Pharmacist-Facilitated Interactive E-Learning for Patients Newly Initiated on Warfarin: A Randomised Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design, Participants and Setting
2.2. Intervention and Standard Care
2.3. Outcomes
- Providing initial education, such as a brief introduction to warfarin and/or providing the warfarin booklet to the patient/carer;
- Providing standard or e-learning warfarin education (including opening the module on the device, if applicable) and answering patient/carer questions about warfarin;
- Discharge education, which included explaining the dose, next INR, and answering final questions immediately prior to discharge.
3. Results
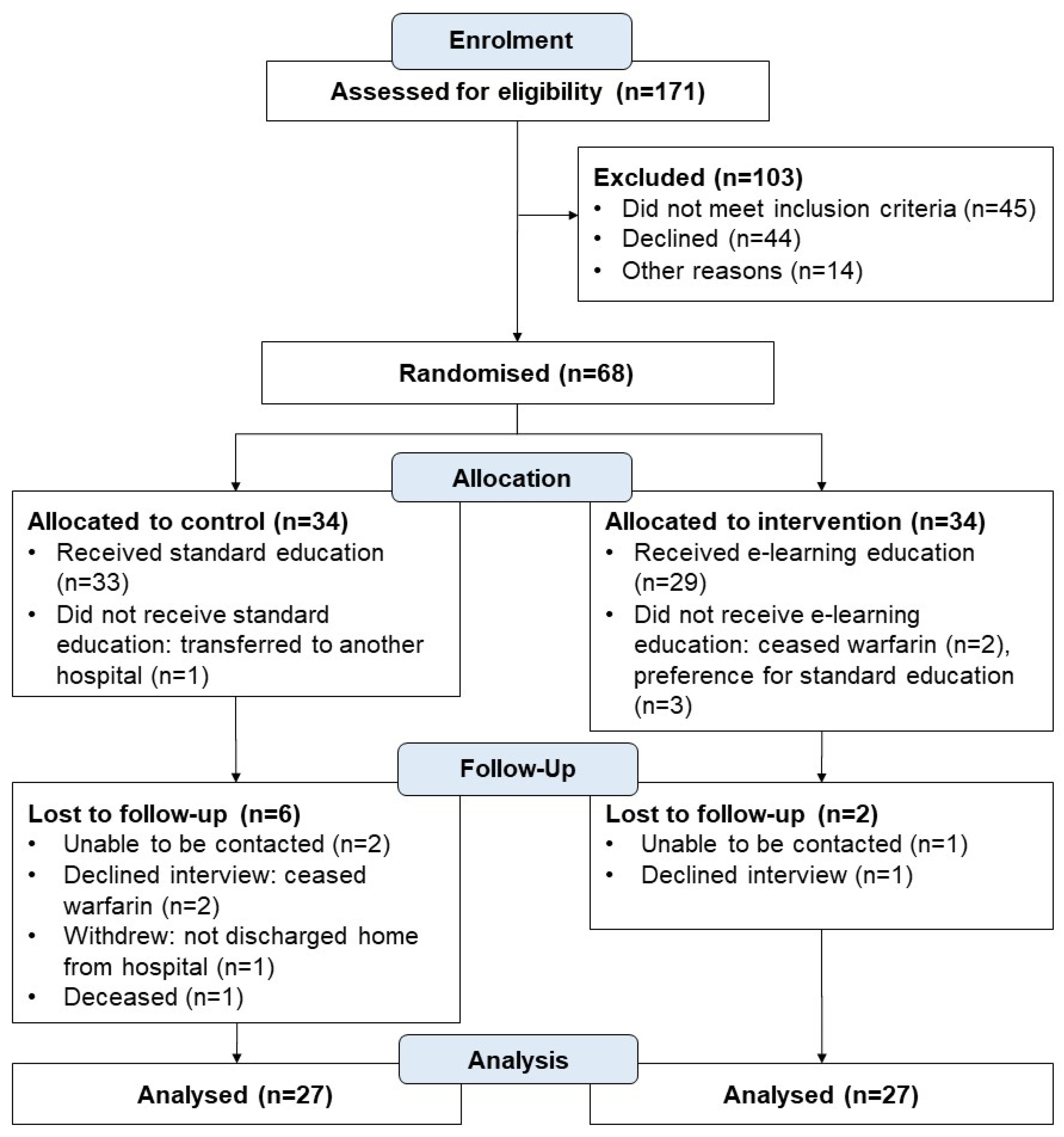
3.1. Participants
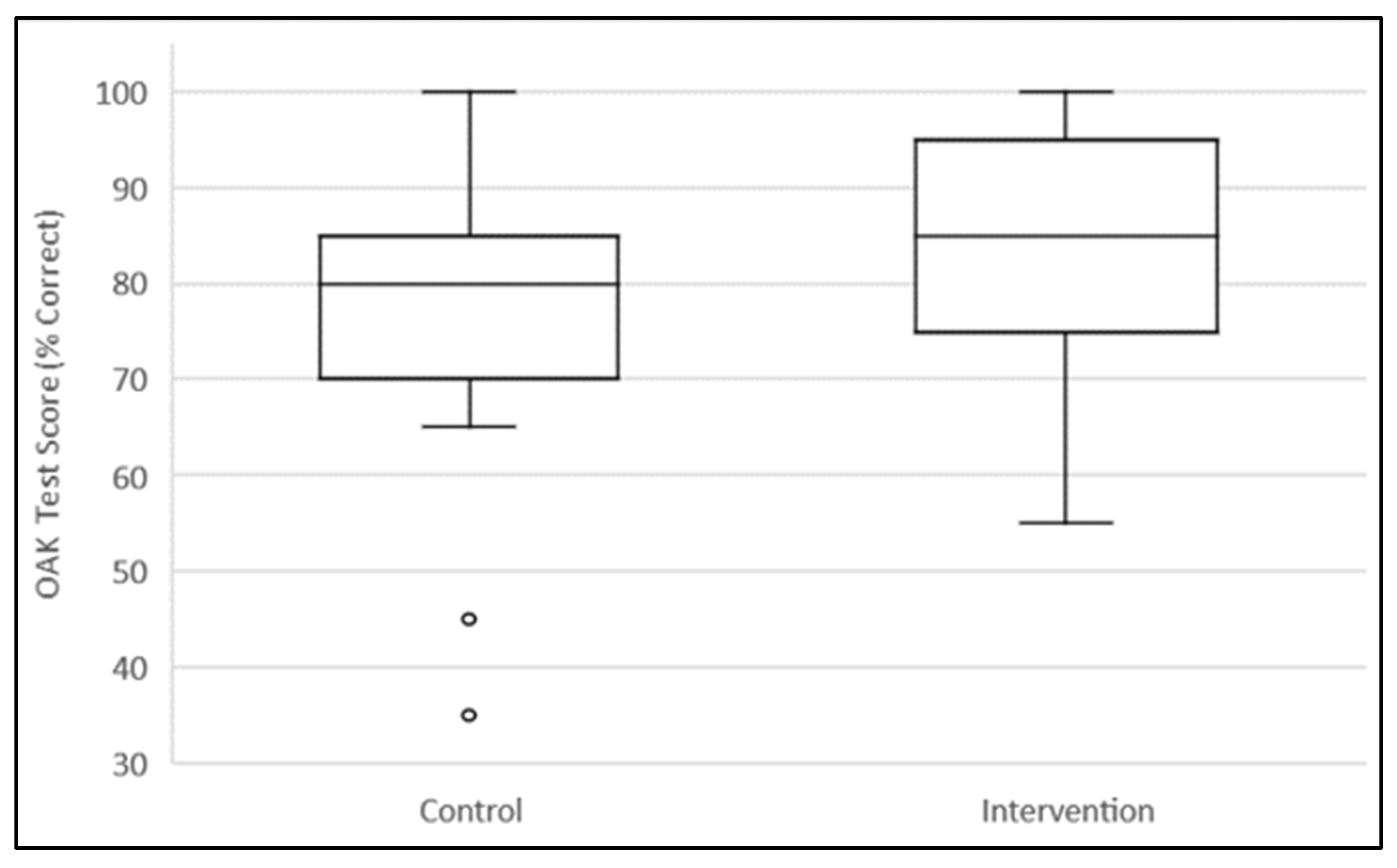
3.2. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Warfarin E-Learning Module Content Outline | |
| About this module Navigating this module What will I learn? What is warfarin? Why is warfarin used? What is an international normalised ratio (INR)? What will happen if my INR is not in my target range? What is an INR test and how do I get one? How often will I need an INR test? Can I choose a brand of warfarin? When should I take my dose? What time should I take my dose? How much warfarin do I take? Halving tablets How can I avoid missing a dose? What to do if I miss a dose? | What can affect my warfarin and/or INR? How do other medications affect my INR? Do I need to change my diet? How does health and lifestyle affect warfarin and/or my INR? Who needs to know I’m taking warfarin? Is it safe to become pregnant? Can I travel? Warfarin and bleeding Warfarin and serious bleeding How do I reduce my chance of bleeding? What are other side effects of warfarin? Will this affect my daily leisure activities? Key points to remember More information |
References
- Kagansky, N.; Knobler, H.; Rimon, E.; Ozer, Z.; Levy, S. Safety of anticoagulation therapy in well-informed older patients. Arch. Intern. Med. 2004, 164, 2044–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, E.O.; Lai, C.S.; Lee, K.K.; Wong, R.S.; Cheng, G.; Chan, T.Y. Relationship between patients’ warfarin knowledge and anticoagulation control. Ann. Pharmacother. 2003, 37, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.L.; Jasser, G.; Wilson, R. Effects of group education on patient satisfaction, knowledge gained, and cost-efficiency in an anticoagulation center. J. Am. Pharm. Assoc. 2003, 43, 264–266. [Google Scholar] [CrossRef]
