Potential Drug-Related Problems in Pediatric Patients—Describing the Use of a Clinical Decision Support System at Pharmacies in Sweden
Abstract
:1. Introduction
- The number and proportion of children (ages 0–12 years) receiving EES analyses over time;
- The type of alerts for potential DRPs being generated by EES for pediatric patients;
- The proportion of EES alerts being resolved for pediatric patients and examine potential differences between the types of alerts;
- What kinds of actions were taken to resolve the generated alerts for pediatric patients.
2. Materials and Methods
2.1. Timeline
2.2. Setting
2.3. Electronic Expert Support System (EES)
2.4. Data and Statistics on the Use of EES
2.4.1. EES: Closing an Alert
2.4.2. National Intervention
- Year 2018 (week 15), focused on the elderly (aged 75 years or older);
- Year 2019 (week 15) focused on the elderly (aged 75 years or older);
- Year 2020 (week 43) focused on the pediatric population (aged 0–12 years);
- Year 2021 (week 16) focused on therapy duplication;
- Year 2022 (week 14) focused on DDIs.
2.5. Data Analysis
2.6. Ethics Statement
3. Results
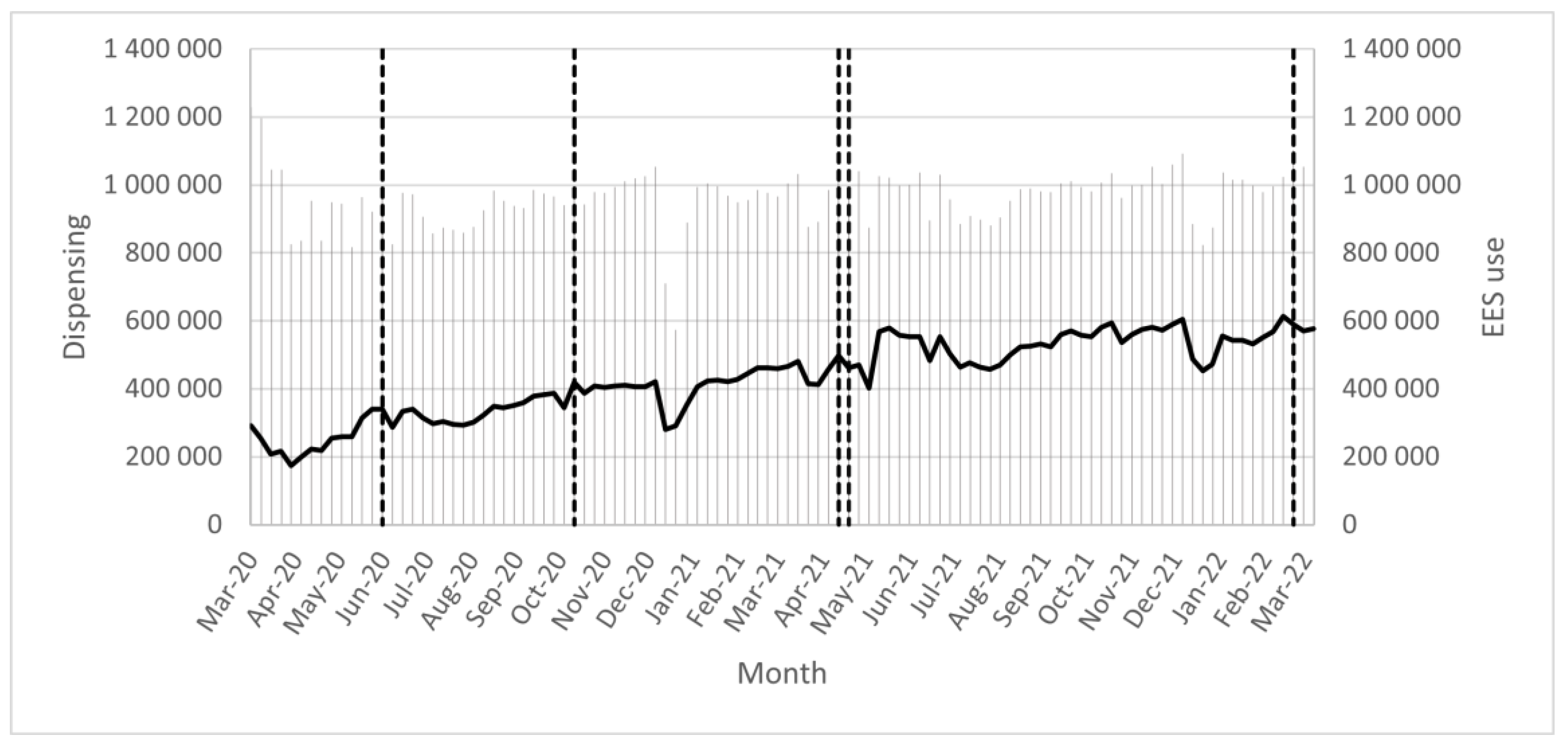
3.1. The Use of EES
3.2. EES Analyses and the Numbers of Alerts Generated and Resolved
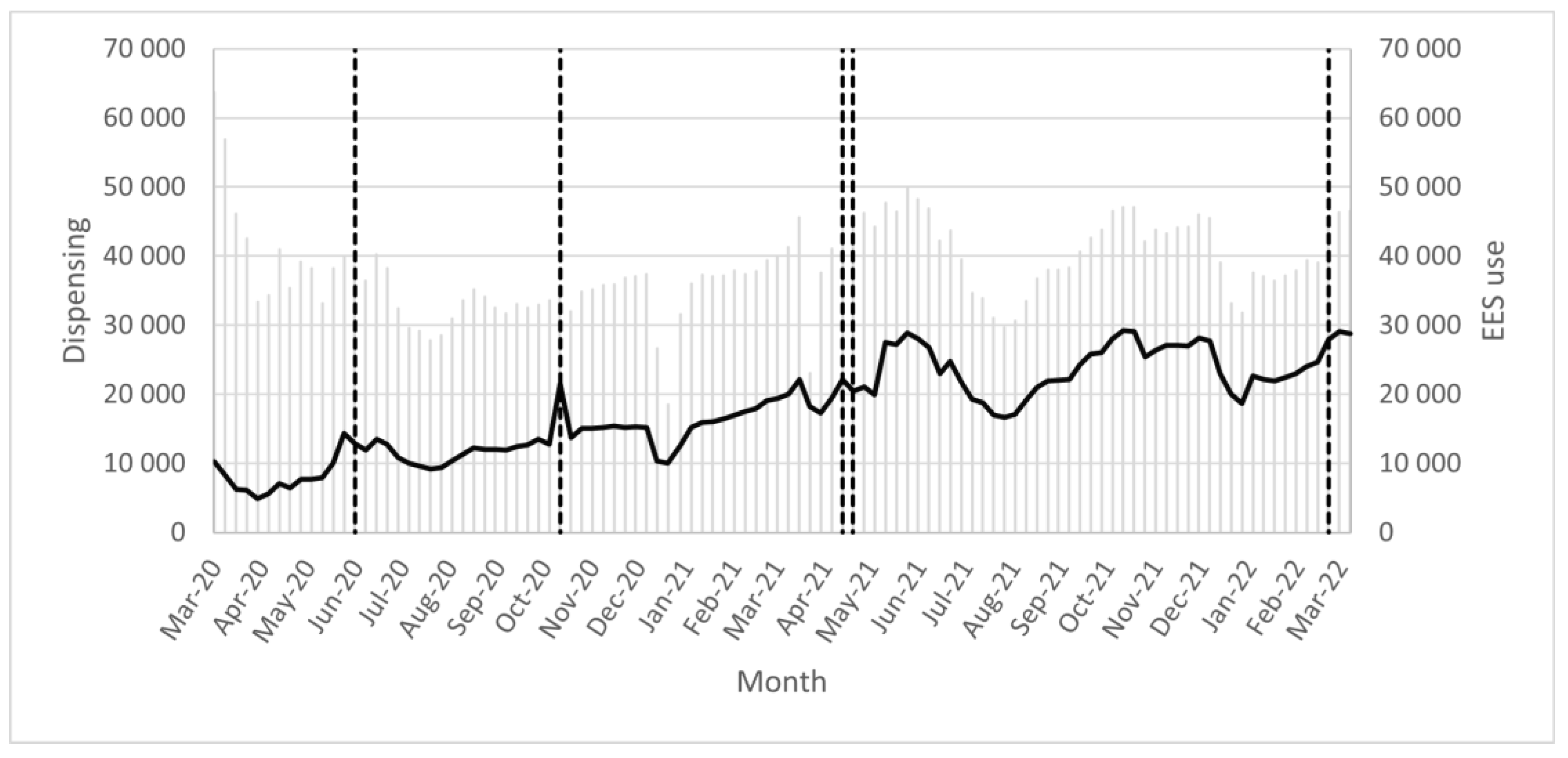
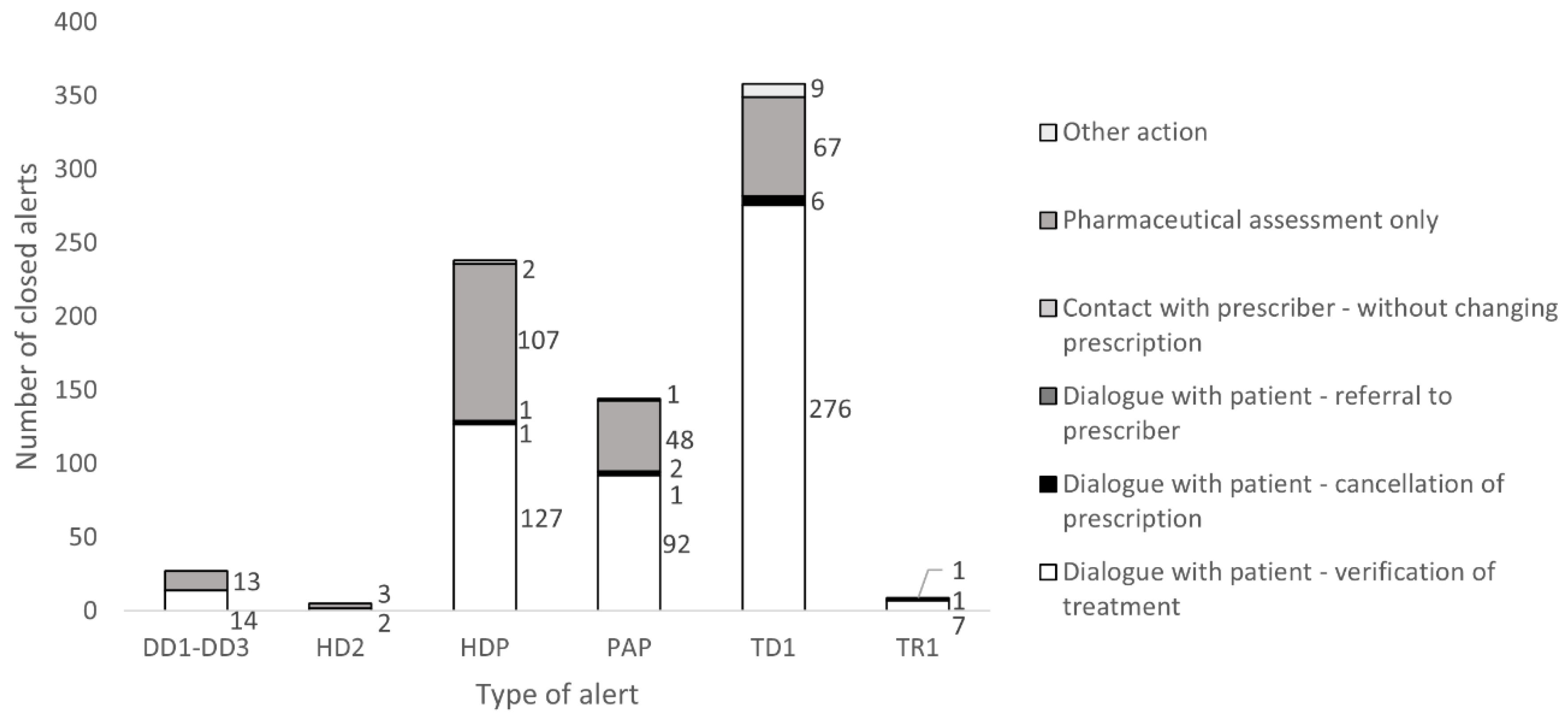
3.3. EES Analyses and the Numbers of Alerts Generated and Resolved for Pediatrics
4. Discussion
4.1. The Increasing Use of EES and Possible Explanations
4.2. Challenges with Non-Relevant Alerts Generated
4.3. DRPs and the Benefits of CDSS and Medication Reviews for Children
4.4. Alerts Being Closed
4.5. Method Discussion (Strengths and Weaknesses)
4.6. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holm, J.; Eiermann, B.; Eliasson, E.; Mannheimer, B. A limited number of prescribed drugs account for the great majority of drug-drug interactions. Eur. J. Clin. Pharmacol. 2014, 70, 1375–1383. [Google Scholar] [CrossRef]
