Analysis of Pharmacy Cardiac Optimization Clinic for Patients with New Onset Atrial Fibrillation Detected via Cardiac Implantable Electronic Device Clinic
Abstract
1. Introduction
2. Materials and Methods
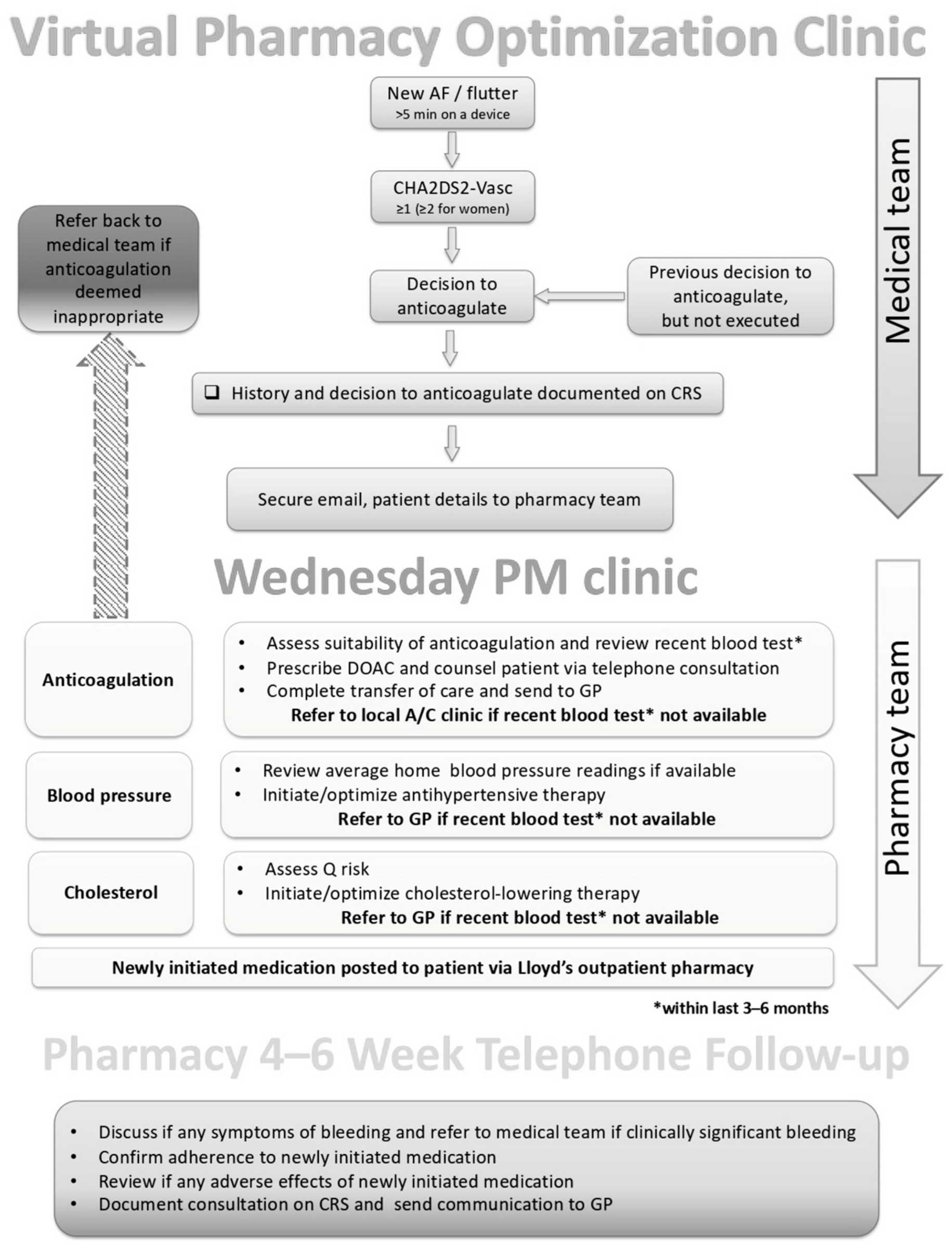
2.1. PCOC Structure
2.2. PCOC Responsibilities
- A cardiovascular review of patient-specific factors. Such factors included: sex, age, weight, smoking status, indication, comorbidities, left ventricular ejection fraction, previous incidence of myocardial infarction or stroke, blood pressure, HbA1c, full blood count, lipid panel, renal function, liver function, international normalized ratio, CHA2DS2VASc score, HAS-BLED score, ORBIT score, QRISK, assess need for concomitant, and all current medications [20,21,22].
