Shortages of Medicines to Treat COVID-19 Symptoms during the First Wave and Fourth Wave: Analysis of Notifications Reported to Registers in Austria, Italy, and Spain
Abstract
1. Introduction
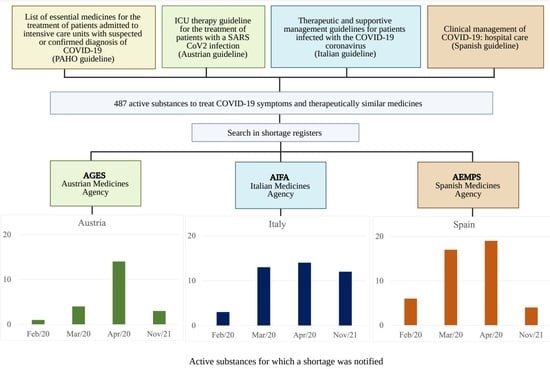
2. Materials and Methods
2.1. Country Selection
2.2. Studied Time Periods
2.3. Product Selection
2.4. Data Sources and Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanaan, A.O.; Sullivan, K.M.; Seed, S.M.; Cookson, N.A.; Spooner, L.M.; Abraham, G.M. In the Eye of the Storm: The Role of the Pharmacist in Medication Safety during the COVID-19 Pandemic at an Urban Teaching Hospital. Pharmacy 2020, 8, 225. [Google Scholar] [CrossRef]
- Watson, K.E.; Lee, D.H.; Nusair, M.B.; Al Hamarneh, Y.N. Impact of the Novel CoronaviruS (COVID-19) on Frontline PharmacIsts Roles and ServicEs: INSPIRE Worldwide Survey. Pharmacy 2023, 11, 66. [Google Scholar] [CrossRef]
- Pauwels, K.; Huys, I.; Casteels, M.; Simoens, S. Drug shortages in European countries: A trade-off between market attractiveness and cost containment? BMC Health Serv. Res. 2014, 14, 438. [Google Scholar] [CrossRef]
- Gray, A.; Manasse, H.R., Jr. Shortages of medicines: A complex global challenge. Bull. World Health Organ. 2012, 90, 158. [Google Scholar] [CrossRef] [PubMed]
- Modisakeng, C.; Matlala, M.; Godman, B.; Meyer, J.C. Medicine shortages and challenges with the procurement process among public sector hospitals in South Africa; findings and implications. BMC Health Serv. Res. 2020, 20, 234. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, R.; Kusama, Y.; Asai, Y.; Yoshiaki, G.; Muraki, Y.; Ohmagari, N. Effects of the cefazolin shortage on the sales, cost, and appropriate use of other antimicrobials. BMC Health Serv. Res. 2021, 21, 1118. [Google Scholar] [CrossRef] [PubMed]
- Acosta, A.; Vanegas, E.A.; Rovira, J.; Godman, B.; Bochenek, T. Medicine Shortages: Gaps between Countries and Global Perspectives. Front. Pharmacol. 2019, 10, 763. [Google Scholar] [CrossRef]
- Hawley, K.L.; Mazer-Amirshahi, M.; Zocchi, M.S.; Fox, E.R.; Pines, J.M. Longitudinal Trends in U.S. Drug Shortages for Medications Used in Emergency Departments (2001–2014). Acad. Emerg. Med. 2016, 23, 63–69. [Google Scholar] [CrossRef]
- Bogaert, P.; Bochenek, T.; Prokop, A.; Pilc, A. A qualitative approach to a better understanding of the problems underlying drug shortages, as viewed from Belgian, French and the European Union’s perspectives. PLoS ONE 2015, 10, e0125691. [Google Scholar] [CrossRef]
- Heiskanen, K.; Ahonen, R.; Kartutunen, P.; Kanerva, R.; Timonen, J. Medicine shortages—A study of community pharmacies in Finland. Health Policy 2015, 119, 232–238. [Google Scholar] [CrossRef]
- Bauters, T.; Claus, B.O.; Norga, K.; Huys, I.; Simoens, S.; Laureys, G. Chemotherapy drug shortages in paediatric oncology: A 14-year single-centre experience in Belgium. J. Oncol. Pharm. Pract. 2016, 22, 766–770. [Google Scholar] [CrossRef]
- Postma, D.J.; De Smet, P.A.G.M.; Gispen-de Wied, C.C.; Leufkens, H.G.M.; Mantel-Teeuwisse, A.K. Drug Shortages from the Perspectives of Authorities and Pharmacy Practice in the Netherlands: An Observational Study. Front. Pharmacol. 2018, 9, 1243. [Google Scholar] [CrossRef]