- Collins, S.B.A.; Sahm, L.J. Pharmacist’s counselling improves patient knowledge regarding warfarin, irrespective of health literacy level. Pharmacy 2014, 2, 10. [Google Scholar]
- Bounda, G.A.; Ngarambe, C.; Ge, W.H.; Yu, F. Assessment and evaluation of a clinical pharmacist-led inpatient warfarin knowledge education program and follow-up at a Chinese tertiary referral teaching hospital. Arch. Pharm. Prac. 2013, 4, 14. [Google Scholar] [CrossRef]
- Kim, J.J.; Mohammad, R.A.; Coley, K.C.; Donihi, A.C. Use of an iPad to provide warfarin video education to hospitalized patients. J. Patient Saf. 2015, 11, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Masnoon, N.; Sorich, W.; Alderman, C.P. A study of consumer retention of key information provided by clinical pharmacists during anticoagulant counselling. J. Pharm. Pract. Res. 2016, 46, 227–244. [Google Scholar] [CrossRef]
- Clarkesmith, D.E.; Pattison, H.M.; Lip, G.Y.; Lane, D.A. Educational intervention improves anticoagulation control in atrial fibrillation patients: The TREAT randomised trial. PLoS ONE 2013, 8, e74037. [Google Scholar] [CrossRef]
- Winans, A.R.; Rudd, K.M.; Triller, D. Assessing anticoagulation knowledge in patients new to warfarin therapy. Ann. Pharmacother. 2010, 44, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Pernod, G.L.J.; Yver, J.; Satger, B.; Allenet, B.; Berremili, T.; Fontaine, M.; Franco, G.; Bosson, J.L. EDUC’AVK: Reduction of oral anticoagulant-related adverse events after patient education: A prospective multicentre open randomized study. J. Gen. Intern. Med. 2008, 23, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyness, M.A. Evaluation of an educational programme for patients taking warfarin. J. Adv. Nurs. 1990, 15, 1052–1063. [Google Scholar] [CrossRef]
- Mazor, K.M.; Baril, J.; Dugan, E.; Spencer, F.; Burgwinkle, P.; Gurwitz, J.H. Patient education about anticoagulant medication: Is narrative evidence or statistical evidence more effective? Patient Educ. Couns. 2007, 69, 145–157. [Google Scholar] [CrossRef]
- Stone, S.; Holden, A.; Knapic, N.; Ansell, J. Comparison between videotape and personalized patient education for anticoagulant therapy. J. Fam. Pract. 1989, 29, 55–57. [Google Scholar] [PubMed]
- Moore, S.J.; Blair, E.A.; Steeb, D.R.; Reed, B.N.; Hull, J.H.; Rodgers, J.E. Impact of video technology on efficiency of pharmacist-provided anticoagulation counseling and patient comprehension. Ann. Pharmacother. 2015, 49, 631–638. [Google Scholar] [CrossRef]
