The Pharmacist’s Role in the Implementation of FASTHUG-MAIDENS, a Mnemonic to Facilitate the Pharmacotherapy Assessment of Critically Ill Patients: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Inclusion and Exclusion Criteria
2.3. Definitions
- Feeding: to determine the type of diet that best suits the characteristics of each patient, the algorithm proposed by Doig et al. was used [9]. In general, the following aspects were evaluated for every patient who received enteral/parental nutrition: the presence of drug-food interactions, protein intake between 1.2–2.0 g/kg (real body weight), daily caloric requirements between 25–30 kcal/kg/day, and an angle of the head of the bed of 30–45° in patients with enteral nutrition [9].
- Analgesia: to determine the level of pain, the Behavioral Pain Scale (BPS) was used, considering a score greater than 5 as significant pain [10]. The Visual Analog Pain Scale (VAS) was used when the patient’s condition allowed it [11]. In addition, opioid dose reductions via the use of adjuvants and significant adverse effects were monitored before performing a painful medical procedure [12].
- Thromboprophylaxis: the clinical guidelines of the HCB and the international treatment guidelines were used. The Padua Scale and the Caprini Risk Analysis Model were used to determine the need for thromboprophylaxis, and the suitability, dose, and route of administration of the selected drug were evaluated, as well as the patient’s contraindications to the use of the medication [14].
- Hyperactive or hypoactive delirium: for the daily monitoring of delirium risk in patients with a RASS score between −2 and 0, the Confusion Assessment Method for the ICU (CAM-ICU) was used. Based on international guidelines, the use of drugs to prevent delirium and the use of pharmacological and non-pharmacological measures for the treatment of delirium were evaluated [15].
- Stress ulcer prophylaxis: the use of medications for this parameter was evaluated in patients with risk factors such as respiratory distress (mechanical ventilation > 48 h), coagulopathy (platelets < 50,000/mm3, INR > 1.5 or TTPa twice the base value) and hepatic or renal disease, among others. The evaluation included doses, route of administration, and adverse effects of medication, especially those of great clinical relevance, such as C. difficile infection or pneumonia.
- Glucose control: the blood glucose level and hypoglycemic medications were reviewed. In addition, compliance with other aspects such as maintenance of glucose level between 140–180 mg/dL, management of hypoglycemia (glucose level < 70 mg/dL), and use of subcutaneous insulin were assessed.
- Medication reconciliation: chronic treatments of the patients were reviewed on the day of admission to the ICU. An evaluation of the need to maintain chronic pathologies under control was made, under the management of the acute condition of the patient (drug interactions and disease impediments).
- Antibiotics or antimicrobial agents: there was a review of whether drug selection, dose, route of administration, and frequency were in the diagnosis and signs and symptoms of the patients. In addition, dose adjustment and de-escalation after the results of cultures were reviewed. For this analysis, the Sanford Guide tool, the IDSA guidelines, and the HCB’s pharmacoepidemiologic reports were used [16,17].
- Indications for medications: it was checked that all medications and their prescribed doses and route of administration had an appropriate indication, using the UpToDate database [18].
- Drug dosing: drug doses were reviewed following the patient’s diagnosis and symptoms. For dose adjustment, the glomerular filtration rate was used as an indicator of renal function, and the Child−Pugh score was used as the liver function parameter.
- Electrolytes, hematology, and other laboratory results: daily revisions of these parameters were made via the electronic hospital records.
- No drug interactions, allergies, duplications, or side effects: the medical history of the patients was reviewed to determine if medications were prescribed for which the patient had previously reported an allergy if there was no duplication of therapy and if side effects were related to the treatment and drug interactions. For drug interactions, UpToDate’s Lexi-Comp® Drug Interactions tool was used.
- Stop dates: the indications given by the treating physicians regarding the duration of treatment were reviewed. In addition, it was verified that the duration of treatment was in accordance with the indication.