- Videau, M.; Chemali, L.; Stucki, C.; Saavedra-Mitjans, M.; Largana, S.; Guerin, A.; Bonnabry, P.; Delhauteur, B.; Van Hees, T.; Lebel, D. Drug shortages in Canada and selected European countries: A cross-sectional, institution-level comparison. Can. J. Hosp. Pharm. 2019, 72, 7–15. [Google Scholar] [CrossRef]
- Bocquet, F.; Albane Degrassat-Théas, A.; Peigné, J.; Paubel, P. The new regulatory tools of the 2016 Health Law to fight drug shortages in France. Health Policy 2017, 121, 471–476. [Google Scholar] [CrossRef]
- Scholz, N. Addressing Shortages of Medicines; European Parliament: Brussels, Belgium, 2020; Available online: https://www.europarl.europa.eu/thinktank/en/document.html?reference=EPRS_BRI(2020)649402 (accessed on 12 March 2023).
- Vis, C.; Pelsy, F.; Dijkstal, F.; Petrosova, L.; Davé, A.; Spit, W.; Varnai, P.; Becker, D.; Jongh, T.; Moulac, M.; et al. Future-Proofing Pharmaceutical Legislation: Study on Medicine Shortages: Final Report; European Commission Directorate-General for Health Food Safety, Publications Office: Brussels, Belgium, 2021; Available online: https://data.europa.eu/doi/10.2875/211485 (accessed on 15 March 2023).
- Kuruc Poje, D.; Kifer, D.; Huys, I.; Miranda, J.; Jenzer, H.; Miljković, N.; Hoppe-Tichy, T.; Bochniarz, M.; Frontini, R.; Schwartz, D.G.; et al. Patients perspectives on drug shortages in six European hospital settings—A cross sectional study. BMC Health Serv. Res. 2021, 21, 689. [Google Scholar] [CrossRef] [PubMed]
- Phuong, J.M.; Penm, J.; Chaar, B.; Oldfield, L.D.; Moles, R. The impacts of medication shortages on patient outcomes: A scoping review. PLoS ONE 2019, 14, e0215837. [Google Scholar] [CrossRef]
- Atif, M.; Sehar, A.; Malik, I.; Mushtaq, I.; Ahmad, N.; Babar, Z.-U.-D. What impact does medicines shortages have on patients? A qualitative study exploring patients’ experience and views of healthcare professionals. BMC Health Serv. Res. 2021, 21, 827. [Google Scholar] [CrossRef]
- Said, A.; Goebel, R.; Ganso, M.; Zagermann-Muncke, P.; Schulz, M. Drug shortages may compromise patient safety: Results of a survey of the reference pharmacies of the Drug Commission of German Pharmacists. Health Policy 2018, 122, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Bochenek, T.; Abilova, V.; Alkan, A.; Asanin, B.; de Miguel Beriain, I.; Besovic, Z.; Vella Bonanno, P.; Bucsics, A.; Davidescu, M.; De Weerdt, E.; et al. Systemic Measures and Legislative and Organizational Frameworks Aimed at Preventing or Mitigating Drug Shortages in 28 European and Western Asian Countries. Front. Pharmacol. 2018, 8, 942. [Google Scholar] [CrossRef] [PubMed]
- Vogler, S.; Fischer, S. How to address medicines shortages: Findings from a cross-sectional study of 24 countries. Health Policy 2020, 124, 1287–1296. [Google Scholar] [CrossRef]
- De Weerdt, E.; De Rijdt, T.; Simoens, S.; Casteels, M.; Huys, I. Time spent by Belgian hospital pharmacists on supply disruptions and drug shortages: An exploratory study. PLoS ONE 2017, 12, e0174556. [Google Scholar] [CrossRef]