- Hammar, T.; Hovstadius, B.; Lidström, B.; Petersson, G.; Eiermann, B. Potential drug related problems detected by electronic expert support system in patients with multi-dose drug dispensing. Int. J. Clin. Pharm. 2014, 36, 943–952. [Google Scholar]
- Danish, A.; Westerlund, T.; Eriksson, T. Use of an electronic expert support system in a Swedish community pharmacy to identify and resolve drug-related problems. Authorea. Available online: https://www.diva-portal.org/smash/get/diva2:1528212/FULLTEXT01.pdf (accessed on 26 May 2020).
- Tolley, C.L.; Slight, S.P.; Husband, A.K.; Watson, N.; Bates, D.W. Improving medication-related clinical decision support. Am. J. Health Pharm. 2018, 75, 239–246. [Google Scholar] [CrossRef]
- Hammar, T.; Zetterholm, M. Patients’ views on information about medications—A pharmacy-based survey focusing on their information sources and experiences of pharmacists using a clinical decision support system. In Proceedings of the 18th International Symposium on Health Information Management Research, Kalmar, Sweden, 17–18 October 2020. [Google Scholar]
- Coleman, J.J.; Van Der Sijs, H.; Haefeli, W.E.; Slight, S.P.; Mcdowell, S.E.; Seidling, H.M.; Eiermann, B.; Aarts, J.; Ammenwerth, E.; Ferner, R.E.; et al. On the alert: Future priorities for alerts in clinical decision support for computerized physician order entry identified from a European workshop. BMC Med. Inform. Decis. Mak. 2013, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Hammar, T.; Hellström, L.; Ericson, L. The use of a decision support system in Swedish pharmacies to identify potential drug-related problems—Effects of a national intervention focused on reviewing elderly patients’ prescriptions. Pharmacy 2020, 8, 118. [Google Scholar] [CrossRef]
- Hammar, T.; Nyström, S.; Petersson, G.; Åstrand, B.; Rydberg, T. Patients satisfied with e-prescribing in Sweden: A survey of a nationwide implementation. J. Pharm. Health Serv. Res. 2011, 2, 97–105. [Google Scholar] [CrossRef]
- Elektroniskt Expertstöd (EES). Available online: https://elektronisktexpertstod.se/ (accessed on 30 April 2022).
