An Integrated Multidisciplinary Circuit Led by Hospital and Community Pharmacists to Implement Clopidogrel Pharmacogenetics in Clinical Practice
Abstract
1. Introduction
1.1. Pharmacogenetics and Pharmacy Practice
1.2. Clopidogrel Pharmacogenetics
1.3. Aims and Justification
- To establish a pilot circuit in which pharmacogenetic markers (variants of CYP2C19) are determined in order to optimize the clopidogrel prescription based on the recommendations of international guidelines (CPIC).
- To evaluate whether the established circuit is feasible and operational.
- To identify patients who are intermediate or poor metabolizers of CYP2C19 who are currently taking clopidogrel, whose treatment will be individually evaluated.
- To identify patients who are carriers of the *17 allele and evaluate whether they have an increased risk of bleeding.
2. Materials and Methods
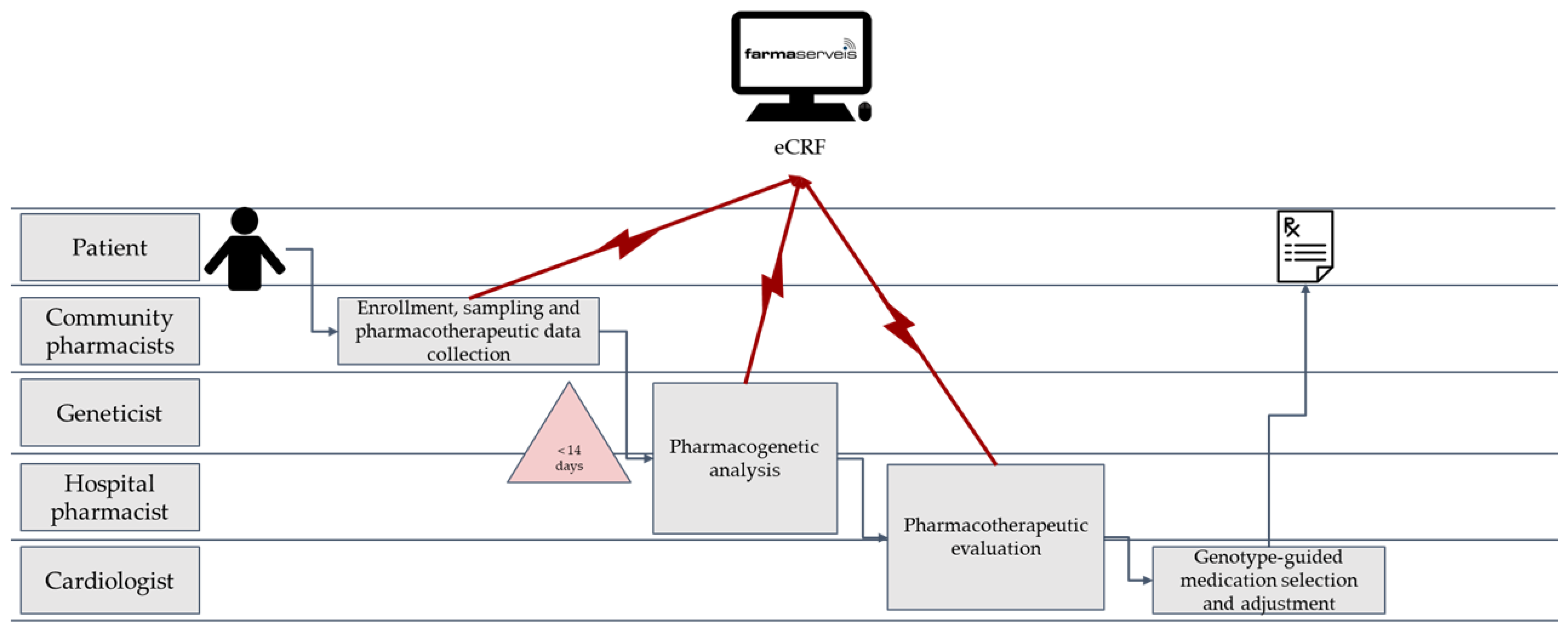
2.1. Study Design
2.2. Healthcare Professionals’ Training for the Study
2.3. Study Population Sampling, Inclusion and Exclusion Criteria
2.4. Ethical Considerations, Information for Subjects, and Informed Consent
2.5. Data Collection
- •
- An interview was held with the patient at the time of recruitment in the community pharmacy. As specified in the informed consent, in order to participate in the study, the patient had to provide the following data: NHS patient identification code, first name and surname, sex, date of birth, phone number, email address, zip code, nationality, and indication for the clopidogrel prescription and any other drugs taken.