- Initiation of anticoagulation. The choice of anticoagulant was facilitated through discussion with the patient and in consideration of individual patient needs. Almost exclusively, a direct oral anticoagulant (DOAC) was selected based on several patient-related factors.
- Assessment of lipid therapy. If there was established cardiovascular disease, the pharmacist would ensure that a high-intensity statin was prescribed (namely atorvastatin 80 mg daily). If there was no established CVD, the 10-year cardiovascular risk (using QRISK) was assessed, and if the risk was greater than 10% then initiation of atorvastatin 20 mg daily was considered, in addition to lifestyle advice [23,24].
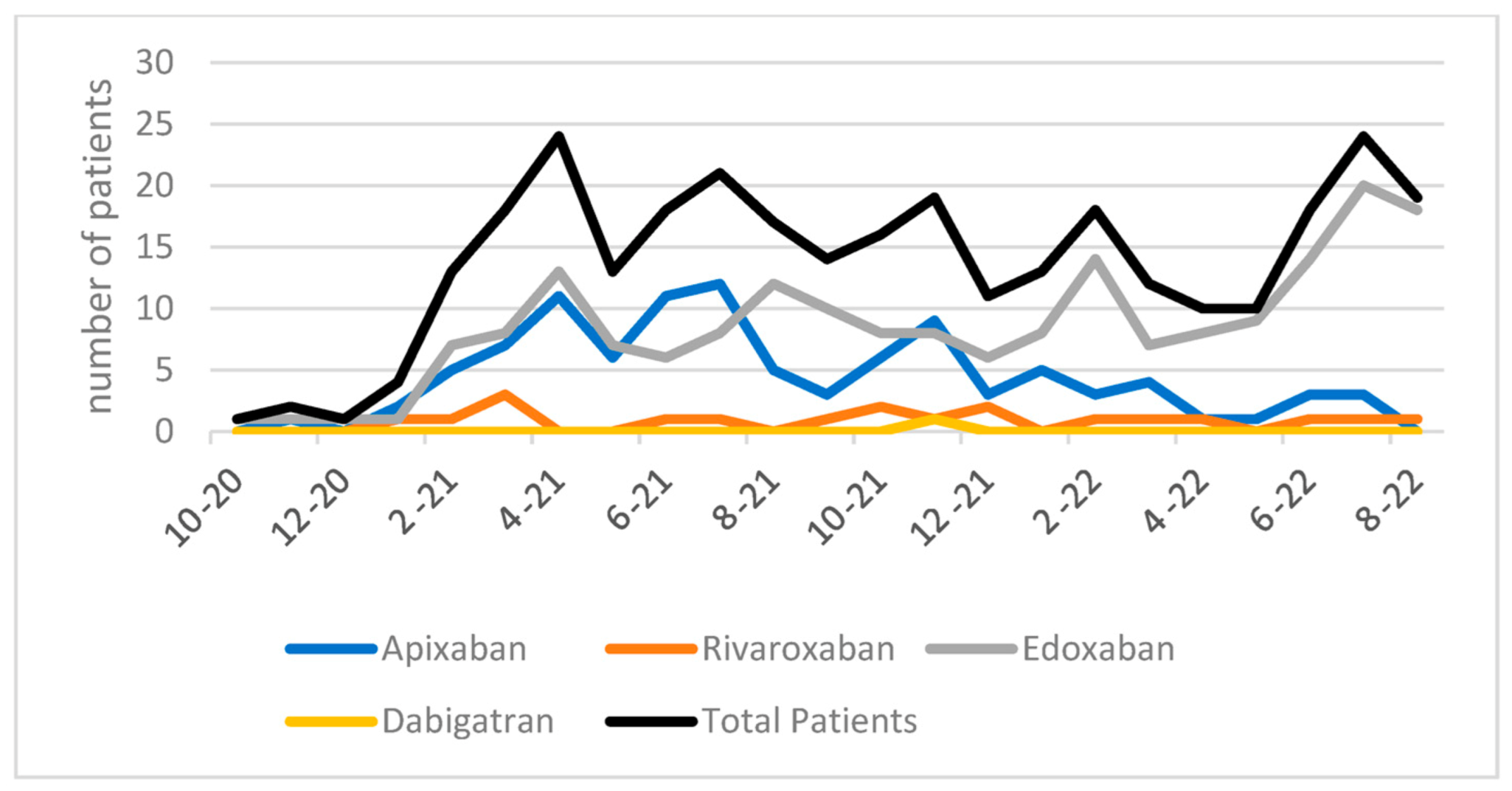
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frankel, D.S.; Parker, S.E.; Rosenfeld, L.E.; Gorelick, P.B. HRS/NSA 2014 Survey of Atrial Fibrillation and Stroke: Gaps in Knowledge and Perspective, Opportunities for Improvement. J. Stroke Cerebrovasc. Dis. 2015, 24, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; Fonarow, G.C. Preventing Stroke in Patients with Atrial Fibrillation—A Steep Climb Away From Achieving Peak Performance. JAMA Cardiol. 2016, 1, 63–64. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef]
- Baranova, E. Oral Anticoagulation in Patients with Non-Valvular Atrial Fibrillation and a CHA2DS2-VASC Score of 1: How to Solve This Problem? European Society of Cardiology. 2020. Available online: https://www.escardio.org/Councils/Council-for-Cardiology-Practice-(CCP)/News/oral-anticoagulation-in-patients-with-non-valvular-atrial-fibrillation-and-a-cha (accessed on 2 November 2022).
- Gao, X.; Cai, X.; Yang, Y.; Zhou, Y.; Zhu, W. Diagnostic Accuracy of the HAS-BLED Bleeding Score in VKA- or DOAC-Treated Patients With Atrial Fibrillation: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 757087. [Google Scholar] [CrossRef]