- Stafford, L.; van Tienen, E.C.; Bereznicki, L.R.; Peterson, G.M. The benefits of pharmacist-delivered warfarin education in the home. Int. J. Pharm. Pract. 2012, 20, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, K.; Sanchez, K.; Hui, C.; Talabi, K.; Perry, M.; Qin, H.; Nguyen, H.; Tatachar, A. Impact of an electronic medium delivery of warfarin education in a low income, minority outpatient population: A pilot intervention study. BMC Public Health 2019, 19, 1050. [Google Scholar] [CrossRef] [Green Version]
- Vormfelde, S.; Abed, M.; Hua, T.; Schneider, S.; Friede, T.; Chenot, J.-F. Educating Orally Anticoagulated Patients in Drug Safety A Cluster-Randomized Study in General Practice. Dtsch. Ärzteblatt Int. 2014, 111, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Sim, V.; Galbraith, K. Effectiveness of multimedia interventions in the provision of patient education on anticoagulation therapy: A review. Patient Educ. Couns. 2020, 103, 2009–2017. [Google Scholar] [CrossRef]
- Fox, M.P. A systematic review of the literature reporting on studies that examined the impact of interactive, computer-based patient education programs. Patient Educ. Couns. 2009, 77, 6–13. [Google Scholar] [CrossRef]
- Ciciriello, S.; Johnston, R.V.; Osborne, R.H.; Wicks, I.; deKroo, T.; Clerehan, R.; O’Neill, C.; Buchbinder, R. Multimedia educational interventions for consumers about prescribed and over-the-counter medications. Cochrane Database Syst. Rev. 2013, 4, Cd008416. [Google Scholar] [CrossRef]
- Guldager, T.B.; Hyldgaard, C.; Hilberg, O.; Bendstrup, E. An E-Learning Program Improves Patients’ Knowledge After Lung Transplantation. Telemed. J. e-Health Off. J. Am. Telemed. Assoc. 2021, 27, 800–806. [Google Scholar] [CrossRef]
- Suhling, H.; Rademacher, J.; Zinowsky, I.; Fuge, J.; Greer, M.; Warnecke, G.; Smits, J.M.; Bertram, A.; Haverich, A.; Welte, T.; et al. Conventional vs. tablet computer-based patient education following lung transplantation—A randomized controlled trial. PLoS ONE 2014, 9, e90828. [Google Scholar] [CrossRef]
- Harrison, J.J.; Badr, S.; Hamandi, B.; Kim, S.J. Randomized Controlled Trial of a Computer-Based Education Program in the Home for Solid Organ Transplant Recipients: Impact on Medication Knowledge, Satisfaction, and Adherence. Transplantation 2017, 101, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Mulders, G.; de Wee, E.M.; Vahedi Nikbakht-Van de Sande, M.C.; Kruip, M.J.; Elfrink, E.J.; Leebeek, F.W. E-learning improves knowledge and practical skills in haemophilia patients on home treatment: A randomized controlled trial. Haemoph. Off. J. World Fed. Hemoph. 2012, 18, 693–698. [Google Scholar] [CrossRef]
