Enteral Delivery of Pravastatin Sodium Tablets: Effect of Compounding into a Liquid Form and Co-Administration of Enteral Nutrition
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. In Vitro Model Study
2.4. Spectrophotometric Determinations of PraNa Concentration
2.5. Statistics
3. Results and Discussion
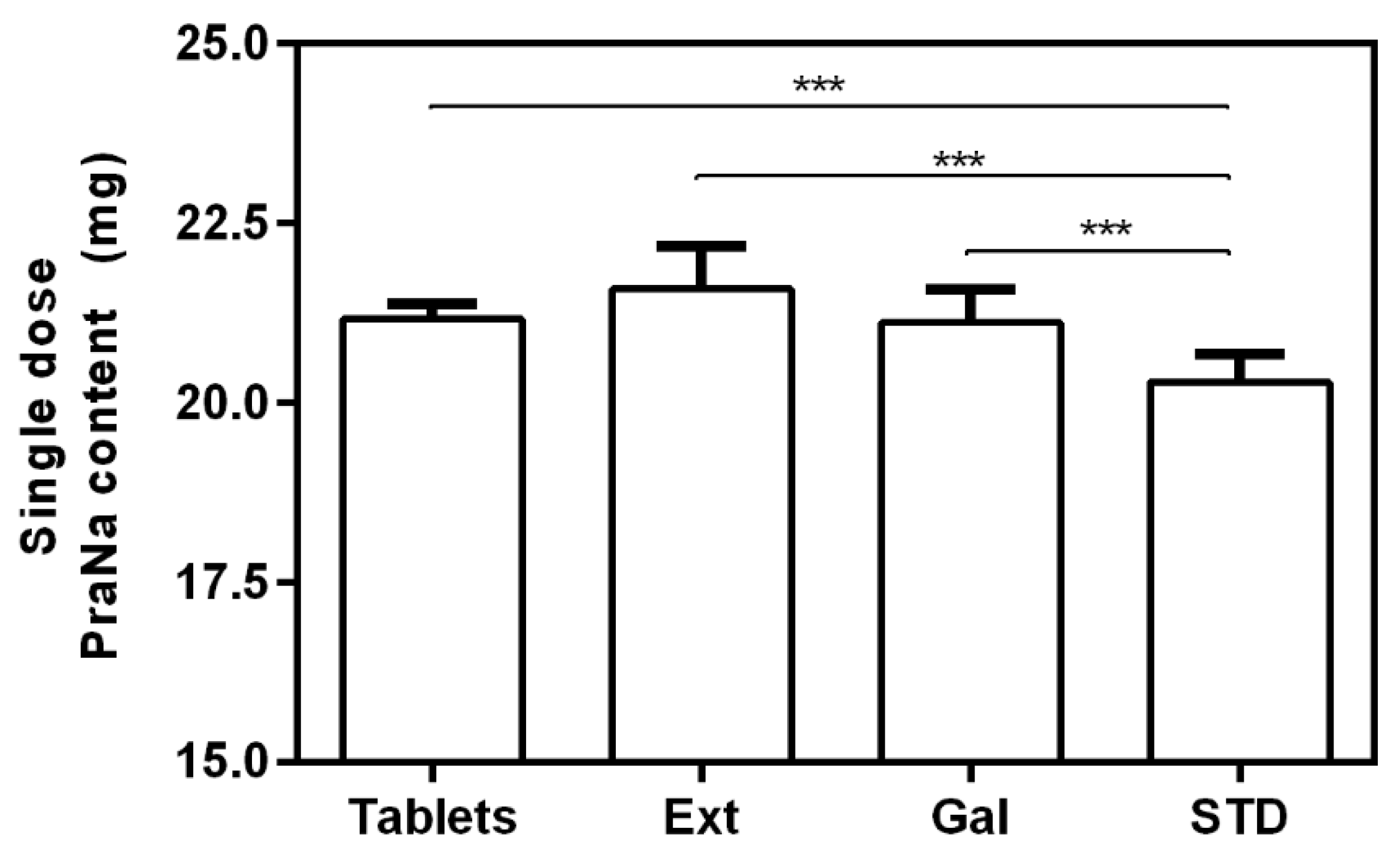
3.1. Effect of the Compounding on PraNa Administration
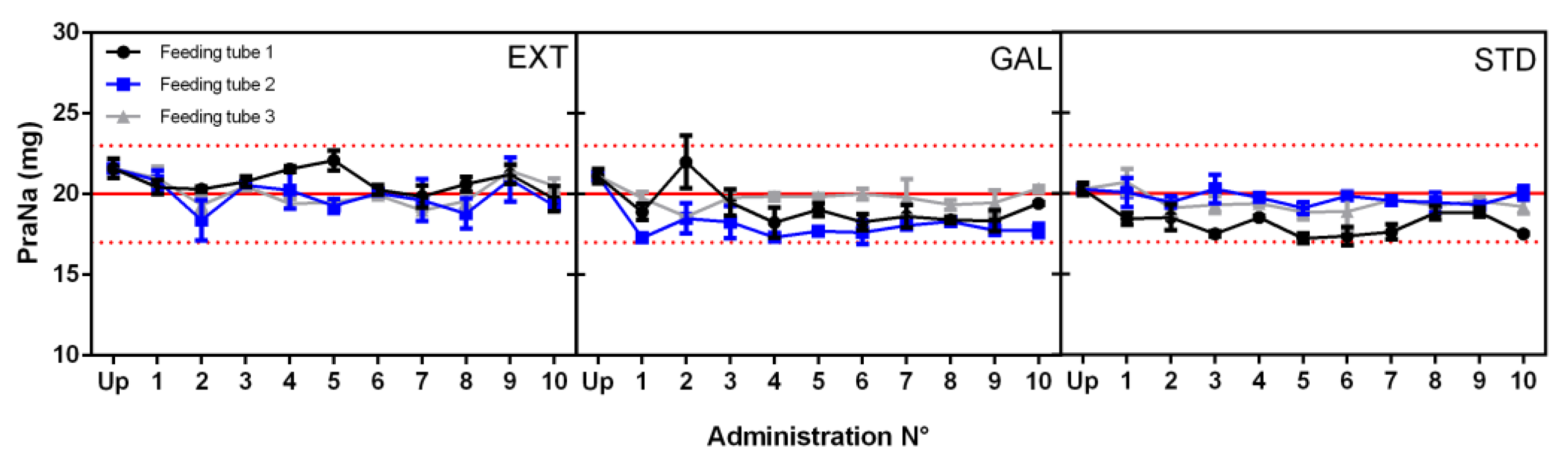
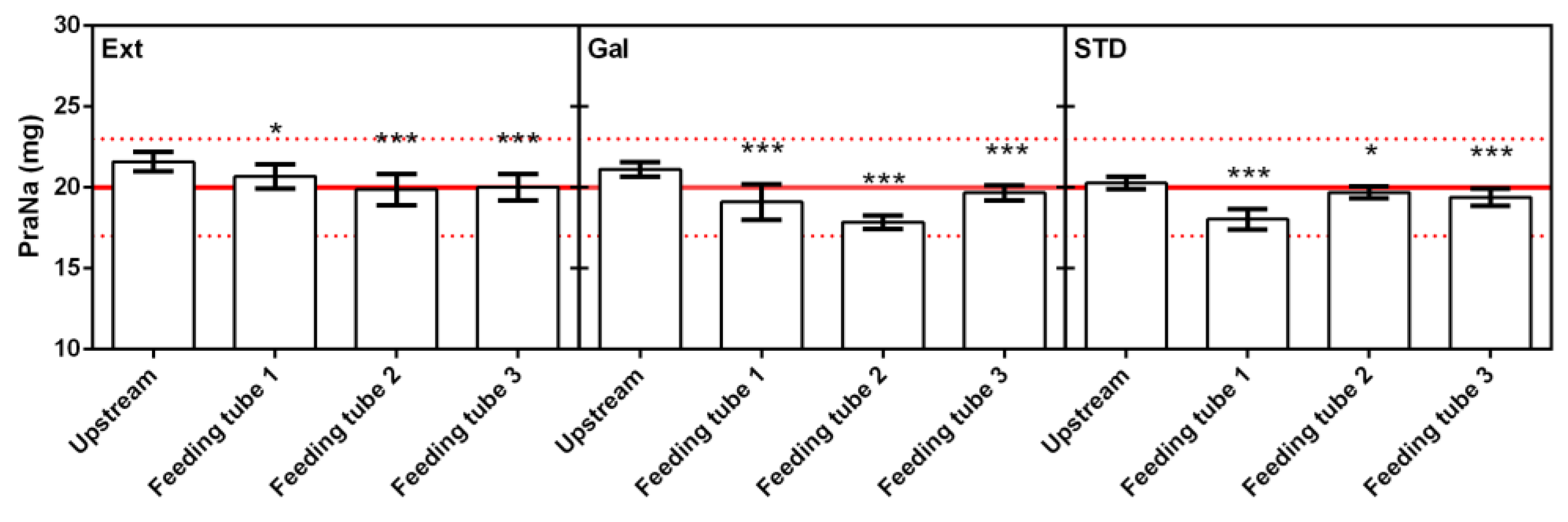
3.2. Nutritional Formulas’ Influence on PraNa Administration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beutin, N.; Schmidt, H. Current Trends and Strategic Options in the Pharma CDMO Market; PricewaterhouseCoopers GmbH Wirtschaftsprüfungsgesellschaft: Frankfurt, Germany, 2019. [Google Scholar]
- Stegemann, S.; Gosch, M.; Breitkreutz, J. Swallowing dysfunction and dysphagia is an unrecognized challenge for oral drug therapy. Int. J. Pharm. 2012, 430, 197–206. [Google Scholar] [CrossRef]
- Harnett, A.; Byrne, S.; O’Connor, J.; Lyons, D.; Sahm, L.J. Adult Patients with Difficulty Swallowing Oral Dosage Forms: A Systematic Review of the Quantitative Literature. Pharmacy 2023, 11, 167. [Google Scholar] [CrossRef]
- Lau, E.T.L.; Steadman, K.J.; Cichero, J.A.Y.; Nissen, L.M. Dosage form modification and oral drug delivery in older people. Adv. Drug Deliv. Rev. 2018, 135, 75–84. [Google Scholar] [CrossRef]
- Conroy, S.; Choonara, I.; Impicciatore, P.; Mohn, A.; Arnell, H.; Rane, A.; Knoeppel, C.; Seyberth, H.; Pandolfini, C.; Raffaelli, M.P.; et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. BMJ 2000, 320, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Logrippo, S.; Ricci, G.; Sestili, M.; Cespi, M.; Ferrara, L.; Palmieri, G.F.; Ganzetti, R.; Bonacucina, G.; Blasi, P. Oral drug therapy in elderly with dysphagia: Between a rock and a hard place! Clin. Interv. Aging 2017, 12, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Salmon, D.; Pont, E.; Chevallard, H.; Diouf, E.; Tall, M.