- Yao, X.; Gersh, B.J.; Sangaralingham, L.R.; Kent, D.M.; Shah, N.D.; Abraham, N.S.; Noseworthy, P.A. Comparison of the CHA2DS2-VASc, CHADS2, HAS-BLED, ORBIT, and ATRIA Risk Scores in Predicting Non-Vitamin K Antagonist Oral Anticoagulants-Associated Bleeding in Patients With Atrial Fibrillation. Am. J. Cardiol. 2017, 120, 1549–1556. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Kaufman, E.S.; Chen, L.Y.; Chung, M.K.; Elkind, M.S.V.; Joglar, J.A.; Leal, M.A.; McCabe, P.J.; Pokorney, S.D.; Yao, X.; et al. Subclinical and Device-Detected Atrial Fibrillation: Pondering the Knowledge Gap: A Scientific Statement from the American Heart Association. Circulation 2019, 140, e944–e963. [Google Scholar] [CrossRef] [PubMed]
- Shoeb, M.; Fang, M.C. Assessing bleeding risk in patients taking anticoagulants. J. Thromb. Thrombolysis 2013, 35, 312–319. [Google Scholar] [CrossRef]
- Cullen, M.W.; Kim, S.; Piccini, J.P., Sr.; Ansell, J.E.; Fonarow, G.C.; Hylek, E.M.; Singer, D.E.; Mahaffey, K.W.; Kowey, P.R.; Thomas, L.; et al. Risks and benefits of anticoagulation in atrial fibrillation: Insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 461–469. [Google Scholar] [CrossRef]
- Ballatore, A.; Matta, M.; Saglietto, A.; Desalvo, P.; Bocchino, P.P.; Gaita, F.; De Ferrari, G.M.; Anselmino, M. Subclinical and Asymptomatic Atrial Fibrillation: Current Evidence and Unsolved Questions in Clinical Practice. Medicina 2019, 55, 497. [Google Scholar] [CrossRef]
- Zhan, C.; Baine, W.B.; Sedrakyan, A.; Steiner, C. Cardiac device implantation in the United States from 1997 through 2004: A population-based analysis. J. Gen. Intern. Med. 2008, 23 (Suppl. S1), 13–19. [Google Scholar] [CrossRef]
- Zeitler, E.P.; Piccini, J.P. Remote monitoring of cardiac implantable electronic devices (CIED). Trends Cardiovasc. Med. 2016, 26, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Ali, S.; Robson, J.; Clements, R.; Theodoulou, A.; Wright, P.; Kearney, M.; Patel, R.; Sohaib, A.; Antoniou, S. Pharmacist-led multidisciplinary approach in preventing strokes in people with atrial fibrillation. Eur. Heart J. 2022, 43, ehac544.613. [Google Scholar] [CrossRef]
