Evaluation of Real-World Vancomycin Dosing and Attainment of Therapeutic Drug Monitoring Targets
Abstract
1. Introduction
2. Materials and Methods
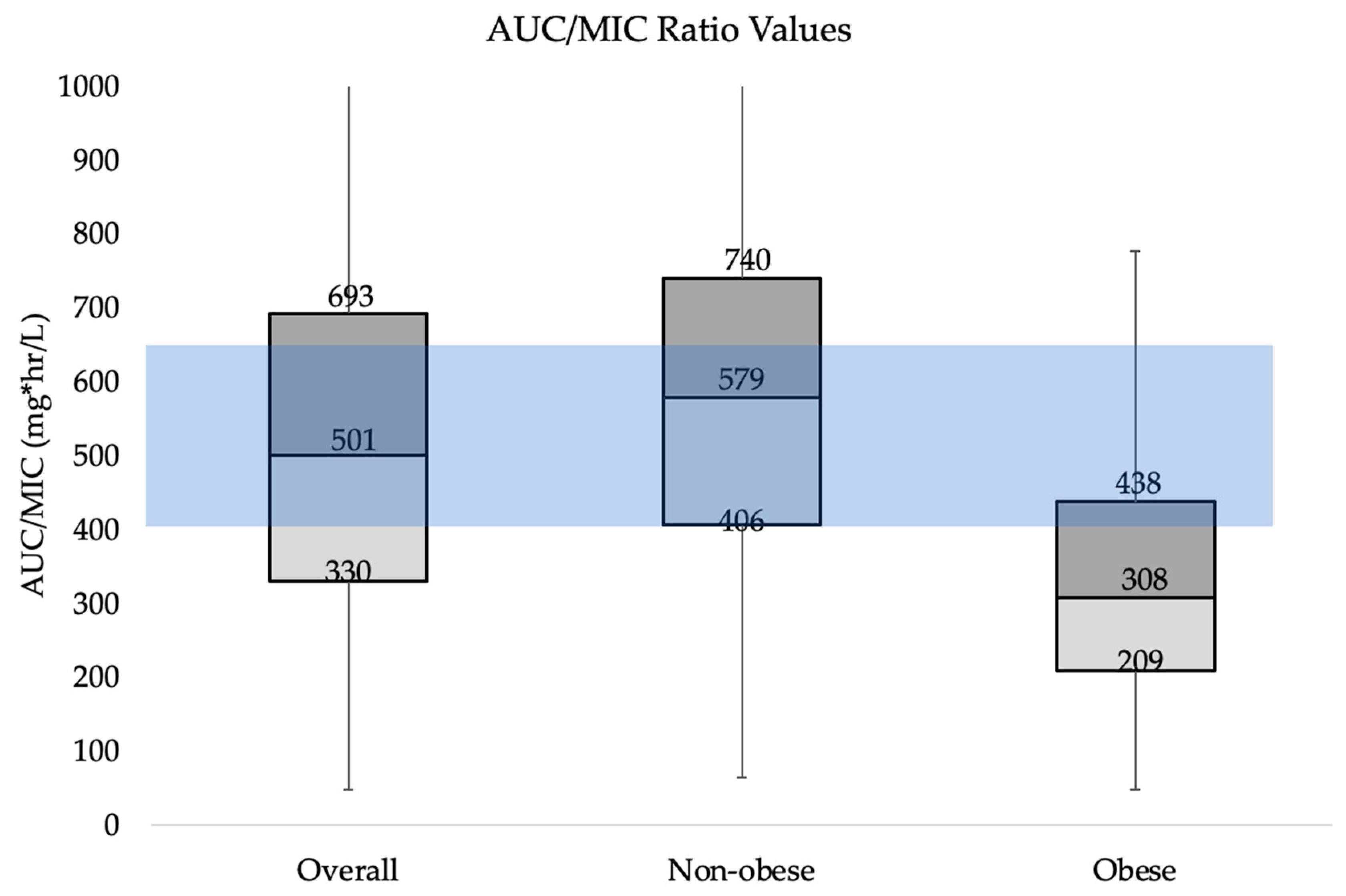
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, S.; Preuss, C.V.; Bernice, F. Vancomycin; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459263/ (accessed on 1 October 2022).
