Notifications and Health Consequences of Unauthorized Pharmaceuticals in Food Supplements
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
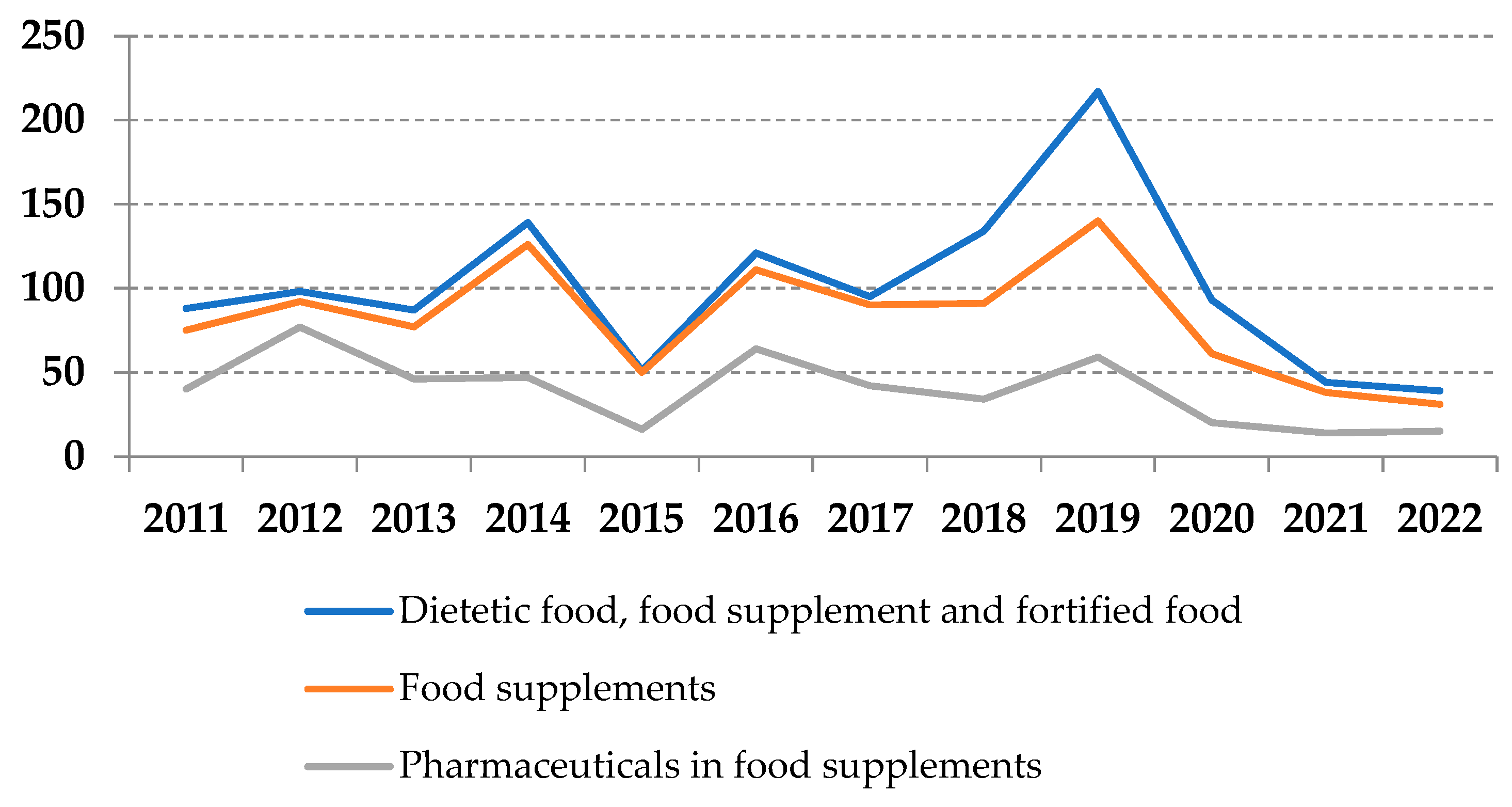
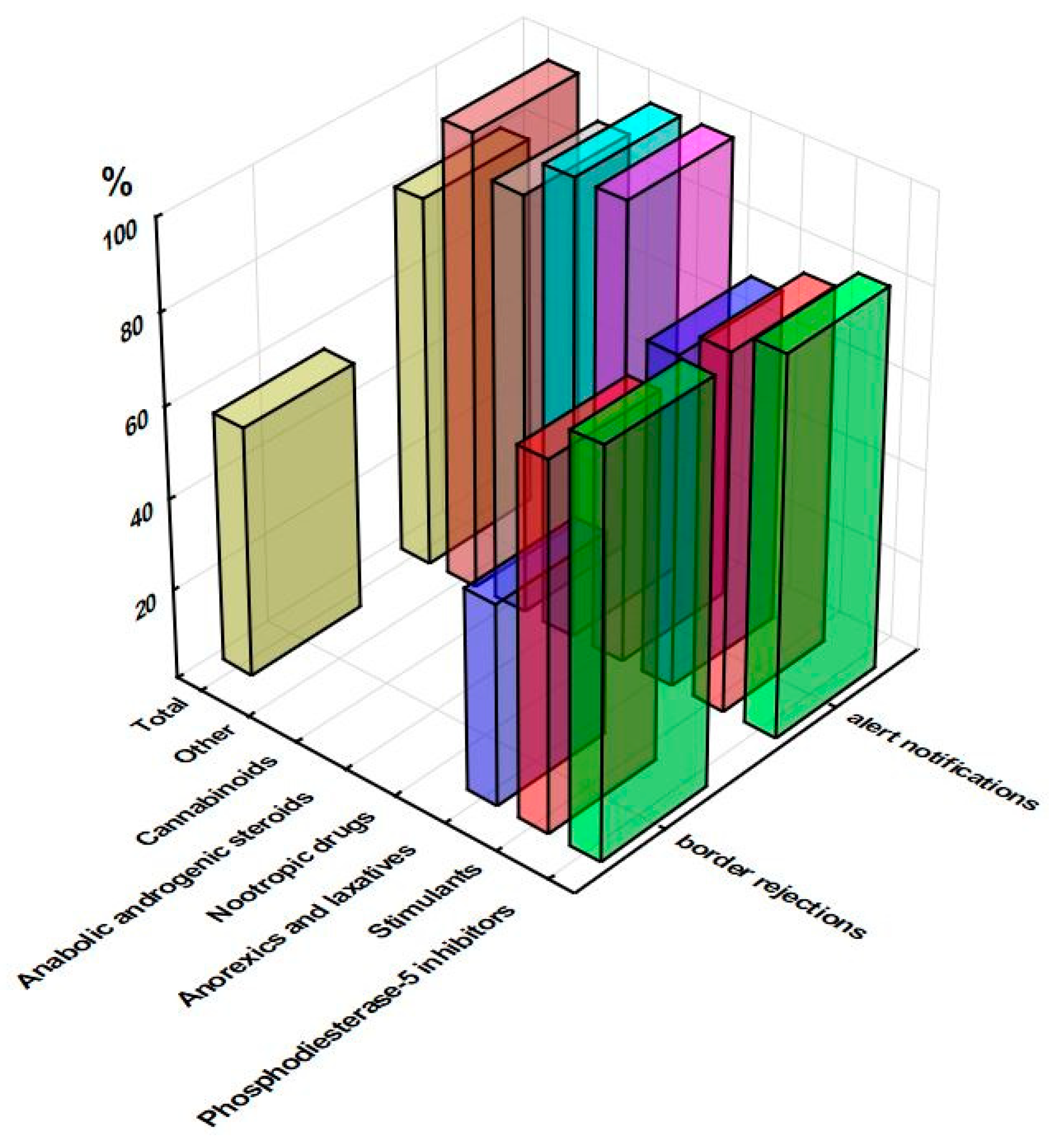
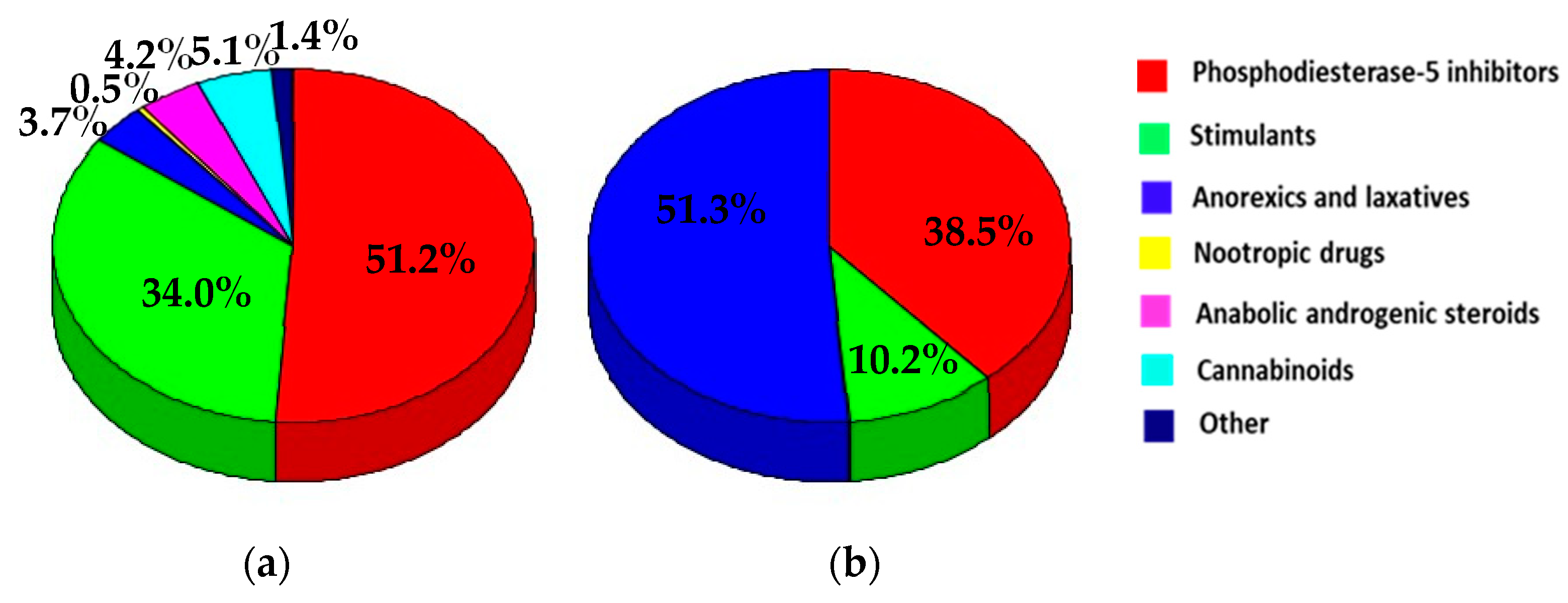
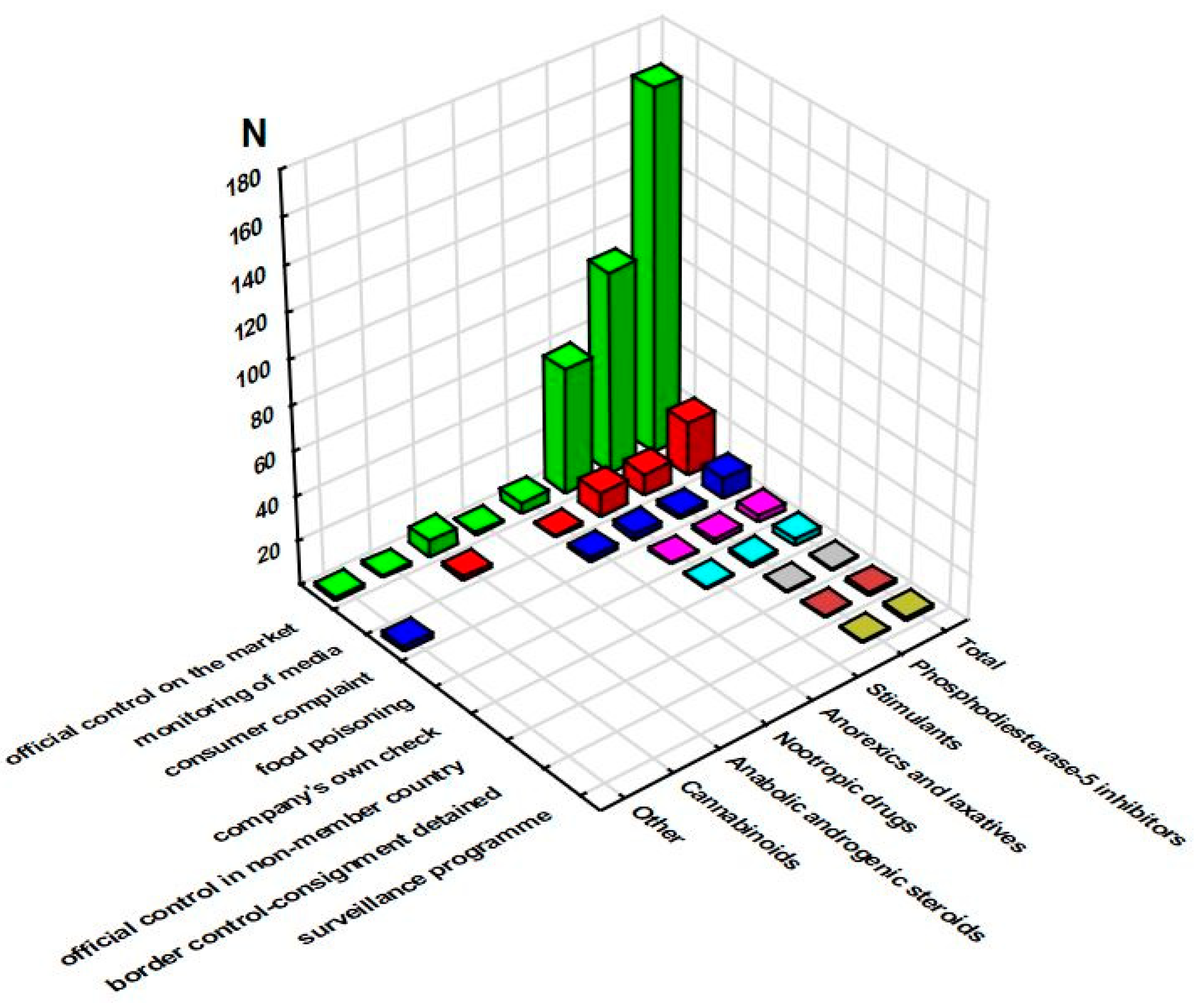
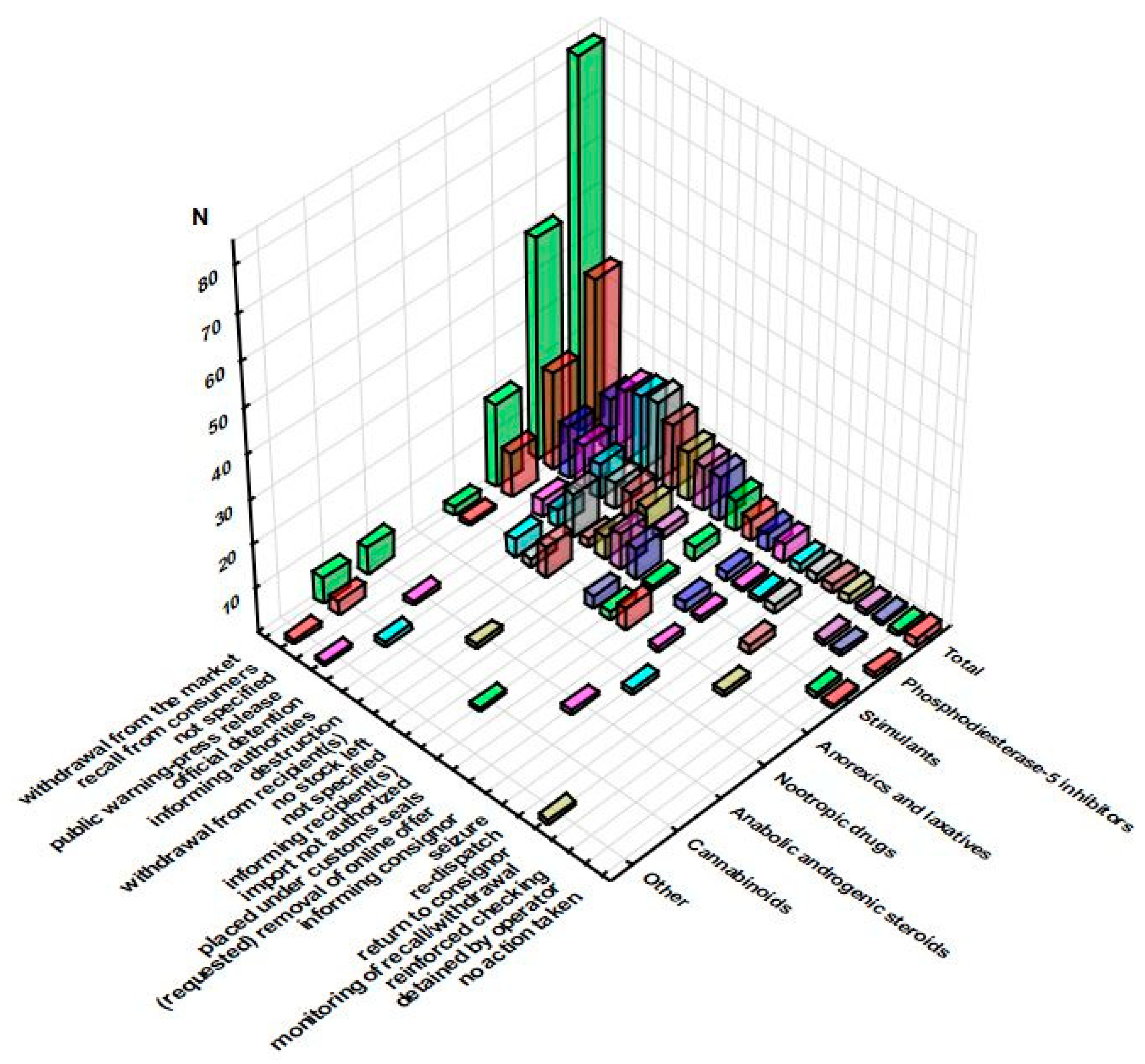
3.1. RASFF Notifications Related to Unathorized Pharmaceuticals in Food Supplements
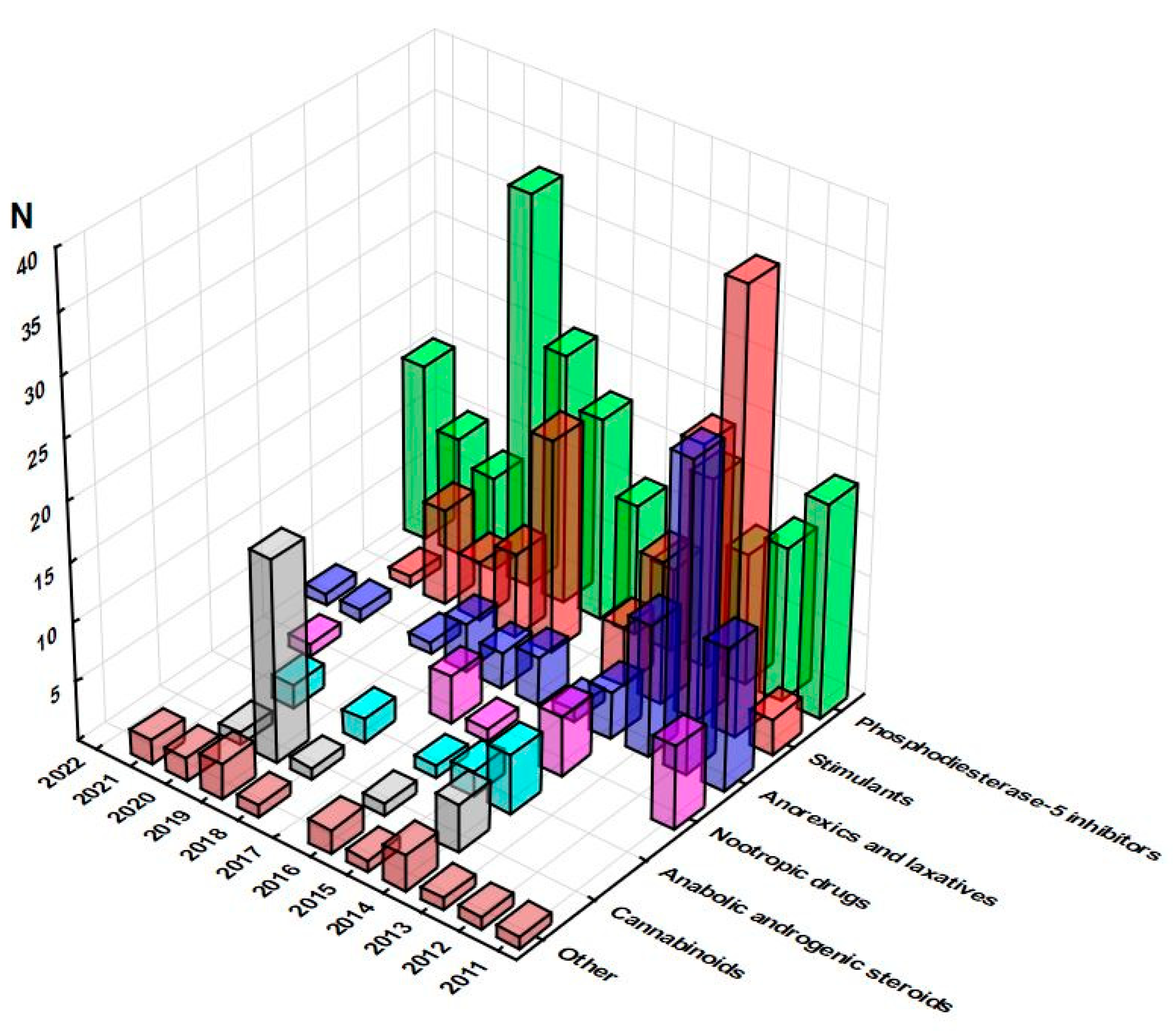
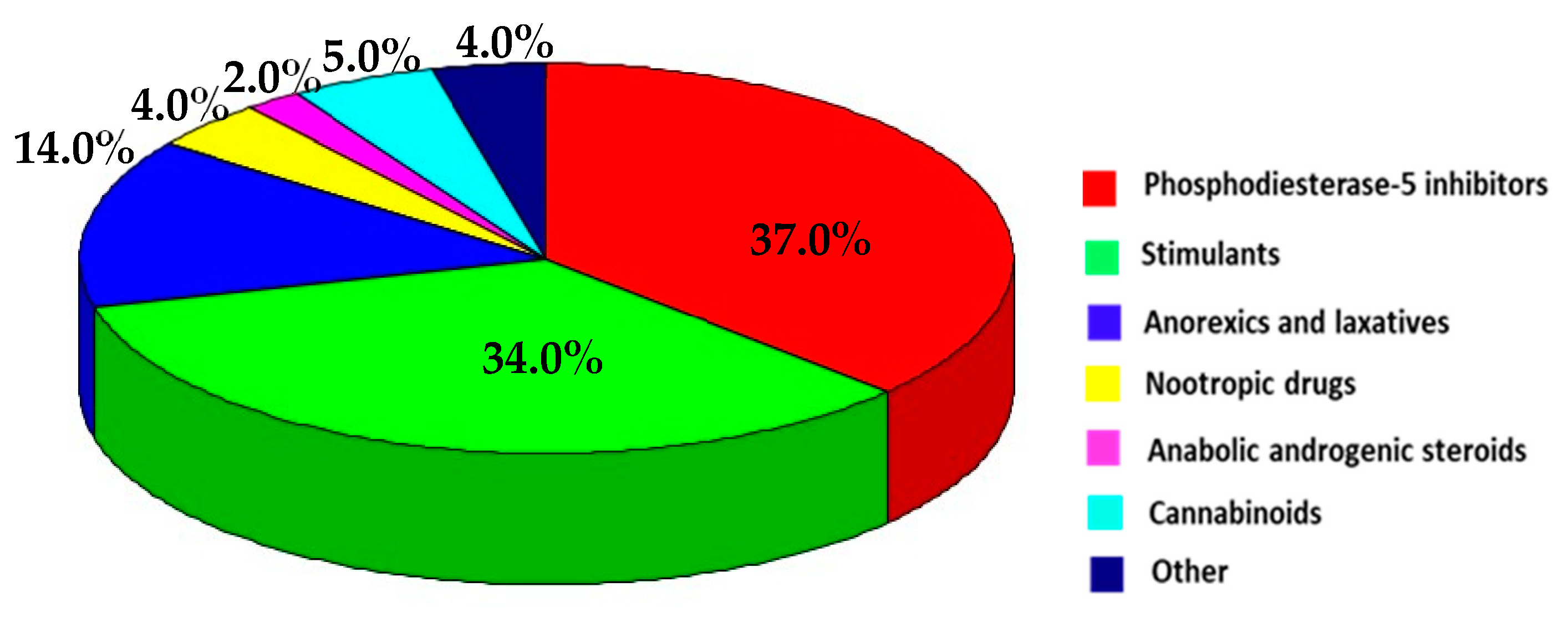
3.2. Pharmaceuticals Identified in Food Supplements
3.2.1. Phosphodiesterase-5 Inhibitors
3.2.2. Stimulants
3.2.3. Anorexics and Laxative
3.2.4. Nootropic Drugs
3.2.5. Anabolic Androgenic Steroids (AAS)
3.2.6. Cannabinoids
3.2.7. Unclassified Pharmaceuticals
3.3. Overall Considerations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission (EC). Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. Off. J. 2002, L183, 51–57. [Google Scholar]
- Zovko Končić, M. Getting More Than You Paid For: Unauthorized “Natural” Substances in Herbal Food Supplements on EU Market. Planta Med. 2012, 84, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Whitsitt, V.; Beehner, C.; Welch, C. The role of good manufacturing practices for preventing dietary supplement adulteration. Anal. Bioanal Chem. 2013, 40, 4353–4358. [Google Scholar] [CrossRef] [PubMed]
- Council for Responsible Nutrition. CRN Reveals Survey Data from 2022 Consumer Survey on Dietary Supplements. Available online: https://www.crnusa.org/newsroom/crn-reveals-survey-data-2022-consumer-survey-dietary-supplements (accessed on 23 March 2023).
