SMART Pharmacists Serving the New Needs of the Post-COVID Patients, Leaving No-One Behind
Abstract
1. Introduction
- “The term “post-COVID conditions” is an umbrella term for the wide range of physical and mental health consequences experienced by some patients that are present four or more weeks after SARS-CoV-2 infection, including by patients who had an initial mild or asymptomatic acute infection.
- Based on current literature, many post-COVID conditions can be managed by the primary care providers, using patient-centered approaches to optimize the quality of life of affected patients.
- Objective laboratory or imaging findings should not be used as the only measure or assessment of a patient’s well-being; as expected laboratory or imaging findings do not exclude the existence, severity, or importance of a patient’s post-COVID symptoms or conditions.
- Healthcare providers and patients are encouraged to set achievable goals through a shared decision-making patient care process, and to choose the treatment by focusing on specific symptoms (e.g., headache) or conditions (e.g., dysautonomia). A comprehensive management plan focusing on improving physical, mental, and social well-being may be helpful for some patients.
- Understanding of post-COVID conditions remains incomplete. The approach to caring for patients with post-COVID conditions will likely change over time as evidence accumulates.”
2. Materials and Methods
2.1. Introducing the New Community Pharmacy-Based Service to Leave No-One Behind
2.2. SMART Pharmacist Program—Educational Activities
2.3. Data Collection
2.4. Patient Counselling
2.5. Statistical Analysis
3. Results
3.1. Analysis of the Most Frequent Symptoms According to Severity, Changes in Self-Reported Patient Symptom Severity and Pharmacist Recommendations to Patients
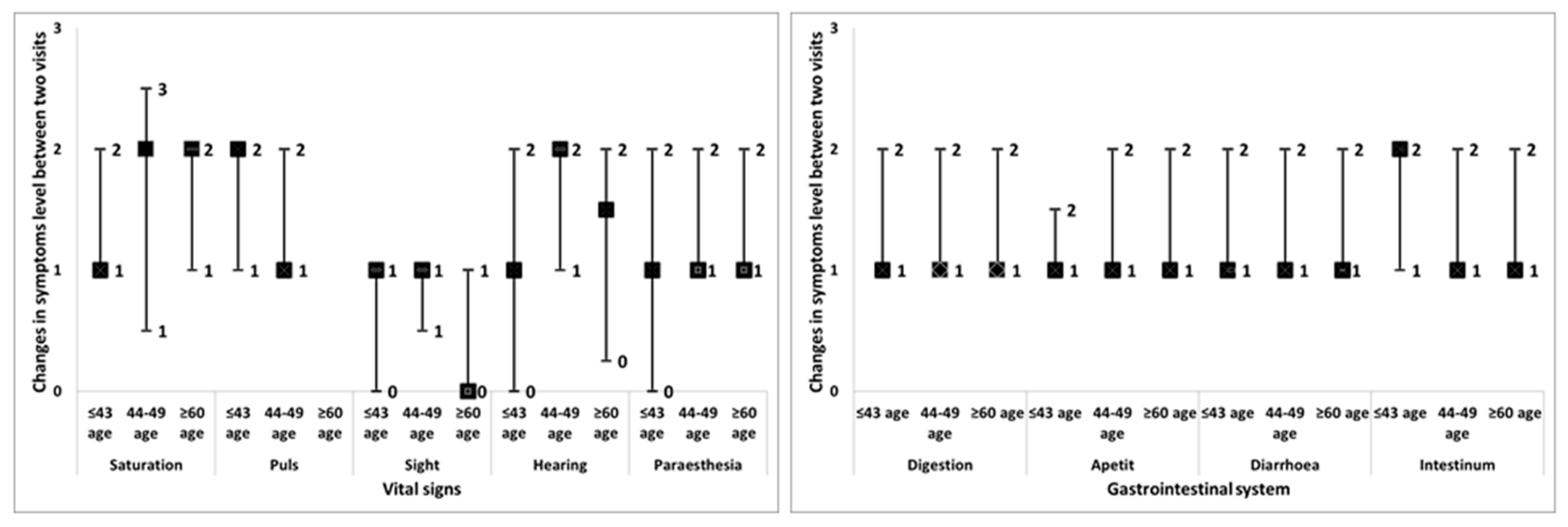
3.2. Analysis of the Self-Reported Symptom Severity Changes and Pharmacist Recommendations in Different Age Groups
4. Discussion
4.1. Comparison of the Reported Symptoms with Other Studies
4.2. Changes Observed in the Patients’ Self-Reported Severity of Symptoms
4.3. Impact of Education on Application in Practice
4.4. Limitations and Advantages of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Institute for Public Health “Dr Milan Jovanovic Batut”. Available online: https://covid19.rs/homepage-english/ (accessed on 13 December 2022).