- Rybak, M.; Lomaestro, B.; Rotscahfer, J.C.; Moellering, R.C.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Vancomycin Therapeutic Guidelines: A summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2009, 49, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus Aureus infections: A revised consensus guideline and review by the American Society of Health-system Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2020, 71, 1361–1364. [Google Scholar] [PubMed]
- Bradley, N.; Lee, Y.; Sadeia, M. Assessment of the implementation of AUC dosing and monitoring practices with vancomycin at hospitals across the United States. J. Pharm. Pract. 2022, 35, 864–869. [Google Scholar] [CrossRef] [PubMed]
- MM.09.01.01; New Antimicrobial Stewardship Standard. The Joint Commission: Oakbrook Terrace, IL, USA, 2016. Available online: https://www.jointcommission.org/-/media/enterprise/tjc/imported-resource-assets/documents/new_antimicrobial_stewardship_standardpdf.pdf?db=web&hash=69307456CCE435B134854392C7FA7D76&hash=69307456CCE435B134854392C7FA7D76 (accessed on 1 October 2022).
- Pai, M.P.; Neely, M.; Rodvold, K.A.; Lodise, T.P. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv. Drug Deliv. Rev. 2014, 77, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Sawchuk, R.J.; Zaske, D.E. Pharmacokinetics of dosing regimens which utilize multiple intravenous infusions: Gentamicin in burn patients. J. Pharmacokinet. Biopharm. 1976, 4, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.P.; Russo, A.; Novelli, A.; Venditti, M.; Falcone, M. Simplified equations using two concentrations to calculate area under the curve for antimicrobials with concentration-dependent pharmacodynamics: Daptomycin as a motivating example. Antimicrob Agents Chemother. 2014, 58, 3162–3167. [Google Scholar] [CrossRef] [PubMed]
- Holmes, N.E.; Turnidge, J.D.; Munckhof, W.J.; Robinson, J.O.; Korman, T.M.; O’Sullivan, M.V.N.; Anderson, T.L.; Roberts, S.A.; Warren, S.J.C.; Gao, W.; et al. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 2013, 57, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kawasaki, K.; Sato, Y.; Tokimatsu, I.; Itoh, H.; Hiramatsu, K.; Takeyama, M.; Kadota, J.-I. Is peak concentration needed in therapeutic drug monitoring of vancomycin? A pharmacokinetic-pharmacodynamic analysis in patients with methicillin-resistant Staphylococcus aureus pneumonia. Chemotherapy 2012, 58, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Patel, N.; Lomaestro, B.M.; Rodvold, K.A.; Drusano, G.L. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin. Infect. Dis. 2009, 15, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.; Payne, K.D.; Bain, A.M.; Rahman, A.P.; Nguyen, S.T.; Eaton, S.A.; Busti, A.J.; Vu, S.L.; Bedimo, R. Multicenter evaluation of vancomycin dosing: Emphasis on obesity. Am. J. Med. 2008, 121, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heggen, T.; Buyle, F.M.; Claus, B.; Somers, A.; Schelstraete, P.; De Paepe, P.; Vanhaesebrouck, S.; De Cock, P.A.J.G. Vancomycin dosing and therapeutic drug monitoring practices: Guidelines versus real-life. Int. J. Clin. Pharm. 2021, 43, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Carland, J.E.; Stocker, S.L.; Baysari, M.T.; Li, C.; Själin, J.; Moran, M.A.; Tang, S.; Sandaradura, I.; Elhage, T.; Gilbey, T.; et al. Are vancomycin dosing guidelines followed? A mixed methods study of vancomycin prescribing practices. Br. J. Clin. Pharmacol. 2021, 87, 4221–4229. [Google Scholar] [CrossRef] [PubMed]
- Van Hal, S.J.; Paterson, D.L.; Lodise, T.P. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob. Agents Chemother. 2013, 57, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Kunming, P.; Xiaotian, J.; Qing, X.; Chenqi, X.; Xiaoqiang, D.; Zhou, L.Q. Impact of pharmacist intervention in reducing vancomycin-associated acute kidney injury: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Joseph, K.; Ramireddy, K.; Madison, G.; Turco, T.; Lui, M. Outcomes of a pharmacist-driven vancomycin monitoring initiative in a community hospital. J. Clin. Pharm. Ther. 2021, 46, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Vancomycin Calculator—ClinCalc.com. 2021. Available online: https://clincalc.com/Vancomycin/Beta.aspx (accessed on 10 January 2023).
- Vancomycin Dosing-Bayesian Analysis. GlobalRPH. 2020. Available online: https://globalrph.com/medcalcs/vancomycin-dosing-bayesian-analysis/ (accessed on 10 January 2023).