- European Public Affairs. Consumer Survey on Food Supplements in the EU. Available online: https://foodsupplementseurope.org/wp-content/uploads/2022/07/FSE-Consumer_Survey-Ipsos-2022.pdf (accessed on 30 July 2023).
- Bjelica, A.; Aleksić, S.; Goločorbin-Kon, S.; Sazdanić, D.; Torović, L.; Cvejić, J. Internet Marketing of Cardioprotective Dietary Supplements. J. Altern. Complement. Med. 2020, 26, 204–211. [Google Scholar] [CrossRef]
- Fortune Business Insights, Europe Dietary Supplements Market Size, Trends|Analysis [2020–2027]. Market Report Research. Available online: https://www.fortunebusinessinsights.com/industry-reports/europe-dietary-supplements-market-101918 (accessed on 5 December 2021).
- Thakkar, S.; Anklam, E.; Xu, A.; Ulberth, F.; Li, Y.; Li, B.; Hugas, M.; Sarma, N.; Crerar, S.; Swift, S.; et al. Regulatory landscape of dietary supplements and herbal medicines from a global perspective. Reg. Toxicol. Pharmacol. 2020, 114, 104647. [Google Scholar] [CrossRef]
- European Commission (EC). Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off. J. 2002, L31, 1–24. [Google Scholar]
- US Food and Drug Administration (FDA). Dietary Supplements. Available online: https://www.fda.gov/food/dietary-supplements (accessed on 27 March 2023).
- Health Canada. Drugs and Health Products. MedEffect Canada. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada.html (accessed on 14 September 2023).
- Vo Van Regnault, G.; Costa, M.C.; Adanić Pajić, A.; Bico, A.P.; Bischofova, S.; Blaznik, U.; Menniti-Ippolito, F.; Pilegaard, K.; Rodrigues, C.; Margaritis, I. The need for European harmonization of Nutrivigilance in a public health perspective: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8230–8246. [Google Scholar] [CrossRef]