2.4. Data Collection
2.5. Statistical Analysis
2.6. Ethics Approval and Consent to Participate
3. Results
3.1. Patient Demographics
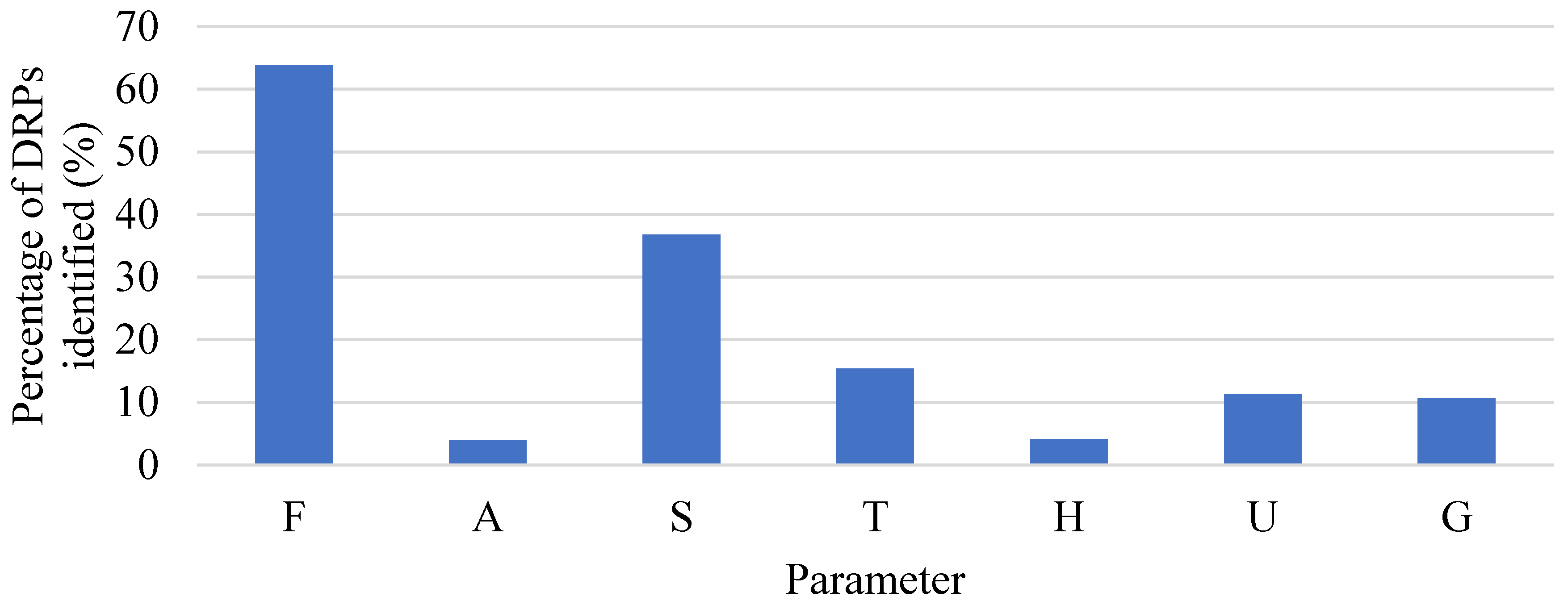
3.2. Assessment Stage
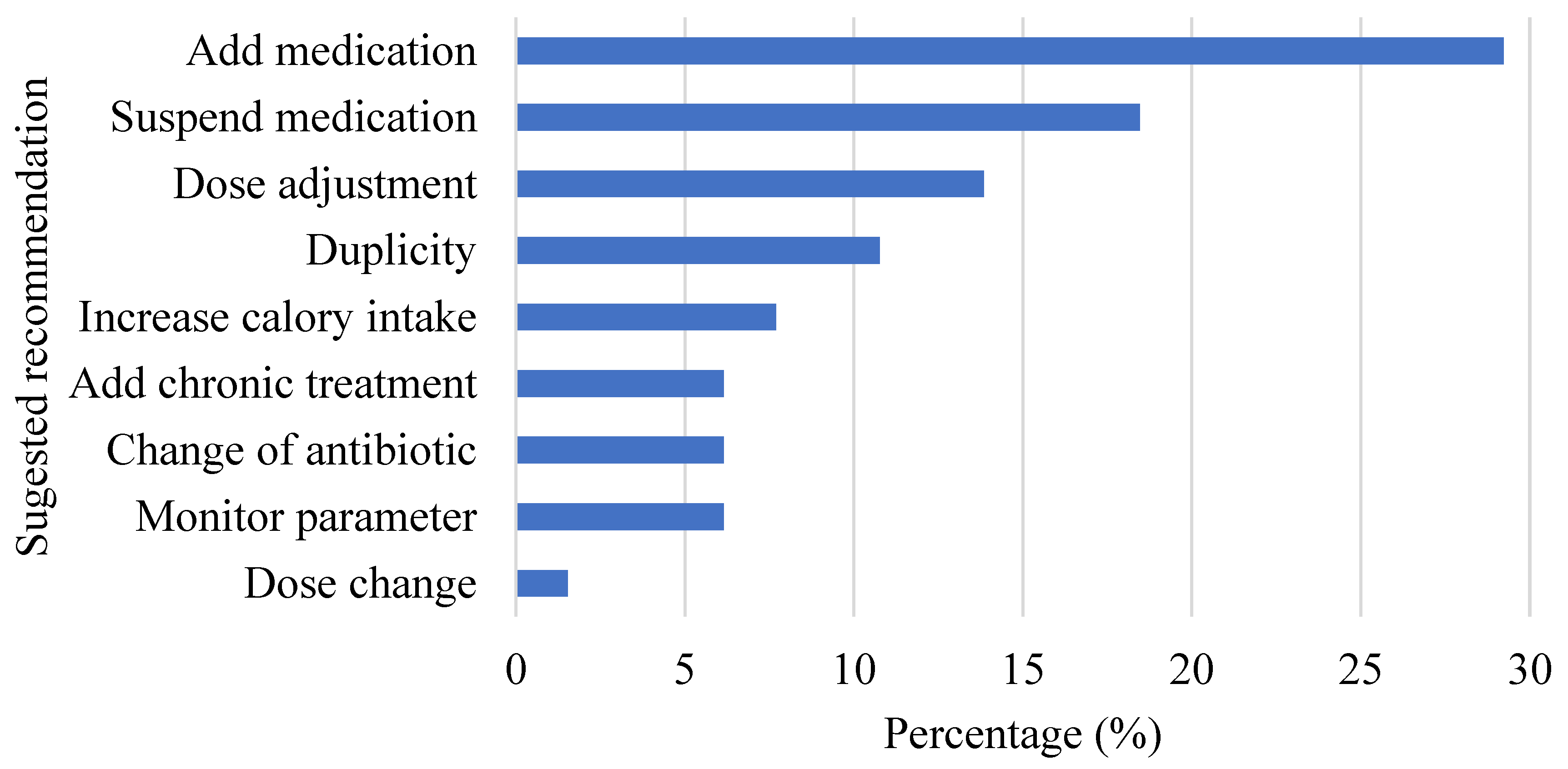
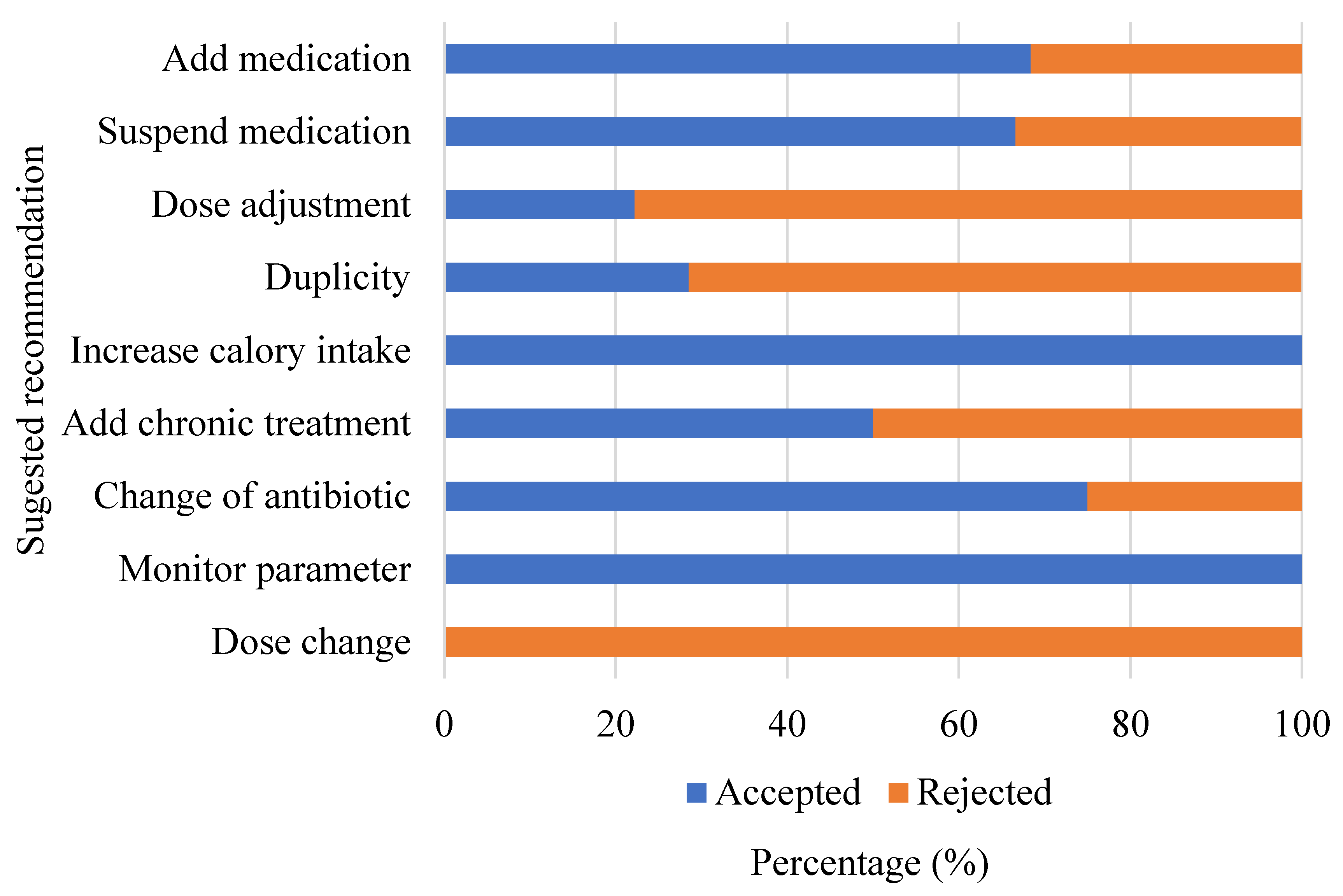
3.3. Implementation of the Protocol
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vincent, J.L. Give your patient a fast hug (at least) once a day. Crit. Care Med. 2005, 33, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Mabasa, V.H.; Malyuk, D.L.; Weatherby, E.M.; Chan, A. A Standardized, Structured Approach to Identifying Drug-Related Problems in the Intensive Care Unit: FASTHUG-MAIDENS. Can. J. Hosp. Pharm. 2011, 64, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson, S.C.; Mabasa, V.H.; Malyuk, D.L.; Perrott, J.L. Validity Evidence for FASTHUG-MAIDENS, a Mnemonic for Identifying Drug-Related Problems in the Intensive Care Unit. Can. J. Hosp. Pharm. 2013, 66, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Millo, O.; Climente-Martí, M.; Galbis-Bernácer, A.M. Clinical impact of an interdisciplinary patient safety program for managing drug-related problems in a long-term care hospital. Int. J. Clin. Pharm. 2017, 39, 1201–1210. [Google Scholar] [CrossRef]
- Bondesson, Å.; Eriksson, T.; Kragh, A.; Holmdahl, L.; Midlöv, P.; Höglund, P. In-hospital medication reviews reduce unidentified drug-related problems. Eur. J. Clin. Pharmacol. 2013, 69, 647–655. [Google Scholar] [CrossRef] [Green Version]
- Abunahlah, N.; Elawaisi, A.; Velibeyoglu, F.M.; Sancar, M. Drug related problems identified by clinical pharmacist at the Internal Medicine Ward in Turkey. Int. J. Clin. Pharm. 2018, 40, 360–367. [Google Scholar] [CrossRef]
- MacLaren, R.; Bond, C.A.; Martin, S.J.; Fike, D. Clinical and economic outcomes of involving pharmacists in the direct care of critically ill patients with infections. Crit. Care Med. 2008, 36, 3184–3189. [Google Scholar] [CrossRef]