-L.; Pivot, C.; Pirot, F. Pharmaceutical and safety considerations of tablet crushing in patients undergoing enteral intubation. Int. J. Pharm. 2013, 443, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-L.; Zhou, Q. Therapeutic concerns when oral medications are administered nasogastrically. J. Clin. Pharm. Ther. 2013, 38, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Sostek, M.B.; Chen, Y.; Skammer, W.; Winter, H.; Zhao, J.; Andersson, T. Esomeprazole administered through a nasogastric tube provides bioavailability similar to oral dosing. Aliment. Pharmacol. Ther. 2003, 18, 581–586. [Google Scholar] [CrossRef]
- Freston, J.W.; Kukulka, M.J.; Lloyd, E.; Lee, C. A novel option in proton pump inhibitor dosing: Lansoprazole orally disintegrating tablet dispersed in water and administered via nasogastric tube. Aliment. Pharmacol. Ther. 2004, 20, 407–411. [Google Scholar] [CrossRef]
- Kotake, T.; Takada, M.; Goto, T.; Komamura, K.; Kamakura, S.; Morishita, H. Serum amiodarone and desethylamiodarone concentrations following nasogastric versus oral administration. J. Clin. Pharm. Ther. 2006, 31, 237–243. [Google Scholar] [CrossRef]
- Clark-Schmidt, A.L.; Garnett, W.R.; Lowe, D.R.; Karnes, H.T. Loss of carbamazepine suspension through nasogastric feeding tubes. Am. J. Hosp. Pharm. 1990, 47, 2034–2037. [Google Scholar] [CrossRef] [PubMed]
- Cacek, A.T.; DeVito, J.M.; Koonce, J.R. In vitro evaluation of nasogastric administration methods for phenytoin. Am. J. Hosp. Pharm. 1986, 43, 689–692. [Google Scholar] [PubMed]