- Choi, J.Y.; Heo, S.; Yoo, G.J.; Park, S.K.; Yoon, C.Y.; Baek, S.Y. Development and validation of an LC-MS/MS method for the simultaneous analysis of 28 specific narcotic adulterants used in dietary supplements. Food Addit. Contam. Part A Chem. Anal. Control. Exp. Risk Assess. 2015, 32, 1029–1039. [Google Scholar] [CrossRef]
- Geller, A.I.; Shehab, N.; Weidle, N.J.; Lovegrove, M.C.; Wolpert, B.J.; Timbo, B.B.; Mozersky, R.P.; Budnitz, D.S. Emergency Department Visits for Adverse Events Related to Dietary Supplements. N. Engl. J. Med. 2015, 373, 1531–1540. [Google Scholar] [CrossRef]
- US Food and Drug Administration (FDA). Center for Food Safety and Applied Nutrition Adverse Event Reporting System. Available online: https://open.fda.gov/data/caers/ (accessed on 16 September 2023).
- Pigłowski, M. Food hazards on the European Union market: The data analysis of the Rapid Alert System for Food and Feed. Food Sci. Nutr. 2020, 8, 1603–1627. [Google Scholar] [CrossRef]
- Wróbel-Harmas, M.; Krysińska, M.; Postupolski, J.; Wysocki, M.J. Food supplement-related risks in the light of internet and RASFF data. Przegl. Epidemiol. 2014, 68, 613–619. [Google Scholar] [PubMed]
- Papapanagiotou, E.P. Serious Notifications on Food Contact Materials in the EU RASFF. Vet. Sci. 2021, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC). Commission implementing regulation (EU) 2019/1715 of 30 September 2019 laying down rules for the functioning of the information management system for official controls and its system components (the “IMSOC” regulation). Off. J. 2019, L261, 37–96. [Google Scholar]