- WHO Regional Office for Europe. Available online: https://covid19.who.int/region/euro/country/rs (accessed on 13 December 2022).
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Qu, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.; Gomersall, C.D.; Fowler, R.A. Care for Critically Ill Patients With COVID-19. JAMA 2020, 323, 1499–1500. [Google Scholar] [CrossRef] [PubMed]
- Olufemi, E. Post-COVID-19 condition: Current evidence and unanswered questions. In The Lancet Global Health; Elsevier: Amsterdam, The Netherlands, 2013; Volume 10, pp. e1210–e1211. ISSN 2214–109X. [Google Scholar]
- National Institute for Health and Care Excellence (NICE), Scottish Intercollegiate Guidelines Network (SIGN) and Royal College of General Practitioners (RCGP). COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 13 December 2022).
- Fernández-de-las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Florencio, L.L. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): An integrative classification. Int. J. Environ. Res. Public Health 2021, 18, 2621. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. (9 July 2021). Post-COVID conditions: Information for healthcare providers. Available online: www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html (accessed on 13 December 2022).
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of post-acute COVID-19 in primary care. BMJ 2020, 370, m3026. Available online: https://www.bmj.com/content/370/bmj.m3026.full (accessed on 20 January 2023). [CrossRef] [PubMed]
- Evaluating and Caring for Patients with Post-COVID Conditions: Interim Guidance. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html#management (accessed on 22 September 2022).
- Good Pharmacy Practice Guideline “Official Gazette of Republic of Serbia” 27, 2021). Available online: https://www.farmkom.rs/farmkom.php?id=69 (accessed on 20 January 2023).
- Rouse, M.J.; Mestrović, A. Learn Today-Apply Tomorrow: The SMART Pharmacist Program. Pharmacy 2020, 8, 139. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: The need for an interdisciplinary approach. Aging Clin. Exp. Res. 2020, 32, 1613–1620. [Google Scholar] [CrossRef]
- Doing What Matters in Times of Stress. Available online: https://www.who.int/publications/i/item/9789240003927 (accessed on 20 January 2023).
- World Health Organization. Support for Rehabilitation: Self-Management after COVID-19-Related Illness; No. WHO/EURO: 2021-855-40590-59892; World Health Organization, Regional Office for Europe: Geneva, Switzerland, 2021. [Google Scholar]
- Guidelines for Pharmacists in Primary Health Care; The Pharmaceutical Chamber of Serbia, The Faculty of Pharmacy, University of Belgrade and The Union of Pharmaceutical Associations of Serbia, 2021. Available online: https://www.zdravlje.gov.rs/view_file.php?file_id=2268&cache=sr (accessed on 20 February 2023).