- Rivkin, A.; Yin, H. Evaluation of the role of the critical care pharmacist in identifying and avoiding or minimizing significant drug-drug interactions in medical intensive care patients. J. Crit. Care 2011, 26, 104. [Google Scholar] [CrossRef]
- Doig, G.S. Effect of Evidence-Based Feeding Guidelines on Mortality of Critically Ill Adults. JAMA 2008, 300, 2731. [Google Scholar] [CrossRef]
- Aïssaoui, Y.; Zeggwagh, A.A.; Zekraoui, A.; Abidi, K.; Abouqal, R. Validation of a Behavioral Pain Scale in Critically Ill, Sedated, and Mechanically Ventilated Patients. Anesth. Analg. 2005, 101, 1470–1476. [Google Scholar] [CrossRef]
- Díez-Álvarez, E.; Arrospide, A.; Mar, J.; Cuesta, M. Valoración del dolor agudo postoperatorio. Rev. Calid. Asist. 2009, 24, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Devlin, J.W.; Skrobik, Y.; Gélinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2018, 46, 825–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, B.A.; Guzman, O.; Campbell, N.L.; Walroth, T.; Tricker, J.L.; Hui, S.L.; Perkins, A.; Zawahiri, M.; Buckley, J.D.; Farber, M.O.; et al. Comparison and Agreement Between the Richmond Agitation-Sedation Scale and the Riker Sedation-Agitation Scale in Evaluating Patients’ Eligibility for Delirium Assessment in the ICU. Chest 2012, 142, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Schünemann, H.J.; Cushman, M.; Burnett, A.E.; Kahn, S.R.; Beyer-Westendorf, J.; Spencer, F.A.; Rezende, S.M.; Zakai, N.A.; Bauer, K.A.; Dentali, F.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018, 2, 3198–3225. [Google Scholar] [CrossRef] [PubMed]
- Gusmao-Flores, D.; Salluh, J.I.F.; Chalhub, R.; Quarantini, L.C. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: A systematic review and meta-analysis of clinical studies. Crit. Care 2012, 16, 115. [Google Scholar] [CrossRef] [Green Version]