- Kowalska, A.; Bieniek, M.; Manning, L. Food supplements’ non-conformity in Europe–Poland: A case study. Trends Food Sci. Technol. 2019, 93, 262–270. [Google Scholar] [CrossRef]
- Koncz, D.; Tóth, B.; Roza, O.; Csupor, D. A systematic review of the European Rapid Alert System for Food and Feed: Tendencies in illegal food supplements for weight loss. Front. Pharmacol. 2021, 11, 611361. [Google Scholar] [CrossRef] [PubMed]
- Rocha, T.; Amaral, J.S.; Oliveira, M.B.P.P. Adulteration of Dietary Supplements by the Illegal Addition of Synthetic Drugs: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 43–62. [Google Scholar] [CrossRef]
- Wróbel, K.; Milewska, A.J.; Marczak, M.; Kozłowski, R. Dietary Supplements Questioned in the Polish Notification Procedure upon the Basis of Data from the National Register of Functional Foods and the European System of the RASFF. Int. J. Environ. Res. Public Health 2022, 19, 8161. [Google Scholar] [CrossRef]
- Giannetti, L.; Gallo, V.; Necci, F.; Marini, F.; Giorgi, A.; Sonego, E.; D’Onofrio, F.; Neri, B. LC-HRMS analysis of 13 classes of pharmaceutical substances in food supplements. Food Addit. Contam. Part B 2023, 16, 253–265. [Google Scholar] [CrossRef]
- Jairoun, A.A.; Al-Hemyari, S.S.; Shahwan, M.; Zyoud, S.H. Adulteration of Weight Loss Supplements by the Illegal Addition of Synthetic Pharmaceuticals. Molecules 2021, 26, 6903. [Google Scholar] [CrossRef]
- European Commission Food Safety ACN Notifications. Available online: https://food.ec.europa.eu/safety/acn/acn-notifications_en (accessed on 12 April 2023).