- Ruzsíková, A.; Součková, L.; Suk, P.; Opatřilová, R.; Kejdušová, M.; Šrámek, V. Quantitative analysis of drug losses administered via nasogastric tube—In vitro study. Int. J. Pharm. 2015, 478, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, R.N. Warfarin resistance and enteral tube feeding: A vitamin K-independent interaction. Nutrition 2008, 24, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, R.N.; Garmon, W.M.; Kuhl, D.A.; Minard, G.; Brown, R.O. Vitamin K-independent warfarin resistance after concurrent administration of warfarin and continuous enteral nutrition. Pharmacotherapy 2008, 28, 308–313. [Google Scholar] [CrossRef]
- Cooper, M.K.; Brock, D.G.; McDaniel, C.M. Interaction between levodopa and enteral nutrition. Ann. Pharmacother. 2008, 42, 439–442. [Google Scholar] [CrossRef]
- Heldt, T.; Loss, S.H. Drug-nutrient interactions in the intensive care unit: Literature review and current recommendations. Rev. Bras. Ter. Intensiv. 2013, 25, 162–167. [Google Scholar] [CrossRef]
- Sugiyama, M.; Takada, K.; Shinde, M.; Matsumoto, N.; Tanaka, K.; Kiriya, Y.; Nishimoto, E.; Kuzuya, M. National survey of the prevalence of swallowing difficulty and tube feeding use as well as implementation of swallowing evaluation in long-term care settings in Japan. Geriatr. Gerontol. Int. 2014, 14, 577–581. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Lai, J.-N.; Kung, W.-M.; Hung, C.-H.; Yip, H.-T.; Chang, Y.-C.; Wei, C.-Y. Nationwide Prevalence and Outcomes of Long-Term Nasogastric Tube Placement in Adults. Nutrients 2022, 14, 1748. [Google Scholar] [CrossRef]
- Italian Medicines Agency (AIFA). Banca Dati Farmaci. Available online: https://farmaci.agenziafarmaco.gov.it/bancadatifarmaci/cerca-per-principio-attivo?princ_att=PRAVASTATINA (accessed on 10 November 2023).