- Sanford Guide—Antimicrobial Stewardship. Antimicrobial Therapy Inc. 2022. Available online: https://www.sanfordguide.com/ (accessed on 20 January 2022).
- IDSA Practice Guidelines. Infectious Diseases Society of America. 2022. Available online: http://www.idsociety.org/Templates/Landing.aspx?id=23622320151 (accessed on 20 January 2022).
- UpToDate: Evidence-Based Clinical Decision Support. Wolters Kluwer. 2022. Available online: https://www.uptodate.com/ (accessed on 20 January 2022).
- Cipolle, R.; Strand, L.; Morley, P. Drug Therapy Problems. In Pharmaceutical Care Practice: The Patient Centered Approach to Medication Management Services, 3rd ed.; Mc Graw Hill: New York, NY, USA, 2012. [Google Scholar]
- Chiang, L.; Huang, Y.; Tsai, T. Clinical pharmacy interventions in intensive care unit patients. J. Clin. Pharm. Ther. 2021, 46, 128–133. [Google Scholar] [CrossRef]
- Papadimos, T.J.; Hensley, S.J.; Duggan, J.M.; A Khuder, S.; Borst, M.J.; Fath, J.J.; Oakes, L.R.; Buchman, D. Implementation of the “FASTHUG” concept decreases the incidence of ventilator-associated pneumonia in a surgical intensive care unit. Patient. Saf. Surg. 2008, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Barrera Jiménez, B.; Correa Jiménez, C.; Ruiz Marines, L.A.; Mendoza Ramirez, M. Aplicación del protocolo FAST-HUG y su asociación con la mortalidad del paciente crítico en UCI. Med. Crítica. 2019, 33, 130–138. [Google Scholar]
- Katoue, M.G. Role of pharmacists in providing parenteral nutrition support: Current insights and future directions. Integr. Pharm. Res. Pract. 2018, 7, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Kang, J.E.; Park, S.H.; Jin, H.K.; Jang, S.M.; Kim, S.A.; Rhie, S.J. Nutrition and Clinical Outcomes of Nutrition Support in Multidisciplinary Team for Critically Ill Patients. Nutr. Clin. Pract. 2018, 33, 633–639. [Google Scholar] [CrossRef]