- Hu, A.C.C.; Dao, D.; Errett, L.; Keith, M. Factors influencing patient knowledge of warfarin therapy after mechanical heart valve replacement. J. Cardiovasc. Nurs. 2006, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Borson, S.; Scanlan, J.M.; Chen, P.; Ganguli, M. The Mini-Cog as a screen for dementia: Validation in a population-based sample. J. Am. Geriatr. Soc. 2003, 51, 1451–1454. [Google Scholar] [CrossRef]
- Zeolla, M.M.; Brodeur, M.R.; Dominelli, A.; Haines, S.T.; Allie, N.D. Development and validation of an instrument to determine patient knowledge: The oral anticoagulation knowledge test. Ann. Pharmacother. 2006, 40, 633–638. [Google Scholar] [CrossRef]
- Brieger, D.; Amerena, J.; Attia, J.; Bajorek, B.; Chan, K.H.; Connell, C.; Freedman, B.; Ferguson, C.; Hall, T.; Haqqani, H.; et al. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian Clinical Guidelines for the Diagnosis and Management of Atrial Fibrillation 2018. Heart Lung Circ. 2018, 27, 1209–1266. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | Control n = 27 (%) | Intervention n = 27 (%) |
|---|---|---|
| Study participant type | ||
| Patient | 26 (96.3) | 26 (96.3) |
| Carer | 1 (3.7) | 1 (3.7) |
| Age (years), mean (SD) | 55 (14.2) | 55 (15.6) |
| Gender (male) | 18 (66.7) | 20 (74.1) |
| English language spoken at home | 25 (92.5) | 26 (96.3) |
| Previously received warfarin therapy (>2 years ago) | 1 (3.7) | 2 (7.4) |
| Highest level of education achieved | ||
| Tertiary | 11 (40.7) | 8 (29.6) |
| Secondary | 10 (37) | 14 (51.9) |
| Primary | 6 (22.2) | 5 (18.5) |
| No School | 0 | 0 |
| Self-rating of confidence with electronic devices (e.g., tablet device) | ||
| Very confident and familiar | 10 (37) | 14 (51.9) |
| Somewhat confident and familiar | 10 (37) | 11 (40.7) |
| Used but not familiar | 7 (25.9) | 2 (7.4) |
| Not used | 0 | 0 |
| Access to electronic device after discharge | 24 (88.9) | 26 (96.3) |
| Access to internet after discharge | 23 (85.2) | 25 (92.6) |
| Survey Statement | Control Group Responses n = 27 (%) | Intervention Group Responses n = 27 (%) | ||||
|---|---|---|---|---|---|---|
| Positive 1 | Neutral | Negative 2 | Positive 1 | Neutral | Negative 2 | |
| I am satisfied with the information I was given about warfarin | 25 (92.6) | 2 (7.4) | 0 | 27 (100) | 0 | 0 |