- •
- Medical history. All participants granted consent for hospital pharmacists and clinicians participating in the study to review their clinical history to extract data of interest regarding the abovementioned clinical variables.
- •
- Online opinion survey. Participating pharmacists were sent a survey through a web-based system at the end of September 2020. The project was resumed briefly in November 2020 when the second peak in the COVID-19 wave had passed.
2.6. Sampling and Analytic Methods
2.7. Data Management and Analysis
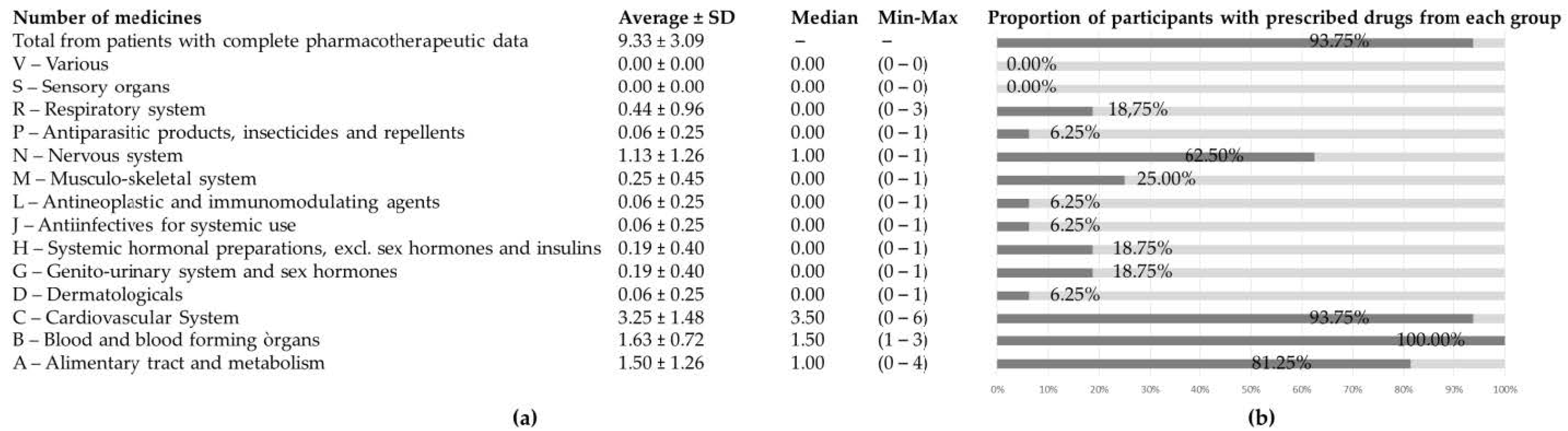
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spear, B.B.; Heath-Chiozzi, M.; Huff, J. Clinical Application of Pharmacogenetics. Trends Mol. Med. 2001, 7, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Slawomirski, L.; Auraaen, A.; Klazinga, N.S. The Economics of Patient Safety: Strengthening a Value-Based Approach to Reducing Patient Harm at National Level; OECD: Paris, France, 2017. [Google Scholar] [CrossRef]
- World Health Organization. Medication Safety in Polypharmacy: Technical Report; Medication without Harm–World Health Organization: Geneva, Switzerland, 2019; pp. 1–63. Available online: https://www.who.int/publications/i/item/WHO-UHC-SDS-2019.11 (accessed on 11 April 2023).