- Almas, T.; Malik, J.; Alsubai, A.K.; Zaidi, S.M.J.; Iqbal, R.; Khan, K.; Ali, M.; Ishaq, U.; Alsufyani, M.; Hadeed, S.; et al. Post-acute COVID-19 syndrome and its prolonged effects: An updated systematic review. Ann. Med. Surg. 2022, 80, 103995. [Google Scholar] [CrossRef]
- Rudroff, T.; Workman, C.D.; Bryant, A.D. Potential Factors That Contribute to Post-COVID-19 Fatigue in Women. Brain Sci. 2022, 12, 556. [Google Scholar] [CrossRef]
- Tortajada, C.; Navarro, A.; Andreu-Ballester, J.C.; Mayor, A.; Añón, S.; Flores, J. Prevalence and duration of symptoms among moderate and severe COVID-19 patients 12 months after discharge. Intern. Emerg. Med. 2022, 17, 929–934. [Google Scholar] [CrossRef]
- Stavem, K.; Ghanima, W.; Olsen, M.K.; Gilboe, H.M.; Einvik, G. Prevalence and determinants of fatigue after COVID-19 in non-hospitalized patients: A population-based study. Int. J. Environ. Res. Public Health 2021, 18, 2030. [Google Scholar] [CrossRef] [PubMed]
- Helman, S.N.; Adler, J.; Jafari, A.; Bennett, S.; Vuncannon, J.R.; Cozart, A.C.; Wise, S.K.; Kuruvilla, M.E.; Levy, J.M. Treatment strategies for postviral olfactory dysfunction: A systematic review. Allergy. Asthma. Proc. 2022, 43, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Kharaba, Z.; Moutraji, S.A.; Khawaldeh, R.A.A.; Alfoteih, Y.; Meslamani, A.Z.A. What has changed in the pharmaceutical care after COVID-19: Pharmacists’ perspective. Pharm. Pract. 2022, 20, 2656. [Google Scholar] [CrossRef]
- Jordan, D.; Guiu-Segura, J.M.; Sousa-Pinto, G.; Wang, L.N. How COVID-19 has impacted the role of pharmacists around the world. Farm. Hosp. 2021, 45, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Perrin, R.; Riste, L.; Hann, M.; Walther, A.; Mukherjee, A.; Heald, A. Into the looking glass: Post-viral syndrome post COVID-19. Med. Hypotheses 2020, 144, 110055. [Google Scholar] [CrossRef]
- International Pharmaceutical Federation. FIP Statement of Policy-Pharmacy: Gateway to Care. The Hague: FIP, 2017. Available online: https://www.fip.org/statements (accessed on 20 January 2023).
- Popescu, M.N.; Berteanu, M.; Beiu, C.; Popa, L.G.; Mihai, M.M.; Iliescu, M.G.; Stănescu, A.M.A.; Ionescu, A.M. Complementary Strategies to Promote Hair Regrowth in Post-COVID-19 Telogen Effluvium. Clin. Cosmet. Investig. Dermatol. 2022, 15, 735–743. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, L.; Dolovich, L. Follow-up in community pharmacy should be routine, not extraordinary. Can. Pharm. J. 2018, 151, 79–81. [Google Scholar] [CrossRef]
- Mestrovic, A.; Rouse, M.J. Pillars and foundations of quality for continuing education in pharmacy. Am. J. Pharm. Educ. 2015, 79, 45. [Google Scholar] [CrossRef]
- International Pharmaceutical Federation. Quality Assurance of Pharmacy Education: The FIP Global Framework. 2014. Available online: https://www.fip.org/files/fip/PharmacyEducation/Quality_Assurance/QA_Framework_2nd_Edition_online_version.pdf (accessed on 25 January 2023).