| AUC Calculation When 2 Levels Available | ||
|---|---|---|
| Step | Description | Equation |
| 1 | Calculate observed Ke from 2 levels | Ke = ln (C1/C2)/t1 − t2 |
| 2 | Calculate true peak (Cmax) | Cmax = C1/(e−Ke(tin)) |
| 3 | Calculate true trough (Cmin) | Cmin = Cmax (e−ke(tau−tin)) |
| 4 | Calculate AUC during infusion using linear trapezoidal rule | AUCinf = tin (Cmax + Cmin/2) |
| 5 | Calculate AUC during elimination using linear trapezoidal rule | AUCelim = (Cmax − Cmin)/Ke |
| 6 | Calculate AUC24 | AUC24 = AUCinf + AUCelim (24/tau) |
| AUC Calculation when 1 level available | ||
| 1 | Estimate Ke using Matzke Equation | Ke = 0.00083 × CrCl + 0.0044 |
| 2 | Estimate true trough (Cmin) | Cmin = Cmax (e−ke(tau−tin)) |
| 3 | Extrapolate level needed, (Cmax) | Cmax = Cmin/(e−Ke(tin)) |
| 4 | Calculate AUC during infusion using linear trapezoidal rule | AUCinf = tin (Cmax + Cmin/2) |
| 5 | Calculate AUC during elimination using linear trapezoidal rule | AUCelim = (Cmax − Cmin)/Ke |
| 6 | Calculate AUC24 | AUC24 = AUCinf + AUCelim (24/tau) |
| Overall (N = 305) | Non-Obese (N = 230) | Obese (N = 75) | |
|---|---|---|---|
| Sex (% Female) | 24.6 | 19.1 | 41.3 |
| Age (years, mean ± SD) | 53.8 ± 18.4 | 55.1 ± 19 | 49.8 ± 15.8 |
| Weight (kg, mean ± SD) | 74.6 ± 20.5 | 67.2 ± 15 | 97.5 ± 22.1 |
| SCr (mg/dL, mean ± SD) | 0.93 ± 0.69 | 0.99 ± 0.77 | 0.78 ± 0.30 |
| CrCl (mL/min, mean ± SD) | 125 ± 91 | 109 ± 75 | 175 ± 91 |
| Vancomycin Considerations | |||
| Dose (mg/kg/dose, mean ± SD) | 16.3 ± 3.9 | 17.5 ± 10.1 | 14.4 ± 2.5 |
| Indication (%) | |||
| Uncomplicated (Cellulitis, UTI) | 88 (28.8%) | 54 (23.5%) | 34 (45.3%) |
| Complicated (CNS, PNA, Bone, BSI, Sepsis) | 198 (64.9%) | 162 (70.4%) | 36 (48%) |
| Unknown | 19 (6.2%) | 14 (6.1%) | 5 (6.6%) |
| Vancomycin Levels | |||
| Availability of appropriately drawn trough level (%) | 201/305 (65.9) | 151/230 (65.7) | 50/75 (66.7) |
| Mean number of serum concentrations per subject | 1.7 | 1.8 | 1.6 |
| Overall (N = 305) | Non-Obese (N = 230) | Obese (N = 75) | X2 | |
|---|---|---|---|---|
| Therapeutic AUC/MIC Ratio (400–600 mg·h/L) | ||||
| n/N (%) | 85/305 (27.9) | 70/230 (30.4) | 15/75 (20) | 3.06, p = 0.08 |
| Median AUC (IQR) | 495 (117.5) | 501 (115.8) | 449 (77) | |
| Below-Goal AUC/MIC Ratio (<400 mg·h/L) | ||||
| n/N (%) | 106/305 (34.8) | 55/230 (23.9) | 51/75 (68) | 48.48, p < 0.00001 |
| Median AUC (IQR) | 270 (142) | 277 (150) | 262 (151) | |
| Above-Goal AUC/MIC Ratio (>600 mg·h/L) | ||||
| n/N (%) | 114/305 (37.4) | 105/230 (45.7) | 9/75 (12) | 27.36, p < 0.00001 |
| Median AUC (IQR) | 758 (375) | 791 (435) | 681 (80) | |
| Rate of AKI | ||||
| n/N (%) | 8/305 (2.6) | 4/230 (1.7) | 4/75 (5.3) | 2.86, p = 0.091 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bradley, N.; Ng, K. Evaluation of Real-World Vancomycin Dosing and Attainment of Therapeutic Drug Monitoring Targets. Pharmacy 2023, 11, 95. https://doi.org/10.3390/pharmacy11030095
Bradley N, Ng K. Evaluation of Real-World Vancomycin Dosing and Attainment of Therapeutic Drug Monitoring Targets. Pharmacy. 2023; 11(3):95. https://doi.org/10.3390/pharmacy11030095
Chicago/Turabian StyleBradley, Nicole, and Kimberly Ng. 2023. "Evaluation of Real-World Vancomycin Dosing and Attainment of Therapeutic Drug Monitoring Targets" Pharmacy 11, no. 3: 95. https://doi.org/10.3390/pharmacy11030095
APA StyleBradley, N., & Ng, K. (2023). Evaluation of Real-World Vancomycin Dosing and Attainment of Therapeutic Drug Monitoring Targets. Pharmacy, 11(3), 95. https://doi.org/10.3390/pharmacy11030095