- Relling, M.V.; Evans, W.E. Pharmacogenomics in the Clinic. Nature 2015, 526, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Krebs, K.; Milani, L. Translating Pharmacogenomics into Clinical Decisions: Do Not Let the Perfect Be the Enemy of the Good. Hum. Genom. 2019, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.H.; Wright, S.; Sharma, V.; Newman, W.G.; Payne, K.; Wilson, P. Characterizing Pharmacogenetic Programs Using the Consolidated Framework for Implementation Research: A Structured Scoping Review. Front. Med. 2022, 9, 945352. [Google Scholar] [CrossRef] [PubMed]
- Oates, J.; Lopez, D. Pharmacogenetics: An Important Part of Drug Development with A Focus on Its Application. Int. J. Biomed. Investig. 2018, 1, 111. [Google Scholar] [CrossRef]
- Formea, C.M.; Nicholson, W.T.; Vitek, C.R. An Inter-Professional Approach to Personalized Medicine Education: One Institution’s Experience. Pers. Med. 2015, 12, 129–138. [Google Scholar] [CrossRef]
- Primorac, D.; Bach-Rojecky, L.; Vađunec, D.; Juginović, A.; Žunić, K.; Matišić, V.; Skelin, A.; Arsov, B.; Boban, L.; Erceg, D.; et al. Pharmacogenomics at the Center of Precision Medicine: Challenges and Perspective in an Era of Big Data. Pharmacogenomics 2020, 21, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Aldaz, A. La Farmacogenética y La Farmacia de Hospital. Farm. Hosp. 2011, 35, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Dávila-Fajardo, C.L.; Díaz-Villamarín, X.; Antúnez-Rodríguez, A.; Fernández-Gómez, A.E.; García-Navas, P.; Martínez-González, L.J.; Dávila-Fajardo, J.A.; Cabeza Barrera, J. Pharmacogenetics in the Treatment of Cardiovascular Diseases and Its Current Progress Regarding Implementation in the Clinical Routine. Genes 2019, 10, 261. [Google Scholar] [CrossRef]
- American Society of Health-System Pharmacists ASHP Statement on the Pharmacist’s Role in Clinical Pharmacokinetic Monitoring. Am. J. Health-Syst. Pharm. 1998, 55, 1726–1727. [CrossRef]
- Rodríguez-Arcas, M.J.; García-Jiménez, E.; Montesinos-Hernández, A.; Martínez-Martínez, F.; Conesa-Zamora, P. Pharmacotherapeutic Follow-up and Pharmacogenetics of CYP2C9 and CYP3A4 in Antihypertensive Therapy: A Pilot Study in a Community Pharmacy. Ther. Innov. Regul. Sci. 2013, 47, 489–494. [Google Scholar] [CrossRef]
- Rendell, T.; Barnett, J.; Wright, D. Co-Designing a Community Pharmacy Pharmacogenomics Testing Service in the UK. BMC Health Serv. Res. 2022, 22, 378. [Google Scholar] [CrossRef] [PubMed]
- Kisor, D.F.; Petry, N.J.; Bright, D.R. Pharmacogenomics in the United States Community Pharmacy Setting: The Clopidogrel- CYP2C19 Example. Pharm. Pers. Med. 2021, 14, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Padgett, L.; O’Connor, S.; Roederer, M.; McLeod, H.; Ferreri, S. Pharmacogenomics in a Community Pharmacy: ACT Now. J. Am. Pharm. Assoc. 2011, 51, 189–193. [Google Scholar] [CrossRef]