- Apikoglu, S.; Selcuk, A.; Ozcan, V.; Balta, E.; Turker, M.; Albayrak, O.D.; Mestrovic, A.; Rouse, M.; Uney, A. Author Correction to: The first nationwide implementation of pharmaceutical care practices through a continuing professional development approach for community pharmacists. Int. J. Clin. Pharm. 2023, 45, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Korff, M.; Jensen, M.P.; Karoly, P. Assessing global pain severity by self-report in clinical and health services research. Spine 2000, 25, 3140–3151. [Google Scholar] [CrossRef]
- Haefeli, M.; Elfering, A. Pain assessment. Eur. Spine J. 2006, 15 (Suppl. S1), S17–S24. [Google Scholar] [CrossRef] [PubMed]
| Condition | Definition |
|---|---|
| Acute COVID-19 | Signs and symptoms of COVID-19 present for up to 4 weeks. |
| Ongoing symptomatic COVID-19 | Signs and symptoms of COVID-19 present from 4 weeks up to 12 weeks. |
| Post-COVID-19 syndrome | Signs and symptoms that develop during or after an infection consistent with COVID-19, which continue for more than 12 weeks and are not explained by an alternative diagnosis. This usually presents with clusters of symptoms, often overlapping, which can fluctuate and change over time and can affect any system in the body. Post-COVID-19 syndrome may be considered before 12 weeks while the possibility of an alternative underlying disease is also being assessed. |
| Symptoms | All N (%) | Females N (%) | Males N (%) | p |
|---|---|---|---|---|
| N | 871 (100) | 534 (62) | 337 (38) | |
| Age, y * | 45 ± 15 (8–92) | 44 ± 15 (8–92) | 47 ± 15 (12–87) | 0.008 |
| Number of symptoms | ||||
| 1 | 300 (34.4) | 178 (33.3) | 121 (36.1) | 0.508 |
| 2 | 170 (19.5) | 107 (20.0) | 63 (18.6) | |
| 3 | 116 (13.4) | 69 (12.9) | 48 (14.2) | |
| >3 | 285 (32.7) | 180 (33.8) | 105 (31.1) | |
| Respiratory system | 476 (51.2) | 288 (53.9) | 184 (54.4) | 0.888 |
| Muscle system | 222 (25.5) | 131 (24.5) | 90 (26.6) | 0.488 |
| Digestive system | 254 (28.2) | 139 (26.0) | 112 (33.1) | 0.024 |
| Insomnia and sleeping disorders | 170 (19.5) | 110 (20.6) | 59 (17.5) | 0.253 |
| Behavioural changes | 121 (13.9) | 76 (14.2) | 35 (10.4) | 0.094 |
| Cognitive functions | 243 (27.9) | 152 (28.8) | 89 (26.3) | 0.493 |
| Immunity status | 280 (32.2) | 117 (33.1) | 100 (29.6) | 0.273 |
| Fatigue and exhaustion | 267 (30.7) | 159 (29.8) | 106 (31.4) | 0.620 |
| CV system disorders | 103 (11.8) | 64 (12.0) | 39 (11.5) | 0.842 |
| Change in vital signs | 124 (14.2) | 71 (13.3) | 52 (15.4) | 0.388 |
| Skin, hair and nail changes | 239 (27.4) | 193 (36.1) | 43 (12.7) | <0.001 |
| Other | 107 (12.3) | 74 (13.9) | 32 (9.50) | 0.053 |
| The Most Frequent Symptoms | The First/Second Visit N | A Follow-Up Rate % | Referred to Physician N (%) * | The Severity of Symptoms Median (Q1–Q3) * | Symptom Severity Changes Median of Difference (Q1–Q3) |
|---|---|---|---|---|---|
| Respiratory tract | |||||
| Cough | 298/252 | 84.6 | 61 (20.5) | 3 (2–3) | 1 (1–2) |
| Loss of taste | 191/157 | 82.2 | 15 (7.8) | 3 (2–4) | 2 (1–2) |
| Loss of smell | 216/183 | 84.7 | 20 (9.3) | 3 (3–4) | 2 (1–2) |