- Hammar, T.; Lidström, B.; Petersson, G.; Gustafson, Y.; Eiermann, B. Potential drug-related problems detected by electronic expert support system: Physicians’ views on clinical relevance. Pharm. Weekbl. 2015, 37, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Hwang, H.; Lee, K.; Lee, H.-Y.; Kim, E.; Kim, M.; Cho, I.Y. Barriers and Facilitators to Implementation of Medication Decision Support Systems in Electronic Medical Records: Mixed Methods Approach Based on Structural Equation Modeling and Qualitative Analysis. JMIR Public Health Surveill. 2020, 8, e18758. [Google Scholar] [CrossRef]
- Damoiseaux-Volman, B.A.; Medlock, S.; van der Meulen, D.M.; de Boer, J.; Romijn, J.A.; van der Velde, N.; Abu-Hanna, A. Clinical validation of clinical decision support systems for medication review: A scoping review. Br. J. Clin. Pharmacol. 2022, 88, 2035–2051. [Google Scholar] [CrossRef]
- Bryant, A.D.; Fletcher, G.S.; Payne, T.H. Drug interaction alert override rates in the meaningfyl use era: No evidence of progress. Appl. Clin. Inform. 2014, 5, 802–813. [Google Scholar] [CrossRef]
- Thiruchelvam, K.; Hasan, S.S.; Pudmenzky, A.; Se, W.P.; Kairuz, T. Development, validation and evaluation of an online medication review tool (MedReview). PLoS ONE 2022, 17, e0269322. [Google Scholar] [CrossRef] [PubMed]
- Heringa, M.; Floor-Schreudering, A.; Tromp, P.C.; de Smet, P.A.G.M.; Bouvy, M.L. Nature and frequency of drug therapy alerts generated by clinical decision support in community pharmacy. Pharmacoepidemiol. Drug Saf. 2015, 25, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Böttiger, Y.; Laine, K.; Andersson, M.L.; Korhonen, T.; Molin, B.; Ovesjö, M.-L.; Tirkkonen, T.; Rane, A.; Gustafsson, L.L.; Eiermann, B. SFINX—A drug-drug interaction database designed for clinical decision support systems. Eur. J. Clin. Pharmacol. 2009, 65, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.; Eiermann, B.; Kimland, E.; Mannheimer, B. Prevalence of potential drug-drug interactions in Swedish pediatric outpatients. PLoS ONE 2019, 14, e0220685. [Google Scholar] [CrossRef]
- Kaushal, R.; Bates, D.W.; Landrigan, C.; McKenna, K.J.; Clapp, M.D.; Federico, F.; Goldmann, D.A. Medication Errors and Adverse Drug Events in Pediatric Inpatients. JAMA 2001, 285, 2114–2120. [Google Scholar] [CrossRef]
- Conn, R.L.; Kearney, O.; Tully, M.P.; Shields, M.D.; Dornan, T. What causes prescribing errors in children? Scoping review. BMJ Open 2019, 9, e028680. [Google Scholar] [CrossRef]
- Ivanovska, V.; Rademaker, C.M.; van Dijk, L.; Mantel-Teeuwisse, A.K. Pediatric Drug Formulations: A Review of Challenges and Progress. Pediatrics 2014, 134, 361–372. [Google Scholar] [CrossRef]
- Mei, M.; Xu, H.; Wang, L.; Huang, G.; Gui, Y.; Zhang, X. Current practice and awareness of pediatric off-label drug use in Shanghai, China -a questionnaire-based study. BMC Pediatr. 2019, 19, 281. [Google Scholar] [CrossRef]
- Kimland, E.; Nydert, P.; Odlind, V.; Böttiger, Y.; Lindemalm, S. Paediatric drug use with focus on off-label prescriptions at Swedish hospitals—A nationwide study. Acta Paediatr. 2012, 101, 772–778. [Google Scholar] [CrossRef]
- Nguyen, T.; Le, V.; Quach, D.; Diep, H.; Nguyen, N.; Lam, A.; Pham, S.; Taxis, K.; Nguyen, T.; Nguyen, P. Drug-Related Problems in Prescribing for Pediatric Outpatients in Vietnam. Healthcare 2021, 9, 327. [Google Scholar] [CrossRef]