- Smith, D.M.; Weitzel, K.W.; Elsey, A.R.; Langaee, T.; Gong, Y.; Wake, D.T.; Duong, B.Q.; Hagen, M.; Harle, C.A.; Mercado, E.; et al. CYP2D6-Guided Opioid Therapy Improves Pain Control in CYP2D6 Intermediate and Poor Metabolizers: A Pragmatic Clinical Trial. Genet. Med. 2019, 21, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Papastergiou, J.; Li, W.; Sterling, C.; van den Bemt, B. Pharmacogenetic-Guided Cannabis Usage in the Community Pharmacy: Evaluation of a Pilot Program. J. Cannabis Res. 2020, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Stäuble, C.K.; Jeiziner, C.; Bollinger, A.; Wiss, F.M.; Hatzinger, M.; Hersberger, K.E.; Ihde, T.; Lampert, M.L.; Mikoteit, T.; Meyer Zu Schwabedissen, H.E.; et al. A Guide to a Pharmacist-Led Pharmacogenetic Testing and Counselling Service in an Interprofessional Healthcare Setting. Pharmacy 2022, 10, 86. [Google Scholar] [CrossRef]
- Aref, H.T.; Makowsky, M.J.; Kung, J.Y.; Guirguis, L.M. Mapping the Implementation of Pharmacogenomic Testing in Community Pharmacies 2002–2021 Using the Theoretical Domains Framework: A Scoping Review. J. Am. Pharm. Assoc. 2022, 63, 459–476.e6. [Google Scholar] [CrossRef] [PubMed]
- Blog COFB. Quin Paper Pot Tenir El Farmacèutic En l’àmbit Dels Tests Genètics?: Vídeo Entrevista al Dr. Manel Rabanal i La Dra. Teresa Pàmpols. 2020. Available online: https://blog.cofb.cat/blog/2020/03/10/quin-paper-pot-tenir-el-farmaceutic-en-lambit-de-la-farmacogenetica-video-entrevistes-al-dr-manel-rabanal-i-la-dra-teresa-pampols/ (accessed on 2 February 2023).
- Blog COFB. Farmacogenètica i Farmàcia Comunitària: La Seva Relació Explicada per Experts. 2017. Available online: https://blog.cofb.cat/blog/2017/11/23/farmacogenetica-farmacia-comunitaria-la-seva-relacio-explicada-per-experts/ (accessed on 2 February 2023).
- Blog COFB. Farmacogenètica de Nivell Avançat al COFB. 2018. Available online: https://blog.cofb.cat/blog/2018/05/22/farmacogenetica-nivell-avancat-cofb/ (accessed on 2 February 2023).
- Colegio de Farmacéuticos de Cuenca. Curso de Farmacogenética y Respuesta a Los Medicamentos. 2019. Available online: https://www.cofcuenca.com/paginas/Noticia.asp?idNoticia=350 (accessed on 3 February 2023).
- Colegio de Farmacéuticos de Valencia. Farmacogenética y Farmacogenómica, el Camino Para Personalizar Los Tratamientos. 2021. Available online: https://www.micof.es/ver/36164/farmacogenetica-y-farmacogenomica--el-camino-para-personalizar-los-tratamientos.html (accessed on 3 February 2023).
- Col·legi de Farmacèutics de les Illes Balears. Curs de Medicina Personalitzada i Farmacogenètica. 2013. Available online: https://www.cofib.es/fitxers_pagines/PROGRAMA_FARMACOGENETICA_BOLETIN.pdf (accessed on 11 April 2023).
- Ruiz, C.; Candás, B.; Padró, A.; Pintó, X. Farmacogenètica de Les Estatines: Cap a Una Medicina Personalitzada. Circ. Farm. 2017, 75, 13–21. Available online: https://www.cofb.org/documents/10136/252210/2017_3.pdf/da3c8c59-efc1-429c-98de-c83aa53e269b (accessed on 12 April 2023).
- Redacción El Global. El COFB Marca Su Estrategia Durante La Diada Del Farmacéutico. El Global. 2018. Available online: https://elglobal.es/farmacia/el-cofb-marca-su-estrategia-durante-la-diada-del-farmaceutico-nd1353593/ (accessed on 11 April 2023).
- Sanofi Winthrop Industrie. Ficha Técnica Iscover 75 Mg Comprimidos Recubiertos Con Película; Agencia Española de Medicamentos y Producto Sanitario: Madrid, Spain, 2022; Available online: https://cima.aemps.es/cima/dochtml/ft/98070001/FT_98070001.html (accessed on 11 April 2023).