- Chahal, J.K.; Antoniou, S.; Earley, M.; Ali, S.; Saja, K.; Singh, H.; MacCallum, P.K.; Robson, J. Preventing strokes in people with atrial fibrillation by improving ABC. BMJ Open Qual. 2019, 8, e000783. [Google Scholar] [CrossRef]
- Das, M.; Panter, L.; Wynn, G.J.; Taylor, R.M.; Connor, N.; Mills, J.D.; Kirchhof, P.; Gupta, D. Primary Care Atrial Fibrillation Service: Outcomes from consultant-led anticoagulation assessment clinics in the primary care setting in the UK. BMJ Open 2015, 9, e009267. [Google Scholar] [CrossRef] [PubMed]
- Virdee, M.S.; Stewart, D. Optimizing the use of oral anticoagulant therapy for atrial fibrilation in primary care: A pharmacist-led intervention. Int. J. Clin. Pharm. 2017, 39, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Shantsila, E.; Wolff, A.; Lip, G.Y.; Lane, D.A. Optimising stroke prevention in patients with atrial fibrillation: Application of the GRASP-AF audit tool in a UK general practice cohort. Br. J. Gen. Pract. 2015, 65, e16–e23. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.M.; Dier, J.G. Comparison of two different models of anticoagulation management services with usual medical care. Pharmacotherapy 2010, 30, 330–338. [Google Scholar] [CrossRef]
- Ahmed, A.; Tanveer, M.; Shrestha, S.; Khatiwada, A.P.; Khanal, S.; Dujaili, J.A.; Paudyal, V. Interventions and Impact of Pharmacist-Delivered Services for People Infected with COVID-19: A Systematic Review. Healthcare 2022, 10, 1630. [Google Scholar] [CrossRef]
- University of Nottingham, EMIS. Welcome to the QRISK 3-2018 Risk Calculator. ClinRisk. 2018. Available online: https://www.qrisk.org/three/ (accessed on 5 November 2022).
- Hwang, C. CHA2DS2-VASc Score for Atrial Fibrillation Stroke Risk. MDCalc. Available online: https://www.mdcalc.com/calc/801/cha2ds2-vasc-score-atrial-fibrillation-stroke-risk (accessed on 22 December 2022).
- Hwang, C. HAS-BLED Score for Major Bleeding Risk. Available online: https://www.mdcalc.com/calc/807/has-bled-score-major-bleeding-risk (accessed on 22 December 2022).