- Alsulaiman, K.; Aljeraisy, M.; Alharbi, S.; Alsulaihim, I.; Almolaiki, M.; Alammari, M. Evaluation of prescribing medication errors in a pediatric outpatient pharmacy. Int. J. Med. Sci. Public Health 2017, 6, 1588–1593. [Google Scholar] [CrossRef]
- Hamadouk, R.M.; Mohammed, F.M.; Albashair, E.D.; Yousef, B.A. Evaluation of community pharmacists’ competences in identifying and resolve drug-related problems in a pediatric prescription using the simulated patient method. Pharmacy 2023, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.W.; Oliveri, L.M.; Ohler, K.H.; Briars, L. Identification of Errors in Pediatric Prescriptions and Interventions to Prevent Errors: A Survey of Community Pharmacists. J. Pediatr. Pharmacol. Ther. 2019, 24, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, F.; van Gelder, T.G.; Bollen, C.W.; Liem, Y.T.; Egberts, T.C. The effect of a decision support system on the incidence of prescription errors in a PICU. J. Clin. Pharm. Ther. 2021, 47, 330–344. [Google Scholar] [CrossRef] [PubMed]
- van Rosse, F.; Maat, B.; Rademaker, C.M.A.; van Vught, A.J.; Egberts, A.C.G.; Bollen, C.W. The Effect of Computerized Physician Order Entry on Medication Prescription Errors and Clinical Outcome in Pediatric and Intensive Care: A Systematic Review. Pediatrics 2009, 123, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Svensk Farmaci. Soon Okey to Use EES without Consent [In Swedish, Snart Okej att Använda EES utan Samtycke]. Available online: https://www.svenskfarmaci.se/2019/12/16/snart-okej-att-anvanda-ees-utan-samtycke/ (accessed on 22 May 2022).
- Sveriges Apoteksförening. Reports about the Pharmacy Industry and Focus Weeks [In Swedish]. Available online: http://www.sverigesapoteksforening.se/apoteksbranschen/ (accessed on 12 April 2022).
- Hammar, T.; Mzil, L.; Eiermann, B. Discrepancies in patients’ medication lists from pharmacies in Sweden: An interview study before the implementation of the Swedish National Medication List. Pharm. Weekbl. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Olsson, J.; Kimland, E.; Pettersson, S.; Odlind, V. Paediatric drug use with focus on off-label prescriptions in Swedish outpatient care—A nationwide study. Acta Paediatr. 2011, 100, 1272–1275. [Google Scholar] [CrossRef]
- Villa Zapata, L.; Subbian, V.; Boyce, R.D.; Hansten, P.D.; Horn, J.R.; Gephart, S.M.; Romero, A.; Malone, D.C. Overriding Drug-Drug Interaction Alerts in Clinical Decision Support Systems: A Scoping Review. Stud. Health Technol. Inform. 2022, 290, 380–384. [Google Scholar] [PubMed]
| Data | Description | Time Period |
|---|---|---|
| Number of EES analyses | Number of times EES is used (calculated once/unique individual/pharmacy/day). * | Per week from week 11 of 2020 to week 11 of year 2022. (T&P) |
| Individuals having prescriptions dispensed | Number of individuals having prescriptions dispensed (calculated once/unique individual/pharmacy/day). * | Per week from week 11 of 2020 to week 11 of year 2022. (T&P) |
| Proportion of individuals getting an EES analysis | Number of EES analyses/Individuals having prescriptions dispensed (%). ** | Per week (week 11 and 36 of 2020, week 11 and 36 of 2021, and week 11 of 2022). (T&P) |