- Sanofi Winthrop Industrie. Ficha Técnica Plavix 75 Mg Comprimidos Recubiertos Con Película; Agencia Española de Medicamentos y Productos Sanitarios: Madrid, Spain, 2022; Available online: https://cima.aemps.es/cima/dochtml/ft/98069001/FT_98069001.HTML (accessed on 11 April 2023).
- Fisher, M. Results of the Management of Atherothrombosis with Clopidogrel in High-Risk Patients Trial: Implications for the Neurologist. Arch. Neurol. 2006, 63, 20–24. [Google Scholar] [CrossRef]
- Ringleb, P.A.; Bhatt, D.L.; Hirsch, A.T.; Topol, E.J.; Hacke, W. Benefit of Clopidogrel Over Aspirin Is Amplified in Patients with a History of Ischemic Events. Stroke 2004, 35, 528–532. [Google Scholar] [CrossRef]
- Sanofi-Aventis. Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance (CHARISMA): A Phase III, Multicenter, Multinational, Randomized, Parallel Group, Double-Blind Trial of Clopidogrel Versus Placebo in High-Risk Patients Aged 45 Years and Older, at Risk of Atherothrombotic Events, and Who Are Receiving Background Therapy Including Low-Dose ASA. ClinicalTrials.gov Identifier: NCT00050817. 2012. Available online: https://www.clinicaltrials.gov/ct2/show/study/NCT00050817 (accessed on 11 April 2023).
- Yusuf, S.; Zhao, F.; Mehta, S.; Chrolavicius, S.; Tognoni, G.; Fox, K. Effects of Clopidogrel in Addition to Aspirin in Patients with Acute Coronary Syndromes without ST-Segment Elevation. N. Engl. J. Med. 2001, 345, 494–502. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Cannon, C.P.; Gibson, C.M.; López-Sendón, J.L.; Montalescot, G.; Theroux, P.; Claeys, M.J.; Cools, F.; Hill, K.A.; Skene, A.M.; et al. Addition of Clopidogrel to Aspirin and Fibrinolytic Therapy for Myocardial Infarction with ST-Segment Elevation. N. Engl. J. Med. 2005, 352, 1179–1189. [Google Scholar] [CrossRef]
- Connolly, S.; Yusuf, S.; Budaj, A.; Camm, J.; Chrolavicius, S.; Commerford, P.; Flather, M.; Fox, K.; Hart, R.; Hohnloser, S.; et al. Rationale and Design of ACTIVE: The Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events. Am. Heart J. 2006, 151, 1187–1193. [Google Scholar] [CrossRef]
- Connolly, S.; Pogue, J.; Hart, R.; Pfeffer, M.; Hohnloser, S.; Chrolavicius, S.; Pfeffer, M.; Hohnloser, S.; Yusuf, S. Clopidogrel plus Aspirin versus Oral Anticoagulation for Atrial Fibrillation in the Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE W): A Randomised Controlled Trial. Lancet 2006, 367, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Zhao, X.; Liu, L.; Wang, D.; Wang, C.; Wang, C.; Li, H.; Meng, X.; Cui, L.; et al. Clopidogrel with Aspirin in Acute Minor Stroke or Transient Ischemic Attack. N. Engl. J. Med. 2013, 369, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Sangkuhl, K.; Klein, T.E.; Altman, R.B. Clopidogrel Pathway. Pharm. Genom. 2010, 20, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Fricke-Galindo, I.; Céspedes-Garro, C.; Rodrigues-Soares, F.; Naranjo, M.E.G.; Delgado; De Andrés, F.; López-López, M.; Peñas-Lledó, E.; Llerena, A. Interethnic Variation of CYP2C19 Alleles, ‘Predicted’ Phenotypes and ‘Measured’ Metabolic Phenotypes across World Populations. Pharm. J. 2015, 16, 113–123. [Google Scholar] [CrossRef]
- Lee, C.R.; Luzum, J.A.; Sangkuhl, K.; Gammal, R.S.; Sabatine, M.S.; Stein, C.M.; Kisor, D.F.; Limdi, N.A.; Lee, Y.M.; Scott, S.A.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2C19 Genotype and Clopidogrel Therapy: 2022 Update. Clin. Pharmacol. Ther. 2022, 112, 959–967. [Google Scholar] [CrossRef]