- Czepielewska, E.; Makarewicz-Wujec, M.; Różewski, F.; Wojtasik, E.; Kozłowska-Wojciechowska, M. Drug adulteration of food supplements: A threat to public health in the European Union? Regul. Toxicol. Pharmacol. 2018, 97, 98–102. [Google Scholar] [CrossRef]
- Zucchi, A.; Costantini, E.; Scroppo, F.I.; Silvani, M.; Kopa, Z.; Illiano, E.; Petrillo, M.G.; Cari, L.; Nocentini, G. The first-generation phosphodiesterase 5 inhibitors and their pharmacokinetic issue. Andrology 2019, 7, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Graziano, S.; Montana, A.; Zaami, S.; Rotolo, M.C.; Minutillo, A.; Busardò, F.P.; Marinelli, E. Sildenafil-associated hepatoxicity: A review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2017, 21 (Suppl. 1), 17–22. [Google Scholar] [PubMed]
- European Medicines Agency. Human Medicine European Public Assessment Report (EPAR): Viagra. Available online: https://www.ema.europa.eu/en/documents/product-information/viagra-epar-product-information_en.pdf (accessed on 27 February 2023).
- Bechara, A.; Casabé, A.; De Bonis, W.; Helien, A.; Bertolino, M.V. Recreational use of phosphodiesterase type 5 inhibitors by healthy young men. J. Sex. Med. 2010, 7, 3736–3742. [Google Scholar] [CrossRef] [PubMed]
- White, C.M. Continued Risk of Dietary Supplements Adulterated With Approved and Unapproved Drugs: Assessment of the US Food and Drug Administration’s Tainted Supplements Database 2007 Through 2021. J. Clin. Pharmacol. 2022, 62, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Farzam, K.; Faizy, R.M.; Saadabadi, A. Stimulants; StatPearls [Internet]: Treasure Island, FL, USA, 2023. [Google Scholar]
- Stohs, S.J.; Badmaev, V. A Review of Natural Stimulant and Non-stimulant Thermogenic Agents. Phytother. Res. 2016, 30, 732–740. [Google Scholar] [CrossRef]
- Broadley, K.J. The vascular effects of trace amines and amphetamines. Pharmacol. Ther. 2010, 125, 363–375. [Google Scholar] [CrossRef]
- Bohn, A.M.; Khodaee, M.; Schwenk, T.L. Ephedrine and other stimulants as ergogenic aids. Curr. Sports Med. Rep. 2003, 2, 220–225. [Google Scholar] [CrossRef]
- Haller, C.A.; Benowitz, N.L. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N. Eng. J. Med. 2000, 343, 1833–1838. [Google Scholar] [CrossRef]
- European Commission (EC). Regulation (EU) 2015/403 of 11 March 2015 amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as regards Ephedra species and Yohimbe (Pausinystalia yohimbe (K. Schum) Pierre ex Beille). Off. J. 2015, L67, 4–5. [Google Scholar]
- Barker, W.D.; Antia, U. A study of the use of Ephedra in the manufacture of methamphetamine. Forensic Sci. Int. 2007, 166, 102–109. [Google Scholar] [CrossRef]
- Stohs, S.J.; Preuss, H.G.; Shara, M. A review of the human clinical studies involving Citrus aurantium (bitter orange) extract and its primary protoalkaloid p-synephrine. Int. J. Med. Sci. 2012, 9, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.A.; Ratamess, N.A.; Stohs, S.J.; Ellis, N.L.; Vought, I.T.; O’Grady, E.A.; Kuper, J.D.; Kang, J.; Faigenbaum, A.D. Acute hematological and mood perception effects of bitter orange extract (p-synephrine) consumed alone and in combination with caffeine: A placebo-controlled, double-blind study. Phytother. Res. 2018, 32, 1593–1607. [Google Scholar] [CrossRef] [PubMed]
- Ratamess, N.A.; Bush, J.A.; Kang, J.; Kraemer, W.J.; Stohs, S.J.; Nocera, V.G.; Leise, M.D.; Diamond, K.B.; Campbell, S.C.; Miller, H.B.; et al. The Effects of Supplementation with p-Synephrine Alone and in Combination with Caffeine on Metabolic, Lipolytic, and Cardiovascular Responses during Resistance Exercise. J. Am. Coll. Nutr. 2016, 35, 657–669. [Google Scholar] [CrossRef]
- de Jonge, M.L.L.; Kieviet, L.C.; Sierts, M.; Egberink, L.B.; van der Heyden, M.A.G. Review of Case Reports on Adverse Events Related to Pre-workout Supplements Containing Synephrine. Cardiovasc. Toxicol. 2023, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, S.C. Martindale: The Complete Drug Reference: The Complete Drug Reference, 36th ed.; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- World Anti-Doping Agency. World Anti-Doping Code International Standard Prohibited List 2023. Available online: https://www.wada-ama.org/sites/default/files/2022-09/2023list_en_final_9_september_2022.pdf (accessed on 13 March 2023).
- Cohen, P.A.; Avula, B.; Venhuis, B.; Travis, J.C.; Wang, Y.H.; Khan, I.A. Pharmaceutical doses of the banned stimulant oxilofrine found in dietary supplements sold in the USA. Drug Test. Anal. 2017, 9, 135–142. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, M.; Avula, B.; Khan, I.A. Detection and quantification of phenethylamines in sports dietary supplements by NMR approach. J. Pharm. Biomed. Anal. 2018, 151, 347–355. [Google Scholar] [CrossRef]
- Stohs, S.J. Physiological functions and pharmacological and toxicological effects of p-octopamine. Drug Chem. Toxicol. 2015, 38, 106–112. [Google Scholar] [CrossRef]
- Kesari, A.N.; Gupta, R.K.; Singh, S.K.; Diwakar, S.; Watal, G. Hypoglycemic and antihyperglycemic activity of Aegle marmelos seed extract in normal and diabetic rats. J. Ethnopharmacol. 2006, 107, 374–379. [Google Scholar] [CrossRef]
- Singh, A.; Srinivasan, A.K.; Chakrapani, L.N.; Kalaiselvi, P. LOX-1, the Common Therapeutic Target in Hypercholesterolemia: A New Perspective of Antiatherosclerotic Action of Aegeline. Oxid. Med. Cell. Longev. 2019, 2019, 8285730. [Google Scholar] [CrossRef]
- Heidemann, L.A.; Navarro, V.J.; Ahmad, J.; Hayashi, P.H.; Stolz, A.; Kleiner, D.E.; Fontana, R.J. Severe Acute Hepatocellular Injury Attributed to OxyELITE Pro: A Case Series. Dig. Dis. Sci. 2016, 61, 2741–2748. [Google Scholar] [CrossRef]
- Pawar, R.S.; Grundel, E. Overview of regulation of dietary supplements in the USA and issues of adulteration with phenethylamines (PEAs). Drug Test. Anal. 2017, 9, 500–517. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Zhou, S.; Li, W.; Li, J.; Yang, L.; Peng, Y.; Zheng, J. Metabolic activation of aegeline mediated by CYP2C19. Xenobiotica 2021, 51, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A.; Avula, B.; Katragunta, K.; Khan, I. Recalls, Availability, and Content of Dietary Supplements Following FDA Warning Letters. JAMA 2022, 328, 393–395. [Google Scholar] [CrossRef]
- Chołbiński, P.; Wicka, M.; Kowalczyk, K.; Jarek, A.; Kaliszewski, P.; Pokrywka, A.; Bulska, E.; Kwiatkowska, D. Detection of β-methylphenethylamine, a novel doping substance, by means of UPLC/MS/MS. Anal. Bioanal. Chem. 2014, 406, 3681–3688. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cohen, P.A.; Bloszies, C.; Yee, C.; Gerona, R. An amphetamine isomer whose efficacy and safety in humans has never been studied, β-methylphenylethylamine (BMPEA), is found in multiple dietary supplements. Drug Test. Anal. 2016, 8, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Venhuis, B.; Keizers, P.; van Riel, A.; de Kaste, D. A cocktail of synthetic stimulants found in a dietary supplement associated with serious adverse events. Drug Test. Anal. 2014, 6, 578–581. [Google Scholar] [CrossRef]
- Cohen, P.A.; Zeijlon, R.; Nardin, R.; Keizers, P.H.; Venhuis, B. Hemorrhagic Stroke Probably Caused by Exercise Combined With a Sports Supplement Containing β-Methylphenyl-ethylamine (BMPEA): A Case Report. Ann. Intern. Med. 2015, 162, 879–880. [Google Scholar] [CrossRef]
- Schindler, C.W.; Thorndike, E.B.; Rice, K.C.; Partilla, J.S.; Baumann, M.H. The Supplement Adulterant β-Methylphenethylamine Increases Blood Pressure by Acting at Peripheral Norepinephrine Transporters. J. Pharmacol. Exp. Ther. 2019, 369, 328–336. [Google Scholar] [CrossRef]
- US Department of Health & Human Services, National Institutes of Health, Inxight Drugs—Deterenol. Available online: https://drugs.ncats.io/drug/BR971OUC9M (accessed on 29 January 2023).