| Xerostomia | 87/75 | 86.2 | 0 (0) | 3 (2–4) | 1 (1–2) |
| Shortness of breath | 122/108 | 88.5 | 45 (36.9) | 3 (2–3) | 1 (1–2) |
| Chest pain (pressure) | 119/98 | 82.3 | 58 (48.7) | 3 (2–3) | 1 (1–2) |
| Cognitive functions | |||||
| Mental tiredness | 112/95 | 84.8 | 5 (4.4) | 3 (2–3) | 1 (1–2) |
| Memory, ability to remember | 115/99 | 86.1 | 7 (5.0) | 3 (2–3) | 1 (1–2) |
| Ability to work, to concentrate | 130/109 | 83.9 | 2 (2.2) | 3 (2–3) | 1 (1–2) |
| Daily sleepiness | 90/61 | 67.8 | 9 (7.8) | 3 (2–3) | 1 (1–2) |
| Immunity status | |||||
| Frequent, repetitive infections | 74/58 | 78.4 | 11 (14.9) | 3 (2–3.25) | 1 (1–2) |
| Weakness and tiredness | 232/184 | 79.3 | 20 (8.5) | 3 (2–3) | 1 (1–2) |
| Autoimmune disease status | 16/15 | 93.8 | 10 (62.5) | 3 (3–3.75) | 1 (1–2) |
| Fatigue and exhaustion | |||||
| Headaches | 115/97 | 84.3 | 12 (10.5) | 3 (2–3) | 1 (1–2) |
| Poor physical strength | 154/113 | 73.4 | 11 (7.2) | 3 (2–3) | 1 (1–2) |
| Loss of energy | 182/135 | 74.2 | 9 (4.9) | 3 (2–3) | 1 (1–2) |
| Skin, hair and nail changes | |||||
| Painful scalp | 27/20 | 74.1 | 0 (0) | 3 (3–4) | 1 (1–2) |
| Hair loss | 163/130 | 81.3 | 6 (3.7) | 4 (3–4) | 1 (1–2) |
| Low hair quality and vitality | 109/80 | 73.4 | 5 (4.6) | 3 (3–4) | 1 (1–2) |
| Dehydrated skin | 93/83 | 89.2 | 2 (1.8) | 3 (2–3) | 1 (1–2) |
| Bruises | 12/10 | 83.3 | 1 (8.3) | 3 (2–3.75) | 1 (0–2) |
| Thick skin | 17/14 | 82.4 | 1 (5.9) | 2 (1–3.5) | 1 (0–1) |
| Visible changes on skin and nails | 25/16 | 64.0 | 1 (4.0) | 3 (2–3) | 1 (0.25–1) |
| Symptoms | N | Recommended Self-Medication 1 | Recommended Self-Medication 2 | Recommended Self-Medication 3 | Other Recommendations |
|---|---|---|---|---|---|
| Cough | 254 | Acetylcysteine 25.6% | Nonprescription antitussives 13.8% | Marshmallow root extract syrup 13.0% | Herbal syrups |
| Loss of taste | 149 | Alpha lipoic acid 52.0% | Supplements (Vit A, D, B and Zn) 13.7% | Different kinds of throat lozenges 12.3% | Olfactory retraining |
| Loss of smell | 167 | Alpha lipoic acid 41.9% | Olfactory retraining 31.2% | AD drops with panthenol 13.2% | Different kinds of throat lozenges, vitamins and omega 3 |
| Xerostomia | 62 | Different kinds of throat lozenges 48.4% | Nonpharmacologic interventions 21% | Mouthwash 12.9% | |
| Shortness of breath | 80 | Nonpharmacologic interventions 73.8% | Coenzyme Q10 7.5% | Inhalations 7.5% | Omega 3 |
| Chest pain (pressure) | 77 | Nonpharmacologic interventions 51.9% | Coenzyme Q10 19.5% | Magnesium 10.4% | OTC analgesics and inhalations |
| Mental tiredness | 93 | Supplements (B, D, E and omega acids) 30.1% | Nonpharmacologic interventions 25.8% | Ginkgo biloba 12.9% | Supplements |
| Memory, ability to remember | 88 | Ginkgo biloba 40.1% | Supplements 29.5% | Nonpharmacologic interventions 13.6% | Rhodiola rosea |
| Ability to work, to concentrate | 91 | Omega 3 and vitamin supplements 29.6% | Ginkgo biloba 22% | Nonpharmacologic interventions 22% | Herbal supplements |
| Daily sleepiness | 54 | Nonpharmacologic interventions 33.3% | Taurine, caffeine and B12 vitamin 14.8% | Iron supplements 11.1% | Other supplements |