- Zeb, I.; Krim, N.; Bella, J. Role of CYP2C19 Genotype Testing in Clinical Use of Clopidogrel: Is It Really Useful? Expert Rev. Cardiovasc. Ther. 2018, 16, 369–377. [Google Scholar] [CrossRef]
- Bauer, T.; Bouman, H.J.; Van Werkum, J.W.; Ford, N.F.; Ten Berg, J.M.; Taubert, D. Impact of CYP2C19 Variant Genotypes on Clinical Efficacy of Antiplatelet Treatment with Clopidogrel: Systematic Review and Meta-Analysis. BMJ 2011, 343, d4588. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.V.; Perel, P.; Shah, T.; Hingorani, A.D.; Casas, J.P. CYP2C19 Genotype, Clopidogrel Metabolism, Platelet Function, and Cardiovascular Events: A Systematic Review and Meta-Analysis. JAMA 2011, 306, 2704–2714. [Google Scholar] [CrossRef] [PubMed]
- Fragoulakis, V.; Bartsakoulia, M.; Díaz-Villamarín, X.; Chalikiopoulou, K.; Kehagia, K.; Ramos, J.G.S.; Martínez-González, L.J.; Gkotsi, M.; Katrali, E.; Skoufas, E.; et al. Cost-Effectiveness Analysis of Pharmacogenomics-Guided Clopidogrel Treatment in Spanish Patients Undergoing Percutaneous Coronary Intervention. Pharm. J. 2019, 19, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.G.; Gruntowicz, D.; Chua, T.; Morlock, R.J. Financial Analysis of CYP2C19 Genotyping in Patients Receiving Dual Antiplatelet Therapy Following Acute Coronary Syndrome and Percutaneous Coronary Intervention. J. Manag. Care Spec. Pharm. 2015, 21, 552–557. [Google Scholar] [CrossRef]
- Col·legi de Farmacèutics de Barcelona; Consell de Col·legis Farmacèutics de Catalunya. Farmaserveis. 2019. Available online: https://www.farmaserveis.cat/ (accessed on 11 April 2023).
- Rother, M.; Shook, J. Learning to See: Value Stream Mapping to Create Value and Eliminate Muda; Lean Enterprise Institute: Boston, MA, USA, 1999. [Google Scholar]
- Jefatura del Estado. Ley Orgánica 3/2018, de 5 de diciembre, de Protección de Datos Personales y Garantía de Los Derechos Digitales. Boletín Oficial Estado 2018, BOE-A-2018-16673, 1–67. Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2018-16673 (accessed on 12 April 2023).
- NICE Satmetrix. What Is Net Promoter? 2021. Available online: https://www.netpromoter.com/know/ (accessed on 7 February 2023).
- Ferreri, S.P.; Greco, A.J.; Michaels, N.M.; O’Connor, S.K.; Chater, R.W.; Viera, A.J.; Faruki, H.; McLeod, H.L.; Roederer, M.W. Implementation of a Pharmacogenomics Service in a Community Pharmacy. J. Am. Pharm. Assoc. 2014, 54, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-T.; Merl, M.Y.; Yang, J.; Zhu, Z.-X.; Li, G.-H. Opportunities for Pharmacists to Integrate Pharmacogenomics into Clinical Practice. Pharm. J. 2020, 20, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Haidar, C.E.; Petry, N.; Oxencis, C.; Douglas, J.S.; Hoffman, J.M. ASHP Statement on the Pharmacist’s Role in Clinical Pharmacogenomics. Am. J. Health Syst. Pharm. 2022, 79, 704–707. [Google Scholar] [CrossRef]
- Suppiah, V.; Lim, C.X.; Hotham, E. Community Pharmacists and Their Role in Pharmacogenomics Testing: An Australian Perspective Drawing on International Evidence. Aust. J. Prim. Health 2018, 24, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Servei Català de la Salut (CatSalut). El CatSalut i el Model Sanitari Català. 2018. Available online: http://catsalut.gencat.cat/ca/coneix-catsalut/presentacio/model-sanitari-catala/ (accessed on 12 April 2023).