- De Weerdt, E.; Simoens, S.; Casteels, M.; Huys, I. Clinical, economic and policy implications of drug shortages in the European Union. Appl. Health Econ. Health Policy 2017, 15, 441–445. [Google Scholar] [CrossRef]
- Pauwels, K.; Simoens, S.; Casteels, M.; Huys, I. Insights into European drug shortages: A survey of hospital pharmacists. PLoS ONE 2015, 10, e0119322. [Google Scholar] [CrossRef]
- EMA. Guidance on Detection and Notification of Shortages of Medicinal Products for Marketing Authorisation Holders (MAHs) in the Union (EEA); EMA/674304/2018; European Medicines Agency and Heads of Medicines Agencies: Amsterdam, The Netherlands, 2019; p. 11. Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guidance-detection-notification-shortages-medicinal-products-marketing-authorisation-holders-mahs_en.pdf (accessed on 14 April 2023).
- De Weerdt, E.; Simoens, S.; Hombroeckx, L.; Casteels, M.; Huys, I. Causes of drug shortages in the legal pharmaceutical framework. Regul. Toxicol. Pharmacol. 2015, 71, 251–258. [Google Scholar] [CrossRef]
- Chapman, S.; Dedet, G.; Lopert, R. Shortages of Medicines in OECD Countries; Organisation for Economic Co-Operation and Development: Paris, France, 2022; Available online: https://www.oecd-ilibrary.org/content/paper/b5d9e15d-en (accessed on 26 March 2023).
- Jee, Y. WHO international health regulations emergency committee for the COVID-19 outbreak. Epidemiol. Health 2020, 42, e2020013. [Google Scholar] [CrossRef] [PubMed]
- ECDC. COVID-19 Situation Dashboard; European Centre for Disease Prevention and Control: Solna, Sweden, 2020; Available online: https://qap.ecdc.europa.eu/public/extensions/COVID-19/COVID-19.html#global-overview-tab (accessed on 25 December 2021).
- Villani, L.; McKee, M.; Cascini, F.; Ricciardi, W.; Boccia, S. Comparison of Deaths Rates for COVID-19 across Europe during the First Wave of the COVID-19 Pandemic. Front. Public Health 2020, 8, 620416. [Google Scholar] [CrossRef] [PubMed]
- Tragaki, A.; Richard, J.L. First wave of SARS-COV2 in Europe: Study and typology of the 15 worst affected European countries. Popul. Space Place 2022, 28, e2534. [Google Scholar] [CrossRef] [PubMed]
- Bezzini, D.; Schiavetti, I.; Manacorda, T.; Franzone, G.; Battaglia, M.A. First Wave of COVID-19 Pandemic in Italy: Data and Evidence. In Coronavirus Therapeutics—Volume II: Clinical Management and Public Health; Asea, A.A.A., Kaur, P., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 91–113. [Google Scholar] [CrossRef]
- Jain, V.; Serisier, A.; Lorgelly, P. The Real-World Impact of Vaccination on COVID-19 Cases During Europe’s Fourth Wave. Int. J. Public Health 2022, 67, 1604793. [Google Scholar] [CrossRef]
- Mordujovich-Buschiazzo, P.; Dorati, C.M.; Marín, G.; Prozzi, G.R.; Buchiazzo, H.O. Lista de Medicamentos Esenciales Para el Manejo de Pacientes que Ingressan a Unidades de Cuidados Intensivos con Sespecha o Diagnóstico Confirmado de COVID-19; Centro Universitario de Farmacología, Centro Colaborador OPS/OMS en el uso Racional de Medicamentos (CUFAR), Facultad de Ciencias Médicas, Universidad Nacional de La Plata: La Plata, Argentina, 2020; Available online: https://www.paho.org/col/dmdocuments/documentos-2020/covid-19/LME-UCI-COVID-19%20final-25-marzo.pdf (accessed on 2 April 2020).