- Graabæk, T.; Kjeldsen, L.J. Medication Reviews by Clinical Pharmacists at Hospitals Lead to Improved Patient Outcomes: A Systematic Review. Basic Clin Pharmacol Toxicol. 2013, 112, 359–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louzon, P.; Jennings, H.; Ali, M. Impact of pharmacist management of pain, agitation, and delirium in the intensive care unit through participation in multidisciplinary bundle rounds. Am. J. Heal. Pharm. 2017, 74, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Lundh, A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst. Rev. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Kwint, H.F.; Bermingham, L.; Faber, A.; Gussekloo, J.; Bouvy, M. The Relationship between the Extent of Collaboration of General Practitioners and Pharmacists and the Implementation of Recommendations Arising from Medication Review. Drugs Aging 2013, 30, 91–102. [Google Scholar] [CrossRef]
- Chen, T.F.; Almeida, A.C. Exploring elements of interprofessional collaboration between pharmacists and physicians in medication review. Pharm. World Sci. 2007, 29, 574–576. [Google Scholar] [CrossRef]
- Fields, W.; Tedeschi, C.; Foltz, J.; Myers, T.; Heaney, K.; Bosak, K.; Rizos, A.; Snyder, R. Reducing Preventable Medication Safety Events by Recognizing Renal Risk. Clin. Nurse Spec. 2008, 22, 73–78. [Google Scholar] [CrossRef]


| Characteristics | Stage 1 (n = 120) | Stage 2 (n = 19) |
|---|---|---|
| Demographics | ||
| Age (years), median (IQR) | 71 (19–100) | 69 (30–91) |
| Respiratory support | ||
| Mechanical Ventilation, n (%) | 30 (25.0) | 8 (42.1) |
| Admission diagnosis, n (%) | ||
| Cardiovascular disorders 1, n (%) | 45 (37.5) | 7 (35.0) |
| Respiratory disorders 2, n (%) | 27 (22.5) | 4 (20.0) |
| Neurologic disorders 3, n (%) | 13 (10.8) | - |
| Gastrointestinal disorders 4, n (%) | 11 (9.2) | - |
| Sepsis, n (%) | 3 (2.5) | 1 (5.0) |
| Trauma 5, n (%) | 6 (5.0) | 1 (5.0) |