| Frequent, repetitive infections | 72 | Supplements (vit B, D, C, Zn, selenium and Mg) 34.4% | Acyclovir 21.3% | Beta glucan 16.4% | |
| Weakness and tiredness | 188 | Supplements 81% | Coenzyme Q10 13.3% | Fatty acids 5.7% | |
| Autoimmune disease status | 13 | Selenium, Zn and Mg supplements 46.0% | Nonpharmacologic interventions 15.4% | Supplements 38.6% | |
| Headaches | 103 | OTC analgesics 62.1% | Magnesium 34.9% | Supplements 3% | |
| Poor physical strength | 108 | Supplements 28% | Nonpharmacologic interventions 24.3% | Vitamin C 18.7% | Herbal products |
| Loss of energy | 122 | Supplements 31.2% | Coenzyme Q10 26.2% | Guarana, Caffeine and Ginkgo 13.1% | |
| Painful scalp | 18 | Hair supplements formulas 66.7% | Local hair products (lotions and shampoos) 33% | Nonpharmacologic interventions | |
| Hair loss | 138 | Hair supplements formulas 57.1% | Minoxidil lotion 24% | Local hair products (lotions and shampoos) and collagen and iron supplements | |
| Low hair quality and vitality | 72 | Hair supplements formulas 45.5% | Nonpharmacologic interventions 39% | Local hair products (lotions and shampoos) and collagen | |
| Dehydrated skin | 86 | Urea products 27.9% | Oil baths 15.1% | Emollients 14% | Nonpharmacologic interventions, supplements and cosmetics |
| Bruises | 9 | Heparin Na gel 55.5% | Chestnut extract gel 22.2% | Nonpharmacologic interventions | |
| Thick skin | 16 | Urea products 57% | Nonpharmacologic interventions, supplements and cosmetics | ||
| Visible changes on skin and nails | 19 | Skin–hair–nail vitamin formulas 57.9% | Antimycotic 15.8% | Collagen, urea creams and nonpharmacologic interventions |
| Symptoms | ≤43 Age | 44–59 Age | ≥60 Age | p * |
|---|---|---|---|---|
| N | 447 (51.3) | 254 (29.2) | 170 (19.5) | |
| Number of symptoms | ||||
| 1 | 156 (34.9) | 94 (37.0) | 50 (29.4) | 0.003 |
| 2 | 90 (20.1) | 47 (18.5) | 33 (19.4) | |
| 3 | 67 (15.0) | 32 (12.6) | 15 (8.8) | |
| Respiratory tract | 244 (54.5) | 139 (54.7) | 88 (51.8) | 0.796 |
| Muscle system | 99 (22.1) | 68 (26.8) | 54 (31.8) | 0.041 |
| Digestive system | 120 (26.8) | 67 (26.4) | 63 (37.1) | 0.027 |
| Insomnia and sleeping disorders | 74 (16.6) | 57 (22.4) | 37 (21.8) | 0.109 |
| Behavioural changes | 53 (11.9) | 37 (14.6) | 21 (12.4) | 0.577 |
| Cognitive functions | 120 (26.8) | 68 (26.8) | 52 (30.6) | 0.614 |
| Immunity status | 142 (31.8) | 75 (29.5) | 60 (35.3) | 0.458 |
| Fatigue and exhaustion | 139 (31.1) | 66 (26.0) | 60 (35.3) | 0.133 |
| CV system disorders | 42 (9.6) | 39 (15.4) | 21 (12.4) | 0.076 |
| Change in vital signs | 42 (9.4) | 41 (16.1) | 40 (23.5) | <0.001 |
| Skin, hair and nail changes and conditions | 123 (27.5) | 73 (28.7) | 39 (22.9) | 0.391 |
| Other | 49 (11.0) | 31 (12.2) | 26 (15.3) | 0.339 |
| The Self-Reported Severity of the Symptoms Median (Q1–Q3) # | Follow-Up Rate % | Referred to Physician % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Symptoms | ≤43 Age | 44–59 Age | ≥60 Age | ≤43 Age | 44–59 Age | ≥60 Age | ≤43 Age | 44–59 Age | ≥60 Age |
| Muscle system | |||||||||