- Mercader, J.; Wanecq, E.; Chen, J.; Carpéné, C. Isopropylnorsynephrine is a stronger lipolytic agent in human adipocytes than synephrine and other amines present in Citrus aurantium. J. Physiol. Biochem. 2011, 67, 443–452. [Google Scholar] [CrossRef]
- Pawar, R.S.; Sagi, S.; Leontyev, D. Analysis of bitter orange dietary supplements for natural and synthetic phenethylamines by LC-MS/MS. Drug Test. Anal. 2020, 12, 1241–1251. [Google Scholar] [CrossRef]
- Cohen, P.A.; Travis, J.C.; Vanhee, C.; Ohana, D.; Venhuis, B.J. Nine prohibited stimulants found in sports and weight loss supplements: Deterenol, phenpromethamine (Vonedrine), oxilofrine, octodrine, beta-methylphenylethylamine (BMPEA), 1,3-dimethylamylamine (1,3-DMAA), 1,4-dimethylamylamine (1,4-DMAA), 1,3-dimethylbutylamine (1,3-DMBA) and higenamine. Clin. Toxicol. (Phila) 2021, 59, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Bovee, T.F.; Mol, H.G.; Bienenmann-Ploum, M.E.; Heskamp, H.H.; Van Bruchem, G.D.; Van Ginkel, L.A.; Kooijman, M.; Lasaroms, J.J.; Van Dam, R.; Hoogenboom, R.L. Dietary supplement for energy and reduced appetite containing the β-agonist isopropyloctopamine leads to heart problems and hospitalisations. Food Addit. Contam. Part A Chem. Anal. Control Exp. Risk Assess. 2016, 33, 749–759. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction. Amphetamine Drug Profile. Available online: https://www.emcdda.europa.eu/publications/drug-profiles/amphetamine_en (accessed on 4 April 2023).
- Heal, D.J.; Smith, S.L.; Gosden, J.; Nutt, D.J. Amphetamine, past and present—A pharmacological and clinical perspective. J. Psychopharmacol. 2013, 27, 479–496. [Google Scholar] [CrossRef]
- Khoramizadeh, M.; Effatpanah, M.; Mostaghimi, A.; Rezaei, M.; Mahjoub, A.; Shishehgar, S. Treatment of amphetamine abuse/use disorder: A systematic review of a recent health concern. Daru 2019, 27, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Perez-Downes, J.; Hritani, A.; Baldeo, C.; Antoun, P. Amphetamine Containing Dietary Supplements and Acute Myocardial Infarction. Case Rep. Cardiol. 2016, 2016, 6404856. [Google Scholar] [CrossRef]
- Cohen, P.A.; Travis, J.C.; Keizers, P.H.J.; Deuster, P.; Venhuis, B.J. Four experimental stimulants found in sports and weight loss supplements: 2-amino-6-methylheptane (octodrine), 1,4-dimethylamylamine (1,4-DMAA), 1,3-dimethylamylamine (1,3-DMAA) and 1,3-dimethylbutylamine (1,3-DMBA). Clin. Toxicol. (Phila) 2018, 56, 421–426. [Google Scholar] [CrossRef]
- Gee, P.; Tallon, C.; Long, N.; Moore, G.; Boet, R.; Jackson, S. Use of recreational drug 1,3 Dimethylamylamine (DMAA) [corrected] associated with cerebral hemorrhage. Ann. Emerg. Med. 2012, 60, 431–434. [Google Scholar] [CrossRef]
- Biesterbos, J.W.H.; Sijm, D.T.H.M.; van Dam, R.; Mol, H.G.J. A health risk for consumers: The presence of adulterated food supplements in the Netherlands. Food Addit. Contam. Part A Chem. Anal. Control. Exp. Risk Assess. 2019, 36, 1273–1288. [Google Scholar] [CrossRef]
- Karnatovskaia, L.V.; Leoni, J.C.; Freeman, M.L. Cardiac arrest in a 21-year-old man after ingestion of 1,3-DMAA-containing workout supplement. Clin. J. Sport Med. 2015, 25, e23–e25. [Google Scholar] [CrossRef]
- Dunn, M. Have prohibition policies made the wrong decision? A critical review of studies investigating the effects of DMAA. Int. J. Drug Policy 2017, 40, 26–34. [Google Scholar] [CrossRef]
- Catalani, V.; Prilutskaya, M.; Al-Imam, A.; Marrinan, S.; Elgharably, Y.; Zloh, M.; Martinotti, G.; Chilcott, R.; Corazza, O. Octodrine: New Questions and Challenges in Sport Supplements. Brain Sci. 2018, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight Fact Sheets. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 23 February 2023).
- European Medicines Agency. European Medicines Agency Updates on Ongoing Safety Review of Sibutramine. Available online: https://www.ema.europa.eu/en/documents/press-release/european-medicines-agency-updates-ongoing-safety-review-sibutramine-weight-loss-medicine-assessed_en.pdf (accessed on 27 February 2023).