| Metabolic disorders 6, n (%) | 5 (4.2) | 1 (5.0) |
| Convalescence phase due to COVID-19 | - | 4 (20.0) |
| Other 7, n (%) | 10 (8.3) | 1 (5.0) |
| Pharmacy interventions | - | 63 |
| Daily interventions, median (IQR) | - | 0.65 (0–4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaverri-Fernández, J.M.; Zavaleta-Monestel, E.; Murillo-Cubero, J.; Díaz-Madriz, J.P.; Leiva-Montero, B.; Arguedas-Chacón, S.; Arguedas-Herrera, R. The Pharmacist’s Role in the Implementation of FASTHUG-MAIDENS, a Mnemonic to Facilitate the Pharmacotherapy Assessment of Critically Ill Patients: A Cross-Sectional Study. Pharmacy 2022, 10, 74. https://doi.org/10.3390/pharmacy10040074
Chaverri-Fernández JM, Zavaleta-Monestel E, Murillo-Cubero J, Díaz-Madriz JP, Leiva-Montero B, Arguedas-Chacón S, Arguedas-Herrera R. The Pharmacist’s Role in the Implementation of FASTHUG-MAIDENS, a Mnemonic to Facilitate the Pharmacotherapy Assessment of Critically Ill Patients: A Cross-Sectional Study. Pharmacy. 2022; 10(4):74. https://doi.org/10.3390/pharmacy10040074
Chicago/Turabian StyleChaverri-Fernández, Jose Miguel, Esteban Zavaleta-Monestel, Josué Murillo-Cubero, José Pablo Díaz-Madriz, Brayan Leiva-Montero, Sebastián Arguedas-Chacón, and Raquel Arguedas-Herrera. 2022. "The Pharmacist’s Role in the Implementation of FASTHUG-MAIDENS, a Mnemonic to Facilitate the Pharmacotherapy Assessment of Critically Ill Patients: A Cross-Sectional Study" Pharmacy 10, no. 4: 74. https://doi.org/10.3390/pharmacy10040074
APA StyleChaverri-Fernández, J. M., Zavaleta-Monestel, E., Murillo-Cubero, J., Díaz-Madriz, J. P., Leiva-Montero, B., Arguedas-Chacón, S., & Arguedas-Herrera, R. (2022). The Pharmacist’s Role in the Implementation of FASTHUG-MAIDENS, a Mnemonic to Facilitate the Pharmacotherapy Assessment of Critically Ill Patients: A Cross-Sectional Study. Pharmacy, 10(4), 74. https://doi.org/10.3390/pharmacy10040074








