 | 0.002 |
| MEDICATION USE | ||||
| Benzodiazepine use | 1.02 | −0.08 | 0.12 | 0.693 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.; Hughes, T.D.; Niznik, J.; Ferreri, S.P. Association of Average Daily Morphine Milligram Equivalents and Falls in Older Adult Chronic Opioid Users. Pharmacy 2024, 12, 62. https://doi.org/10.3390/pharmacy12020062
Hwang S, Hughes TD, Niznik J, Ferreri SP. Association of Average Daily Morphine Milligram Equivalents and Falls in Older Adult Chronic Opioid Users. Pharmacy. 2024; 12(2):62. https://doi.org/10.3390/pharmacy12020062
Chicago/Turabian StyleHwang, Stephanie, Tamera D. Hughes, Joshua Niznik, and Stefanie P. Ferreri. 2024. "Association of Average Daily Morphine Milligram Equivalents and Falls in Older Adult Chronic Opioid Users" Pharmacy 12, no. 2: 62. https://doi.org/10.3390/pharmacy12020062
APA StyleHwang, S., Hughes, T. D., Niznik, J., & Ferreri, S. P. (2024). Association of Average Daily Morphine Milligram Equivalents and Falls in Older Adult Chronic Opioid Users. Pharmacy, 12(2), 62. https://doi.org/10.3390/pharmacy12020062