- Martin, K.; Gardner, G. Home Enteral Nutrition: Updates, Trends, and Challenges. Nutr. Clin. Pract. 2017, 32, 712–721. [Google Scholar] [CrossRef]
- Robertson, J.A.; De Monredon, F.D.; Dysseler, P.; Guillon, F.; Amadò, R.; Thibault, J.-F. Hydration properties of dietary fibre and resistant starch: A European collaborative study. LWT-Food Sci. Technol. 2000, 33, 72–79. [Google Scholar] [CrossRef]
- Deng, J.; Zhu, X.; Chen, Z.; Fan, C.H.; Kwan, H.S.; Wong, C.H.; Shek, K.Y.; Zuo, Z.; Lam, T.N. A Review of Food–Drug Interactions on Oral Drug Absorption. Drugs 2017, 77, 1833–1855. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.T. Medication administration through enteral feeding tubes. Am. J. Health-Syst. Pharm. 2008, 65, 2347–2357. [Google Scholar] [CrossRef] [PubMed]
- Bonacucina, G.; Logrippo, S.; Cespi, M.; Ganzetti, R.; Casettari, L.; Sestili, M.; Perinelli, D.R.; Ricciutelli, M.; Marziali, A.; Polidori, C.; et al. Chemical and microbiological stability studies of an aqueous solution of pravastatin sodium salt for drug therapy of the dysphagic patients. Eur. J. Hosp. Pharm. 2016, 23, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Mitsuru, E.; Yasushi, M. Gel Preparation for Oral Administration. US Patent 2008/0160087 A1, 3 July 2008. [Google Scholar]
- Pancorbo-Hidalgo, P.L.; García-Fernandez, F.P.; Ramírez-Pérez, C. Complications associated with enteral nutrition by nasogastric tube in an internal medicine unit. J. Clin. Nurs. 2001, 10, 482–490. [Google Scholar] [CrossRef]
- Rudmin, J.W. Calculating the Exact Pooled Variance. arXiv 2010, arXiv:1007.1012. [Google Scholar]
- Uniformity of mass of single-dose preparations. In European Pharmacopoeia 10 Edition 2.9.5; European Directorate for the Quality of Medicines: Strasbourg, France, 2020.



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logrippo, S.; Ganzetti, R.; Sestili, M.; Perinelli, D.R.; Cespi, M.; Bonacucina, G. Enteral Delivery of Pravastatin Sodium Tablets: Effect of Compounding into a Liquid Form and Co-Administration of Enteral Nutrition. Pharmacy 2024, 12, 32. https://doi.org/10.3390/pharmacy12010032
Logrippo S, Ganzetti R, Sestili M, Perinelli DR, Cespi M, Bonacucina G. Enteral Delivery of Pravastatin Sodium Tablets: Effect of Compounding into a Liquid Form and Co-Administration of Enteral Nutrition. Pharmacy. 2024; 12(1):32. https://doi.org/10.3390/pharmacy12010032
Chicago/Turabian StyleLogrippo, Serena, Roberta Ganzetti, Matteo Sestili, Diego Romano Perinelli, Marco Cespi, and Giulia Bonacucina. 2024. "Enteral Delivery of Pravastatin Sodium Tablets: Effect of Compounding into a Liquid Form and Co-Administration of Enteral Nutrition" Pharmacy 12, no. 1: 32. https://doi.org/10.3390/pharmacy12010032
APA StyleLogrippo, S., Ganzetti, R., Sestili, M., Perinelli, D. R., Cespi, M., & Bonacucina, G. (2024). Enteral Delivery of Pravastatin Sodium Tablets: Effect of Compounding into a Liquid Form and Co-Administration of Enteral Nutrition. Pharmacy, 12(1), 32. https://doi.org/10.3390/pharmacy12010032