- Nathan, P.J.; O’Neill, B.V.; Napolitano, A.; Bullmore, E.T. Neuropsychiatric adverse effects of centrally acting antiobesity drugs. CNS Neurosci. Ther. 2011, 17, 490–505. [Google Scholar] [CrossRef] [PubMed]
- Stypułkowska, K.; Błażewicz, A.; Maurin, J.; Sarna, K.; Fijałek, Z. X-ray powder diffractometry and liquid chromatography studies of sibutramine and its analogues content in herbal dietary supplements. J. Pharm. Biomed. Anal. 2011, 56, 969–975. [Google Scholar] [CrossRef]
- Costa, J.G.; Vidovic, B.; Saraiva, N.; do Céu Costa, M.; Del Favero, G.; Marko, D.; Oliveira, N.G.; Fernandes, A.S. Contaminants: A dark side of food supplements? Free Radic. Res. 2019, 53 (Suppl. 1), 1113–1135. [Google Scholar] [CrossRef]
- Haslam, D. Weight management in obesity—Past and present. Int. J. Clin. Pract. 2016, 70, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Grundlingh, J.; Dargan, P.I.; El-Zanfaly, M.; Wood, D.M. 2,4-dinitrophenol (DNP): A weight loss agent with significant acute toxicity and risk of death. J. Med. Toxicol. 2011, 7, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Li, J.D.; Yan, C. An update on vinpocetine: New discoveries and clinical implications. Eur. J. Pharmacol. 2018, 819, 30–34. [Google Scholar] [CrossRef]
- Panda, P.K.; Ramachandran, A.; Panda, P.; Sharawat, I.K. Safety and Efficacy of Vinpocetine as a Neuroprotective Agent in Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Neurocrit. Care 2022, 37, 314–325. [Google Scholar] [CrossRef]
- Fink, J.; Schoenfeld, B.J.; Hackney, A.C.; Matsumoto, M.; Maekawa, T.; Nakazato, K.; Horie, S. Anabolic-androgenic steroids: Procurement and administration practices of doping athletes. Phys. Sportsmed. 2019, 47, 10–14. [Google Scholar] [CrossRef]
- Havnes, I.A.; Jørstad, M.L.; Wisløff, C. Anabolic-androgenic steroid users receiving health-related information; health problems, motivations to quit and treatment desires. Subst. Abuse Treat. Prev. Policy 2019, 14, 20. [Google Scholar] [CrossRef]
- Duiven, E.; van Loon, L.J.C.; Spruijt, L.; Koert, W.; de Hon, O.M. Undeclared Doping Substances are Highly Prevalent in Commercial Sports Nutrition Supplements. J. Sports Sci. Med. 2021, 20, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Carrasquillo, R.; Chu, K.; Ramasamy, R. Novel Therapy for Male Hypogonadism. Curr. Urol. Rep. 2018, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Rahnema, C.D.; Crosnoe, L.E.; Kim, E.D. Designer steroids—Over-the-counter supplements and their androgenic component: Review of an increasing problem. Andrology 2015, 3, 150–155. [Google Scholar] [CrossRef] [PubMed]
- van Amsterdam, J.; Opperhuizen, A.; Hartgens, F. Adverse health effects of anabolic-androgenic steroids. Regul. Toxicol. Pharmacol. 2010, 57, 117–123. [Google Scholar] [CrossRef]
- Torrisi, M.; Pennisi, G.; Russo, I.; Amico, F.; Esposito, M.; Liberto, A.; Cocimano, G.; Salerno, M.; Li Rosi, G.; Di Nunno, N.; et al. Sudden Cardiac Death in Anabolic-Androgenic Steroid Users: A Literature Review. Medicina (Kaunas) 2020, 56, 587. [Google Scholar] [CrossRef]
- Patanè, F.G.; Liberto, A.; Maria Maglitto, A.N.; Malandrino, P.; Esposito, M.; Amico, F.; Cocimano, G.; Rosi, G.L.; Condorelli, D.; Nunno, N.D.; et al. Nandrolone Decanoate: Use, Abuse and Side Effects. Medicina (Kaunas) 2020, 56, 606. [Google Scholar] [CrossRef]
- Rabijewski, M.; Papierska, L.; Binkowska, M.; Maksym, R.; Jankowska, K.; Skrzypulec-Plinta, W.; Zgliczynski, W. Supplementation of dehydroepiandrosterone (DHEA) in pre- and postmenopausal women—Position statement of expert panel of Polish Menopause and Andropause Society. Ginekol. Pol. 2020, 91, 554–562. [Google Scholar] [CrossRef]
- Wierman, M.E.; Kiseljak-Vassiliades, K. Should Dehydroepiandrosterone Be Administered to Women? J. Clin. Endocrinol. Metab. 2022, 107, 1679–1685. [Google Scholar] [CrossRef]
- Maughan, R.J. Contamination of dietary supplements and positive drug tests in sport. J. Sports Sci. 2005, 23, 883–889. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. Cannabis Legislation in Europe: An Overview; Office for Official Publications of the European Communities: Luxembourg, 2017. [Google Scholar]
- Busse, J.W.; Wang, L.; Kamaleldin, M.; Craigie, S.; Riva, J.J.; Montoya, L.; Mulla, S.M.; Lopes, L.C.; Vogel, N.; Chen, E.; et al. Opioids for Chronic Noncancer Pain: A Systematic Review and Meta-analysis. JAMA 2018, 320, 2448–2460. [Google Scholar] [CrossRef]
- Mücke, M.; Phillips, T.; Radbruch, L.; Petzke, F.; Häuser, W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2018, 3, CD012182. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, P.; Elsohly, M.; Hill, K.P. Cannabidiol Interactions with Medications, Illicit Substances, and Alcohol: A Comprehensive Review. J. Gen. Intern. Med. 2021, 36, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Damkier, P.; Lassen, D.; Christensen, M.M.H.; Madsen, K.G.; Hellfritzsch, M.; Pottegård, A. Interaction between warfarin and cannabis. Basic Clin. Pharmacol. Toxicol. 2019, 124, 28–31. [Google Scholar] [CrossRef]
- Jacobsen, J.P.R.; Krystal, A.D.; Krishnan, K.R.R.; Caron, M.G. Adjunctive 5-Hydroxytryptophan Slow-Release for Treatment-Resistant Depression: Clinical and Preclinical Rationale. Trends Pharmacol. Sci. 2016, 37, 933–944. [Google Scholar] [CrossRef]
- Erland, L.A.; Saxena, P.K. Melatonin Natural Health Products and Supplements: Presence of Serotonin and Significant Variability of Melatonin Content. J. Clin. Sleep Med. 2017, 13, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. Int. J. Mol. Sci. 2020, 22, 181. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (EFSA NDA Panel); Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of betaine as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05658. [Google Scholar] [CrossRef]
- Levy, I.; Attias, S.; Ben-Arye, E.; Schiff, E. Use and safety of dietary and herbal supplements among hospitalized patients: What have we learned and what can be learned?—A narrative review. Eur. J. Integr. Med. 2017, 16, 39–45. [Google Scholar] [CrossRef]
- US Food and Drug Administration (FDA). Tainted Products Marketed as Dietary Supplements_CDER. Available online: https://www.accessdata.fda.gov/scripts/sda/sdnavigation.cfm?filter=&sortColumn=1d&sd=tainted_supplements_cder&page=1 (accessed on 4 January 2023).
- US Food and Drug Administration (FDA). Medication Health Fraud. Available online: https://www.fda.gov/drugs/buying-using-medicine-safely/medication-health-fraud (accessed on 4 January 2023).
- Bandara, S.B.; Urban, A.; Liang, L.G.; Parker, J.; Fung, E.; Maier, A. Active pharmaceutical contaminants in dietary supplements: A tier-based risk assessment approach. Regul. Toxicol. Pharmacol. 2021, 123, 104955. [Google Scholar] [CrossRef]
- White, C.M. Dietary supplements pose real dangers to patients. Ann. Pharmacother. 2020, 54, 815–819. [Google Scholar] [CrossRef]