| Pain and cramps | 3 (2–3) | 3 (2–3) | 3 (3–3) | 82.8 | 91.2 | 96.3 | 8.0 | 11.8 | 20.4 |
| Digestive system | |||||||||
| Digestion | 3 (2–3) | 3 (2–3) | 3 (2–3) | 88.6 | 88.0 | 84.2 | 7.2 | 8.0 | 15.0 |
| Appetite | 3 (2–3) | 3 (2–3) | 3 (2–3) | 84.1 | 93.0 | 84.6 | 6.4 | 10 | 3.8 |
| Diarrhoea | 3 (2–3) | 2 (2–3) | 2.5 (2–4) | 89.6 | 83.3 | 96.2 | 14.5 | 7.5 | 23.1 |
| Intestinum (flatulence in intestines) | 3 (2–3.5) | 2 (2–3) | 3 (2–4) | 90.5 | 90.0 | 94.1 | 9.5 | 5.0 | 11.8 |
| Vital signs | |||||||||
| Saturation | 2 (2–3) | 3 (2.8–4) | 3 (2–3) | 100 | 83.3 | 100 | 50.0 | 17.0 | 28.6 |
| Pulse | 3 (2–3.75) | 3 (2–4) | 2.5 (2–3) | 75.0 | 78.6 | 75.0 | 66.6 | 57.1 | 75.0 |
| Sight | 3 (2.5–3.3) | 2(1.8–3.3) | 3 (1.5–3) | 10 | 100 | 76.9 | 66.6 | 50.0 | 30.8 |
| Hearing | 3 (3–4) | 3 (2–4) | 3 (2.5–4) | 60.0 | 100 | 80.0 | 70.0 | 40.0 | 60.0 |
| Paraesthesia | 3 (3–4) | 3 (2–3) | 3 (2–3) | 92.9 | 92.9 | 100 | 28.6 | 20.0 | 5.0 |
| Symptom | N | Recommended Self-Medication 1 | Recommended Self-Medication 2 | Recommended Self-Medication 3 | Other Recommendations |
|---|---|---|---|---|---|
| Digestion | 86 | Probiotics and/or prebiotics 43% | Herbal laxatives 19.7% | Plant fibres 10.6% | Supplements |
| Appetite | 84 | Vitamin B 69% | Regulated diet 11.9% | Probiotics 10.7% | Supplements |
| Diarrhoea | 108 | Probiotics and/or prebiotics 88.1% | Activated charcoal 10.2% | Regulated diet | |
| Intestinum (flatulence in the intestines) | 69 | Probiotics and/or prebiotics 37.7% | Herbal extracts 15.9% | Simethicone 14.5% | Regulated diet |
| Saturation | 16 | Outdoor activities 56.3% | Oximeter and more frequent checks 18.8% | Breathing exercises and hyperbaric chamber 24.9% | |
| Pulse | 10 | Coenzyme Q10 50% | Magnesium 30% | Astaxanthin and other antioxidant formulas 20% | |
| Sight | Eyedrops 70% | Vitamin eye formulas 30% | |||
| Hearing | 13 | Ginkgo 46% | Ear spray 31% | Supplements 23% | |
| Paraesthesia | 50 | Alpha lipoic acid 48% | B complex 26% | Massages and physical therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šipetić, T.; Rajković, D.; Bogavac Stanojević, N.; Marinković, V.; Meštrović, A.; Rouse, M.J. SMART Pharmacists Serving the New Needs of the Post-COVID Patients, Leaving No-One Behind. Pharmacy 2023, 11, 61. https://doi.org/10.3390/pharmacy11020061
Šipetić T, Rajković D, Bogavac Stanojević N, Marinković V, Meštrović A, Rouse MJ. SMART Pharmacists Serving the New Needs of the Post-COVID Patients, Leaving No-One Behind. Pharmacy. 2023; 11(2):61. https://doi.org/10.3390/pharmacy11020061
Chicago/Turabian StyleŠipetić, Tatjana, Dragana Rajković, Nataša Bogavac Stanojević, Valentina Marinković, Arijana Meštrović, and Michael J. Rouse. 2023. "SMART Pharmacists Serving the New Needs of the Post-COVID Patients, Leaving No-One Behind" Pharmacy 11, no. 2: 61. https://doi.org/10.3390/pharmacy11020061
APA StyleŠipetić, T., Rajković, D., Bogavac Stanojević, N., Marinković, V., Meštrović, A., & Rouse, M. J. (2023). SMART Pharmacists Serving the New Needs of the Post-COVID Patients, Leaving No-One Behind. Pharmacy, 11(2), 61. https://doi.org/10.3390/pharmacy11020061