| I am satisfied with the way warfarin information was presented to me | 24 (88.9) | 3 (11.1) | 0 | 26 (96.3) | 0 | 1 (3.7) |
| I am satisfied that the information provided was clear and easy to understand | 23 (85.2) | 3 (11.1) | 1 (3.7) | 25 (92.6) | 2 (7.4) | 0 |
| I am satisfied that I had enough opportunity to ask questions about my warfarin therapy | 25 (92.6) | 1 (3.7) | 1 (3.7) | 23 (85.2) | 2 (11.1) | 2 (11.1) |
| Overall, I am satisfied with the manner in which the information was provided | 24 (88.9) | 3 (11.1) | 0 | 25 (92.6) | 2 (7.4) | 0 |
| Survey Statement | Pharmacist Responses n = 12 (%) | ||
|---|---|---|---|
| Positive 1 | Neutral | Negative 2 | |
| I found it easy to enable study participant access to the interactive warfarin e-learning module | 11 (91.7) | 1 (8.3) | 0 |
| I considered the information delivered via the interactive warfarin e-learning module to be clear and easily understood by study participants | 12 (100) | 0 | 0 |
| I found I could identify gaps in study participant knowledge following use of the interactive warfarin e-learning module | 4 (33.3) | 5 (41.7) | 3 (25.0) |
| I found the interactive warfarin e-learning module took less of my time compared to when I deliver standard warfarin education | 9 (75) | 1 (8.3) | 2 (16.7) |
| E-Learning Module | No Preference | Standard Education | |
| Overall preference for warfarin education delivery mode | 5 (41.7) | 5 (41.7) | 2 (16.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, J.; Nalder, M.J.; Gorelik, A.; Elliott, R.A. Pharmacist-Facilitated Interactive E-Learning for Patients Newly Initiated on Warfarin: A Randomised Controlled Study. Pharmacy 2022, 10, 3. https://doi.org/10.3390/pharmacy10010003
Young J, Nalder MJ, Gorelik A, Elliott RA. Pharmacist-Facilitated Interactive E-Learning for Patients Newly Initiated on Warfarin: A Randomised Controlled Study. Pharmacy. 2022; 10(1):3. https://doi.org/10.3390/pharmacy10010003
Chicago/Turabian StyleYoung, Joanne, Michelle J. Nalder, Alexandra Gorelik, and Rohan A. Elliott. 2022. "Pharmacist-Facilitated Interactive E-Learning for Patients Newly Initiated on Warfarin: A Randomised Controlled Study" Pharmacy 10, no. 1: 3. https://doi.org/10.3390/pharmacy10010003
APA StyleYoung, J., Nalder, M. J., Gorelik, A., & Elliott, R. A. (2022). Pharmacist-Facilitated Interactive E-Learning for Patients Newly Initiated on Warfarin: A Randomised Controlled Study. Pharmacy, 10(1), 3. https://doi.org/10.3390/pharmacy10010003