- Köstenberger, M.; Hasibeder, W.; Dankl, D.; Germann, R.; Hörmann, C.; Joannidis, M.; Markstaller, K.; Müller-Muttonen, S.-O.; Neuwersch-Sommeregger, S.; Schaden, E.; et al. ICU Therapy Guideline for the Treatment of Patients with a SARS CoV2 Infection, Version 29.3.2020; 2020. Available online: https://www.anaesthesie.news/wp-content/uploads/%C3%96GARI-FASIM-%C3%96GIAIN-Guideline-NEU-Covid19-290320.pdf (accessed on 19 June 2023).
- SIMIT. Linee Guida Sulla Gestione Terapeutica e di Supporto per Pazienti con Infezione da Coronavirus COVID-19. Edizione Marzo 2020; Società Italiana di Malattie Infettive e Tropicali: Milano, Italy, 2020; Available online: http://www.fvcalabria.unicz.it/COVID-19/LINEE-GUIDA/linee-guida-SIMIT-marzo-2020.pdf (accessed on 19 June 2023).
- Ministerio de Sanidad. Documento Técnico. Manejo Clínico del COVID-19: Atención Hospitalaria. 19 de Marzo de 2020; Centro de Coordinación de Alertas y Emergencias Sanitarias, Dirección General de Salud Pública, Calidad e Innovación: Madrid, Spain, 2020; Available online: https://chguv.san.gva.es/documents/138129/1312271/19-3-2020++Protocolo_manejo_clinico_atencion+hospitalaria+_COVID-19.pdf/e3ebf581-9f9e-407c-a97f-a44a8c29bc78 (accessed on 19 June 2023).
- WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index. 2020; WHO Collaborating Centre for Drug Statistics Methodology, Norwegian Institute of Public Health: Oslo, Norway, 2020; Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 15 March 2020).
- WHO. World Health Organization Model List of Essential Medicines; 22nd List; World Health Organization: Geneva, Switzerland, 2021; Available online: https://apps.who.int/iris/rest/bitstreams/1374779/retrieve (accessed on 13 April 2023).
- BASG. Shortages Catalogue; Austrian Federal Office for Safety in Health Care: Vienna, Austria, 2020; Available online: https://medicineshortage.basg.gv.at/vertriebseinschraenkungen (accessed on 14 April 2023).
- AIFA. Farmaci Carenti; Agenzia Italiana del Farmaco: Roma, Italy, 2020. Available online: https://www.aifa.gov.it/en/farmaci-carenti (accessed on 12 December 2021).
- AEMSP. Problemas de Summistro Activos; Agencia Española de Medicamentos y Productos Sanitarios: Madrid, Spain, 2021; Available online: https://cima.aemps.es/cima/publico/listadesabastecimiento.html (accessed on 12 December 2021).
- Pauwels, K.; Huys, I.; Casteels, M.; Larsson, K.; Voltz, C.; Penttila, K.; Morel, T.; Simoens, S. Are products with an orphan designation for oncology indications different from products for other rare indications? A retrospective analysis of European orphan designations granted between 2002–2012. Orphanet J Rare Dis 2017, 12, 36. [Google Scholar] [CrossRef]
- Consejo General de Colegios Oficiales de Farmacéuticos. Desabastecimiento. 2020. Available online: https://www.portalfarma.com/Paginas/LoginPortalFarma.aspx?ReturnUrl=%252FProfesionales%252Fmedicamentos%252FCISMED%252F_layouts%252FAuthenticate.aspx%253FSource%253D%25252FProfesionales%25252Fmedicamentos%25252FCISMED%25252FPaginas%25252FListados%25252DMedicamentos%25252Dproblemas%252 (accessed on 13 September 2020).