- Swen, J.J.; van der Wouden, C.H.; Manson, L.E.; Abdullah-Koolmees, H.; Blagec, K.; Blagus, T.; Böhringer, S.; Cambon-Thomsen, A.; Cecchin, E.; Cheung, K.C.; et al. A 12-gene pharmacogenetic panel to prevent adverse drug reactions: An open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet 2023, 401, 347–356. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Pegge, N.; Kinnaird, T. Spartan RX Point-of-Care CYP2C19 Test to Guide Treatment in Acute Coronary Syndrome. National Institute for Health and Care Excellence (NICE). 2020. Available online: https://www.nice.org.uk/advice/mib223 (accessed on 4 April 2023).
- Ministerio de la Presidencia, Relaciones con las Cortes y Memoria Democrática. Real Decreto 463/2020, de 14 de Marzo, por el que se declara el Estado de Alarma para la gestión de la situación de crisis sanitaria ocasionada por el COVID-19. Boletín Oficial Estado 2020, BOE-A-2020-3692, 25390–25400. Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2020-3692 (accessed on 11 April 2023).
| Enrolled Patients | Assessed Patients | |||||
|---|---|---|---|---|---|---|
| Total patients | N = 16 | N = 120 | ||||
| Gender | n | % | n | % | ||
| Male | 11 | 68.8% | 71 | 59.2% | ||
| Female | 5 | 31.2% | 49 | 40.8% | ||
| Avg ± SD | Median | Range (Min–Max) | Avg ± SD | Median | Range (Min–Max) | |
| Age | 71.8 ± 12.4 | 73.0 | (51–94) | 68.3 ± 13.3 | 72.0 | (41–95) |
| Total Enrolled Patients | N = 16 | |
|---|---|---|
| Tobacco use | n | % |
| Smoker | 1 | 6.3% |
| Former smoker | 8 | 50.0% |
| Never smoker | 7 | 43.8% |
| Cardiovascular risk factors | n | % |
| Hypertension | 10 | 62.5% |
| Dyslipemia | 16 | 100.0% |
| Type-2 diabetes | 8 | 50.0% |
| Family history of cardiac ischemia | 7 | 43.8% |
| Personal history of cardiac ischemia | 6 | 37.5% |
| Personal history of stroke | 2 | 12.5% |
| Peripheral vascular disease | 2 | 12.5% |
| Chronic kidney disease | 1 | 6.3% |
| Clinical situation | n | % |
| ST-elevation myocardial infarction | 3 | 18.8% |
| Non-ST-elevation myocardial infarction | 1 | 6.3% |
| Chronic angina | 3 | 18.8% |
| Unstable angina | 3 | 18.8% |
| Unavailable data | 6 | 37.5% |
| Coronary disease type | n | % |
| 1 blood vessel | 3 | 18.8% |
| 2 blood vessels | 2 | 12.5% |
| Truncus arteriousus | 1 | 6.3% |
| Unavailable data | 10 | 62.5% |
| Coronary revascularization technique | n | % |
| Drug-eluting stents | 5 | 31.3% |
| Conventional stents | 2 | 12.5% |
| Surgical intervention | 3 | 18.8% |
| None | 4 | 25.0% |
| Unavailable data | 2 | 12.5% |
| Other clinical information | Avg ± SD | Median |
| Left ventricle ejection fraction | 39.7 ± 30.5 | 42.0 |
| Number of thrombotic events | 0.00 ± 0.00 | 0.00 |
| Number of hemorragic events | 0.29 ± 0.47 | 0.00 |
| Time from Sampling to Lab Sample Reception (Days) | Avg ± SD | Median | Range (Min–Max) | |
|---|---|---|---|---|
| Pre-COVID-19 period | 7.7 ± 5.5 | 7.0 | (1–18) | p < 0.001 |
| Post-COVID-19 period | 64.1 ± 36.8 | 85.0 | (7–91) | |