- Chróinín, D.N.; Asplund, K.; Åsberg, S.; Callaly, E.; Cuadrado-Godia, E.; Díez-Tejedor, E.; Di Napoli, M.; Engelter, S.T.; Furie, K.L.; Giannopoulos, S.; et al. Statin therapy and outcome after ischemic stroke: Systematic review and meta-analysis of observational studies and randomized trials. Stroke 2013, 44, 448–456. [Google Scholar] [CrossRef]
- Till, L.T.; Voris, J.C.; Horst, J.B. Assessment of clinical pharmacist management of lipid-lowering therapy in a primary care setting. J. Manag. Care Pharm. 2003, 9, 269–273. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Sapko, M.T. Blood pressure lowering treatment for preventing stroke recurrence: A systematic review and meta-analysis. Int. Arch. Med. 2009, 2, 30. [Google Scholar] [CrossRef] [PubMed]
- Vivian, E.M. Improving Blood Pressure Control in a Pharmacist-Managed Hypertension Clinic. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2002, 22, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Atrial Fibrillation: Diagnosis and Management. 2021. Available online: https://www.nice.org.uk/guidance/ng196 (accessed on 22 December 2022).
- National Health Service. Operational Note: Commissioning Recommendations for National Procurement for DOACs. 2022. Available online: https://www.england.nhs.uk/wp-content/uploads/2022/01/B1279-national-procurement-for-DOACs-commissioning-recommendations-v1.pdf (accessed on 19 February 2023).
| Mean Age | 76 years old (42–100 years) | |
| Sex | Male | 225 (71.4%) |
| Female | 90 (28.6%) | |
| Current Smokers | 26 (8.3%) | |
| Mean CHA2DS2VASc | Male | 3.5 |
| Female | 4.2 | |
| % with LVSD/HCM | 101 (32.0%) | |
| % with hypertension | 209 (66.3%) | |
| % with diabetes | 87 (27.6%) | |
| % with history of CVA/TIA | 51 (16.1%) | |
| % with history of IHD/PVD | 124 (39.4%) | |
| Mean HASBLED | 1.2 | |
| On lipid therapy at time of PCOC referral | 234 (74.3%) | |
| On antiplatelet therapy at time of PCOC referral | 190 (60.3%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schellhase, E.; Stanko, M.; Kinstler, N.; Miller, M.L.; Antoniou, S.; Fhadil, S.; Patel, M.; Wright, P. Analysis of Pharmacy Cardiac Optimization Clinic for Patients with New Onset Atrial Fibrillation Detected via Cardiac Implantable Electronic Device Clinic. Pharmacy 2023, 11, 48. https://doi.org/10.3390/pharmacy11020048
Schellhase E, Stanko M, Kinstler N, Miller ML, Antoniou S, Fhadil S, Patel M, Wright P. Analysis of Pharmacy Cardiac Optimization Clinic for Patients with New Onset Atrial Fibrillation Detected via Cardiac Implantable Electronic Device Clinic. Pharmacy. 2023; 11(2):48. https://doi.org/10.3390/pharmacy11020048
Chicago/Turabian StyleSchellhase, Ellen, Madeline Stanko, Natalie Kinstler, Monica L. Miller, Sotiris Antoniou, Sadeer Fhadil, Mital Patel, and Paul Wright. 2023. "Analysis of Pharmacy Cardiac Optimization Clinic for Patients with New Onset Atrial Fibrillation Detected via Cardiac Implantable Electronic Device Clinic" Pharmacy 11, no. 2: 48. https://doi.org/10.3390/pharmacy11020048
APA StyleSchellhase, E., Stanko, M., Kinstler, N., Miller, M. L., Antoniou, S., Fhadil, S., Patel, M., & Wright, P. (2023). Analysis of Pharmacy Cardiac Optimization Clinic for Patients with New Onset Atrial Fibrillation Detected via Cardiac Implantable Electronic Device Clinic. Pharmacy, 11(2), 48. https://doi.org/10.3390/pharmacy11020048