| Number of EES alerts | Total number of alerts from EES. Each time a pharmacist presses the EES button, EES analyses the patient’s prescriptions and may generate a number of alerts. * | Week 11 of 2022 (P) |
| Average number of alerts per EES analysis | Number of EES alerts/number of EES analyses. The average number of generated alerts each time EES is used. ** | Week 11 of 2022 (P) |
| Closed (resolved) EES alerts | Total number of closed alerts from EES. The pharmacist can close an alert after resolving it. * | Per week from week 11 of 2020 to week 11 of year 2022. (T&P) |
| Proportion of alerts being closed | Number of closed alerts/number of alerts (%). ** | Week 11 of 2022 (P) |
| Type of alert generated, resolved, and documented action | The pharmacist can close an alert after resolving it and provide the reason for closing it according to the eHealth Agency’s available reasons. * | Week 11 of 2022 (P) |
| Week 11 2020 | Week 36 2020 | Week 11 2021 | Week 36 2021 | Week 11 2022 | ||
|---|---|---|---|---|---|---|
| Total population | Number of EES analyses | 290,678 | 345,003 | 466,984 | 531,675 | 576,510 |
| Individuals having prescriptions dispensed | 1,227,797 | 953,236 | 1,004,752 | 980,988 | 1,019,349 | |
| Proportion of individuals receiving an EES analysis (%) | 24% | 36% | 46% | 54% | 57% | |
| 0–12 years old | Number of EES analyses | 10,257 | 12,015 | 20,051 | 22,000 | 28,748 |
| Individuals having prescriptions dispensed * | 69,042 | 37,417 | 41,337 | 41,475 | 50,827 | |
| Proportion of individuals receiving an EES analysis (%) | 15% | 32% | 49% | 53% | 57% | |
| Number of closed EES alerts | 576 | 290 | 461 | 479 | 858 |
| Alert Categories | Number of Alerts | Proportion of All Alerts (%) | Number of Closed Alerts | Proportion Being Closed (%) |
|---|---|---|---|---|
| High dose pediatric | 9339 | 30.3 | 238 | 2.55 |
| Therapy duplication | 7779 | 25.2 | 358 | 4.6 |
| Age warning pediatric | 7328 | 23.8 | 144 | 1.97 |
| Drug–drug interactions | 5605 | 18.2 | 27 | 0.48 |
| Supplementary review | 531 | 1.7 | 9 | 1.69 |
| High dose | 227 | 0.74 | 5 | 2.2 |
| Total | 30,809 | 100 | 781 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulkadir, S.A.; Wettermark, B.; Hammar, T. Potential Drug-Related Problems in Pediatric Patients—Describing the Use of a Clinical Decision Support System at Pharmacies in Sweden. Pharmacy 2023, 11, 35. https://doi.org/10.3390/pharmacy11010035
Abdulkadir SA, Wettermark B, Hammar T. Potential Drug-Related Problems in Pediatric Patients—Describing the Use of a Clinical Decision Support System at Pharmacies in Sweden. Pharmacy. 2023; 11(1):35. https://doi.org/10.3390/pharmacy11010035
Chicago/Turabian StyleAbdulkadir, Sazan Abass, Björn Wettermark, and Tora Hammar. 2023. "Potential Drug-Related Problems in Pediatric Patients—Describing the Use of a Clinical Decision Support System at Pharmacies in Sweden" Pharmacy 11, no. 1: 35. https://doi.org/10.3390/pharmacy11010035
APA StyleAbdulkadir, S. A., Wettermark, B., & Hammar, T. (2023). Potential Drug-Related Problems in Pediatric Patients—Describing the Use of a Clinical Decision Support System at Pharmacies in Sweden. Pharmacy, 11(1), 35. https://doi.org/10.3390/pharmacy11010035