| Time from Reception of Lab Sample to Analysis (days) | Avg ± SD | Median | Range (Min–Max) | |
| Pre-COVID-19 period | 6.1 ± 1.1 | 7.0 | (5–7) | p = 0.099 |
| Post-COVID-19 period | 20.3 ± 31.9 | 11.0 | (0–92) | |
| Total Time from Sampling to Analysis (days) | Avg ± SD | Median | Range (Min–Max) | |
| Pre-COVID-19 period | 13.8 ± 5.4 | 14.0 | (8–23) | p < 0.001 |
| Post-COVID-19 period | 84.4 ± 31.3 | 96.0 | (14–102) | |
| CYP2C19 Genotype (Expected Phenotype) | n | % | ||
| CYP2C19*1/*1 (normal metabolizer) | 7 | 43.75% | ||
| CYP2C19*1/*17 (ultrarapid metabolizer) | 3 | 18.75% | ||
| CYP2C19*1/*2 (intermediate metabolizer) | 5 | 31.25% | ||
| CYP2C19*2/*17 (intermediate metabolizer) | 1 | 6.25% | ||
| How Do You Rate... (from 0 to 10) | Avg ± SD | Median | Range (Min–Max) |
|---|---|---|---|
| ... the support received from the COFB Help Desk? | 8.7 ± 0.9 | 9.0 | (7–10) |
| ... the support received from the Research Institute? | 7.1 ± 2.5 | 8.0 | (2–9) |
| ... the electronic case report form in Farmaserveis App? | 6.8 ± 2.1 | 7.5 | (3–9) |
| ... the pharmacogenetic report generation system? | 5.8 ± 2.6 | 6.5 | (2–9) |
| How likely is it that you would recommend a fellow pharmacist to participate in this pharmacogenetic circuit? | |||
| (0, not likely at all; 10, extremely likely) | 7.3 ± 2.7 | 7.5 | (2–10) |
| Distribution of respondent groups | n | % | |
| Promoter pharmacists (10–9) | 4 | 40% | |
| Passive pharmacists (8–7) | 3 | 30% | |
| Detractor pharmacists (6–0) | 3 | 30% | |
| Net Promoter Score (from −100% to +100%) | +10% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mir, J.F.; Rodríguez-Caba, C.; Estrada-Campmany, M.; Fernández de Gamarra-Martínez, E.; Mangues, M.A.; Bagaría, G.; Riera, P. An Integrated Multidisciplinary Circuit Led by Hospital and Community Pharmacists to Implement Clopidogrel Pharmacogenetics in Clinical Practice. Pharmacy 2023, 11, 76. https://doi.org/10.3390/pharmacy11020076
Mir JF, Rodríguez-Caba C, Estrada-Campmany M, Fernández de Gamarra-Martínez E, Mangues MA, Bagaría G, Riera P. An Integrated Multidisciplinary Circuit Led by Hospital and Community Pharmacists to Implement Clopidogrel Pharmacogenetics in Clinical Practice. Pharmacy. 2023; 11(2):76. https://doi.org/10.3390/pharmacy11020076
Chicago/Turabian StyleMir, Joan Francesc, Cristina Rodríguez-Caba, Maria Estrada-Campmany, Edurne Fernández de Gamarra-Martínez, Maria Antònia Mangues, Guillermo Bagaría, and Pau Riera. 2023. "An Integrated Multidisciplinary Circuit Led by Hospital and Community Pharmacists to Implement Clopidogrel Pharmacogenetics in Clinical Practice" Pharmacy 11, no. 2: 76. https://doi.org/10.3390/pharmacy11020076
APA StyleMir, J. F., Rodríguez-Caba, C., Estrada-Campmany, M., Fernández de Gamarra-Martínez, E., Mangues, M. A., Bagaría, G., & Riera, P. (2023). An Integrated Multidisciplinary Circuit Led by Hospital and Community Pharmacists to Implement Clopidogrel Pharmacogenetics in Clinical Practice. Pharmacy, 11(2), 76. https://doi.org/10.3390/pharmacy11020076