- Cameron, E.E.; Bushell, M.-J.A. Analysis of drug shortages across two countries during pre-pandemic and pandemic times. Res. Soc. Adm. Pharm. 2021, 17, 1570–1573. [Google Scholar] [CrossRef]
- Romano, S.; Galante, H.; Figueira, D.; Mendes, Z.; Rodrigues, A.T. Time-trend analysis of medicine sales and shortages during COVID-19 outbreak: Data from community pharmacies. Res. Soc. Adm. Pharm. 2021, 17, 1876–1881. [Google Scholar] [CrossRef] [PubMed]
- Nowak, B.M.; Kamiński, M.; Owczarek, B.; Szulińska, M.; Bogdański, P. Availability of Cardiodiabetological Drugs in Poland during the First Year of COVID-19 Pandemic: Retrospective Study. BioMed 2022, 2, 117–126. [Google Scholar] [CrossRef]
- Aljadeed, R.; AlRuthia, Y.; Balkhi, B.; Sales, I.; Alwhaibi, M.; Almohammed, O.; Alotaibi, A.J.; Alrumaih, A.M.; Asiri, Y. The Impact of COVID-19 on Essential Medicines and Personal Protective Equipment Availability and Prices in Saudi Arabia. Healthcare 2021, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Turbucz, B.; Major, M.; Zelko, R.; Hanko, B. Proposal for handling of medicine shortages based on a comparison of retrospective risk analysis. Int. J. Environ. Res. Public Health 2022, 19, 4102. [Google Scholar] [CrossRef] [PubMed]
- Silverman, E. A New COVID-19 Problem: Shortages of Medicines Needed for Placing Patients on Ventilators. STAT. 2020. Available online: https://www.statnews.com/pharmalot/2020/03/31/a-new-covid-19-problem-shortages-of-medicines-needed-for-placing-patients-on-ventilators/ (accessed on 25 December 2021).
- Miljković, N.; Gibbons, N.; Batista, A.; Fitzpatrick, R.W.; Underhill, J.; Horák, P. Results of EAHP’s 2018 survey on medicines shortages. Eur. J. Hosp. Pharm. 2019, 26, 60–65. [Google Scholar] [CrossRef]
- Badreldin, H.A.; Atallah, B. Global drug shortages due to COVID-19: Impact on patient care and mitigation strategies. Res. Soc. Adm. Pharm. 2020, 17, 1946–1949. [Google Scholar] [CrossRef]
- Cundell, T.; Guilfoyle, D.; Kreil, T.R.; Sawant, A. Controls to Minimize Disruption of the Pharmaceutical Supply Chain during the COVID-19 Pandemic. PDA J. Pharm. Sci. Technol. 2020, 74, 468–494. [Google Scholar] [CrossRef]
- Regulation (EU) 2022/123 of the European Parliament and of the Council of 25 January 2022 on a Reinforced Role for the European Medicines Agency in Crisis Preparedness and Management for Medicinal Products and Medical Devices. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2022:020:FULL (accessed on 25 June 2023).