- Kowalska, A.; Manning, L. Using the rapid alert system for food and feed: Potential benefits and problems on data interpretation. Crit. Rev. Food Sci. Nutr. 2021, 61, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Weesepoel, Y.; Erasmus, S.; Sinkeldam, J.; Piccinelli, A.L.; van Ruth, S. A multi-analyte screening method for the rapid detection of illicit adulterants in dietary supplements using a portable SERS analyzer. Helyon 2023, 9, 18509. [Google Scholar] [CrossRef] [PubMed]

| Unauthorized Substance | Trade-Name 1 | Indications/Use | N |
|---|---|---|---|
| Phosphodiesterase-5 inhibitors (PDE-i) | |||
| Sildenafil | Viagra | Erectile dysfunction, pulmonary arterial hypertension | 99 |
| -sildenafil thiono analogue | 46 | ||
| -sildenafil analogue | 2 | ||
| -dimethylsildenafil | 7 | ||
| -nor-acetildenafil | 1 | ||
| Tadalafil | Cialis | Erectile dysfunction | 63 |
| -desmethyl tadalafil | 1 | ||
| -nortadalafil | 3 | ||
| Vardenafil | Levitra | Erectile dysfunction | 11 |
| -pseudovardenafil | 1 | ||
| Avanafil | Spedra | Erectile dysfunction | 1 |
| Stimulants | |||
| Natural phenylethylamines (PEA) | |||
| Ephedrine, Norephedrine, Ephedra | Efedrosan, Neo Rhinovit, Rhinolex, Kondon’s Nasal | Sympathomimetic relief in nasal congestion, bronchodilatation in asthma therapy | 5 |
| Synephrine | Neo-Synephrine, Ocu-Phrin, Visadron | Decongestant, mydriatic, vasopressor agent in surgical procedures | 53 |
| Octopamine | Norphen | Hypotension therapy and cardiotonic | 4 |
| Aegeline | Oxyelite Pro, VERSA-1 | Designer stimulant | 1 |
| Synthetic phenylethylamines (PEA) | |||
| Oxilofrine | Carnigen | Hypotension therapy, antitussive preparations | 8 |
| β-Methylphenylethylamine (BMPEA) | Dexaprine, Jacket Power, MM Sports | Designer stimulant | 6 |
| Isopropyloctopamine | Dexaprine | Designer stimulant | 3 |
| α-Methylphenylethylamine (amphetamine) | Adderall | Therapy of ADHD and other neurological disorders | 1 |
| Alkylamines | |||
| 1,3-Dimethylamine (DMAA) | OxyElite Pro, Jack3d, Napalm | Designer stimulant | 97 |
| 2-Amino-6-methylheptane (DMHA, octodrine) | Ambredin | Designer stimulant | 6 |
| Anorexics and laxatives | |||
| Sibutramine | Reductil, Reduxade, Zelium | Obesity | 45 |
| N-Didesmethyl sibutramine | - | - | 7 |
| Phenolphthalein | Alin, Laksafenol, Fam-Lax, Agoral | Constipation | 18 |
| 2,4-Dinitrophenol (DNP) | - | Obesity | 6 |
| Nootropic drugs | |||
| Vinpocetine | Cavinton | Cerebrovascular disorders, mental function impairment | 18 |
| Vincamine | Cerebroxine, Vincapan | 3 | |
| Vinburnine | Cervoxan | 3 | |
| Anabolic androgenic steroids (AAS) | |||
| Androstenedione | Metharmon-F | Hormone replacement therapy | 6 |
| Stanozolol | Winstrol | Hereditary angioedema, vascular disorders | 2 |
| Dehydroepiandrosterone (DHEA) | Biosteron | Hormone replacement therapy | 2 |
| Testosterone | Andriol, Androgel | Testosterone replacement therapy | 1 |
| Methasterone | - | Designer ASS | 1 |
| 4,6-Androstadien-17-ol-3-one | - | Designer AAS | 1 |
| 4,6-Androstadien-3,17-dione | - | Designer AAS | 1 |
| 5-Androsten-17-ol-3-one | - | Designer AAS | 1 |
| 4,9-Estradien-3,17-dione | - | Designer AAS | 1 |
| Cannabinoids | |||
| Delta-9-tetrahydrocannabinol (THC) | Marinol, Cesamet | Cancer treatment, AIDS, multiple sclerosis | 23 |
| Cannabidiol (CBD) | Epydiolex | Lennox–Gastaut syndrome, Dravet syndrome | 14 |
| THC + CBD | Sativex | Multiple sclerosis | 13 |
| Others (Unclassified pharmaceuticals) | |||
| 5-HTP | Levotonine, Trip-OH | Depression, neurological disorders | 7 |
| Betaine | Cystadane | Homocystinuria | 7 |
| Zopiclone | Zimovane | Hypnotic, Insomnia | 1 |
| Ciprofloxacin | Ciproxin, Ciloxan, Cetraxal | Antibiotic | 1 |
| Unauthorized Substance | Number of Samples | Concentration | Dosage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conc. Not Available/Quantified | “Suspected” | “Detected” | mg/Item | mg/cps | mg/dosage | mg/kg | mg/kg Dry Matter | mg/100 mL | mg | % | mg/Day | |
| Phosphodiesterase-5 inhibitors | ||||||||||||
| Sildenafil | 45/44 | 10 | >7–146.3 | 0.318–104.7 | 36.2–58.34 | 2.6–244,000 | 72,650–349,820 | 2.8 | ||||
| Sildenafil thiono analogue | 34/11 | 1 | >20–114.7 | 0.07–140 | 347 | 2.25–14.7 | ||||||
| Sildenafil analogue | 0/0 | 2 | ||||||||||
| Dimethylsildenafil | 4/3 | 67–78 | 89 | |||||||||
| Nor-acetildenafil | 0/1 | 106 | ||||||||||
| Tadalafil | 26/30 | 7 | 0.023–20 | 4.6–147,000 | 80.6 | 14–49.2 | ||||||
| Desmethyl tadalafil | 0/0 | 1 | ||||||||||
| Nortadalafil | 0/1 | 0.107–120,000 | 34 | |||||||||
| Vardenafil | 9/3 | 1 | > 5 | |||||||||
| Pseudovardenafil | 0/0 | 1 | ||||||||||
| Avanafil | 1/0 | |||||||||||
| Stimulants | ||||||||||||
| DMAA | 72/25 | 12.5–270 | 36.5 | 60 | 2.95–55,300 | |||||||
| DMHA | 6/0 | |||||||||||
| Synephrine | 34/19 | 23.23–44.1 | 75–21,220 | 646 | 75–76 | |||||||
| Ephedrine, Norephedrine and Ephedra | 5/0 | |||||||||||
| Octopamine | 1/3 | 200 | 46.1 | 107,500 | ||||||||
| Aegaline | 1/0 | |||||||||||
| Oxilofrine | 4/4 | 4300–45,740 | ||||||||||
| BMPEA | 4/2 | 9800–10,800 | ||||||||||
| Isopropyloctopamine | 2/1 | 7000 | ||||||||||
| Amphetamine | 1/0 | |||||||||||
| Anorexics and laxatives | ||||||||||||
| Sibutramine | 16/29 | 0.053–17.4 | 16.2 | 15.9 | 63–22,830 | |||||||
| N-Didesmethyl sibutramine | 1/6 | 0.531–3020 | ||||||||||
| Phenolphthalein | 4/14 | 5.1 | 3.8–6093 | 950 | ||||||||
| 2,4-Dintrophenol | 5/1 | 11 | ||||||||||
| Nootropic drugs | ||||||||||||
| Vinpocetine | 16/1 | 1 | 2.5 | |||||||||
| Vincamine | 3/0 | |||||||||||
| Vinburnine | 3/0 | |||||||||||
| Anabolic androgenic steroids | ||||||||||||
| Androstendione | 1/5 | 0.103–5.57 | ||||||||||
| Stanozolol | 0/2 | 1.7–11 | ||||||||||
| DHEA | 1/1 | 20 | ||||||||||
| Testosterone | 0/1 | 2403 | ||||||||||
| Methasterone | 0/1 | 7.6 | ||||||||||
| 4,6-Androstadien-17-ol-3-one | 0/1 | 5142 | ||||||||||
| 4,6-Androstadien-3,17-dione | 0/1 | 199 | ||||||||||
| 5-Androsten-17-ol-3-one | 0/1 | 6327 | ||||||||||
| 4,9-Estradien-3,17-dione | 0/1 | 28,606 | ||||||||||
| Cannabinoids | ||||||||||||
| THC | 0/23 | 0.101–2980 | 324 | |||||||||
| CBD | 8/6 | 5 | 12,000–32,100 | |||||||||
| Other | ||||||||||||
| 5-HTP | 5/2 | 100 | 30 | |||||||||
| Betaine | 7/0 | |||||||||||
| Zopiclone | 1/0 | |||||||||||
| Ciprofloxacin | 1/0 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amidžić, M.; Banović Fuentes, J.; Banović, J.; Torović, L. Notifications and Health Consequences of Unauthorized Pharmaceuticals in Food Supplements. Pharmacy 2023, 11, 154. https://doi.org/10.3390/pharmacy11050154
Amidžić M, Banović Fuentes J, Banović J, Torović L. Notifications and Health Consequences of Unauthorized Pharmaceuticals in Food Supplements. Pharmacy. 2023; 11(5):154. https://doi.org/10.3390/pharmacy11050154
Chicago/Turabian StyleAmidžić, Maja, Jelena Banović Fuentes, Jovica Banović, and Ljilja Torović. 2023. "Notifications and Health Consequences of Unauthorized Pharmaceuticals in Food Supplements" Pharmacy 11, no. 5: 154. https://doi.org/10.3390/pharmacy11050154
APA StyleAmidžić, M., Banović Fuentes, J., Banović, J., & Torović, L. (2023). Notifications and Health Consequences of Unauthorized Pharmaceuticals in Food Supplements. Pharmacy, 11(5), 154. https://doi.org/10.3390/pharmacy11050154