| Country | Database Provider | Scope of Shortage Information | Update Frequency | Format and Accessibility | Start and End Date | Reason for Shortage | Mitigation Measures |
|---|---|---|---|---|---|---|---|
| Austria | Bundesamt für Sicherheit im Gesundheitswesen (BASG) | Unavailable and partially unavailable medicines as reported by the MAH | Constantly, published on the day after notification of the shortage | Publicly accessible web-based database | Start dates and expected and actual end dates are included | Included | Not included, but information is available whether, or not, an export ban has been imposed on the medicine in short supply |
| Italy | Agenzia Italiana del Farmaco (AIFA) | Temporary and permanent shortages as reported by the MAH | Every 2–3 weeks | Publicly accessible website that provides the latest updated Excel® file | Start dates and expected end dates are included (no actual end dates) | Included | Included (e.g., alternative medicines, export ban) |
| Spain | Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) | “Ongoing shortages” and “solved shortages” as reported by the MAH | Constantly, published on the day after notification of the shortage | Publicly accessible web-based database | Expected start dates and expected end dates are included (no actual end dates) | Not included | Included (e.g., alternative medicines, exceptional marketing) |
| Country | February 2020 | March 2020 | April 2020 | November 2021 | ||||
|---|---|---|---|---|---|---|---|---|
| Austria | 1 (1) | Amoxicillin/ clavulanic acid | 4 (5) | Amoxicillin/ clavulanic acid Pantoprazole Oseltamivir Cisatracurium | 14 (27) | Famotidine Pantoprazole Rabeprazole Esomeprazole Granisetron Sodium chloride Dexamethasone Amoxicillin/clavulanic acid Combinations of penicillins Ciprofloxacin Lamivudine/abacavir Rocuronium bromide Paracetamol Midazolam | 3 (4) | Omeprazole Methylprednisolone Ciprofloxacin |
| Italy | 3 (4) | Pantoprazole Esomeprazole Roxithromycin | 13 (40) | Pantoprazole Methylprednisolone Roxithromycin Ciprofloxacin Lopinavir/ritonavir Darunavir/cobicistat Atracurium Rocuronium bromide Mivacurium chloride Cisatracurium Propofol Fentanyl Paracetamol | 14 (36) | Pantoprazole Esomeprazole Ondansetron Clarithromycin Lamivudine/abacavir Tocilizumab Rocuronium bromide Mivacurium chloride Cisatracurium Propofol Morphine Fentanyl Paracetamol Midazolam | 12 (19) | Pantoprazole Lansoprazole Heparin Cefotaxime Clarithromycin Azithromycin Ciprofloxacin Lamivudine/abacavir Lopinavir/ritonavir Rocuronium bromide Propofol Fentanyl |
| Spain | 6 (9) | Omeprazole Pantoprazole Ondansetron Granisetron Propofol Paracetamol | 17 (46) | Omeprazole Ondansetron Chlorhexidine Hydrocortisone Piperacilin/tazobactam Ceftazidime Clarithromycin Azithromycin Levofloxacin Moxifloxacin Atracurium Cisatracurium Propofol Morphine Fentanyl Paracetamol Haloperidol | 19 (67) | Omeprazole Ondansetron Enoxaparin Sodium bicarbonate Chlorhexidine Ethanol Methylprednisolone Prednisone Hydrocortisone Amoxicillin/ clavulanic acid Piperacilin/tazobactam Cefixime Cefditoren Azithromycin Levofloxacin Suxamethonium Rocuronium bromide Propofol Paracetamol | 4 (6) | Sodium chloride Moxifloxacin Vancomycin Paracetamol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, D.I.R.; Vogler, S. Shortages of Medicines to Treat COVID-19 Symptoms during the First Wave and Fourth Wave: Analysis of Notifications Reported to Registers in Austria, Italy, and Spain. Pharmacy 2023, 11, 120. https://doi.org/10.3390/pharmacy11040120
Sánchez DIR, Vogler S. Shortages of Medicines to Treat COVID-19 Symptoms during the First Wave and Fourth Wave: Analysis of Notifications Reported to Registers in Austria, Italy, and Spain. Pharmacy. 2023; 11(4):120. https://doi.org/10.3390/pharmacy11040120
Chicago/Turabian StyleSánchez, Diana Ivonne Rodríguez, and Sabine Vogler. 2023. "Shortages of Medicines to Treat COVID-19 Symptoms during the First Wave and Fourth Wave: Analysis of Notifications Reported to Registers in Austria, Italy, and Spain" Pharmacy 11, no. 4: 120. https://doi.org/10.3390/pharmacy11040120
APA StyleSánchez, D. I. R., & Vogler, S. (2023). Shortages of Medicines to Treat COVID-19 Symptoms during the First Wave and Fourth Wave: Analysis of Notifications Reported to Registers in Austria, Italy, and Spain. Pharmacy, 11(4), 120. https://doi.org/10.3390/pharmacy11040120