Clinical Update on Patient-Controlled Analgesia for Acute Postoperative Pain
Abstract
1. Introduction
2. Methods
3. Results/Discussion
3.1. Predictors of Postoperative Pain
3.2. Indications and Benefits of PCA
| PCA IV and/or Other Systemic Therapies * | Side Specific Infiltration or Block with or without Regional Catheters ** | Neuraxial Anesthetic Techniques *** | |
|---|---|---|---|
| Thoracotomy/thoracoscopy | ++ | Paravertebral block ++ | +++ |
| Laparotomy | ++ | Infiltration catheters TAP block + | +++ |
| Laparoscopy | +++ | Infiltration ++ | + |
| Hip | +++ | ++ | ++ |
| Knee | ++ | +++ | ++ |
| Shoulder/upper arm | ++ | +++ | |
| Spinal fusion | +++ | ++ | |
| Cesarean section | ++ | TAP block ++ | +++ |
| Breast surgery | ++ | Paravertebral block +++ | |
| CABG | +++ | ||
| Cervicofacial surgery | +++ | +++ When indicated |
3.3. Interindividual Variability
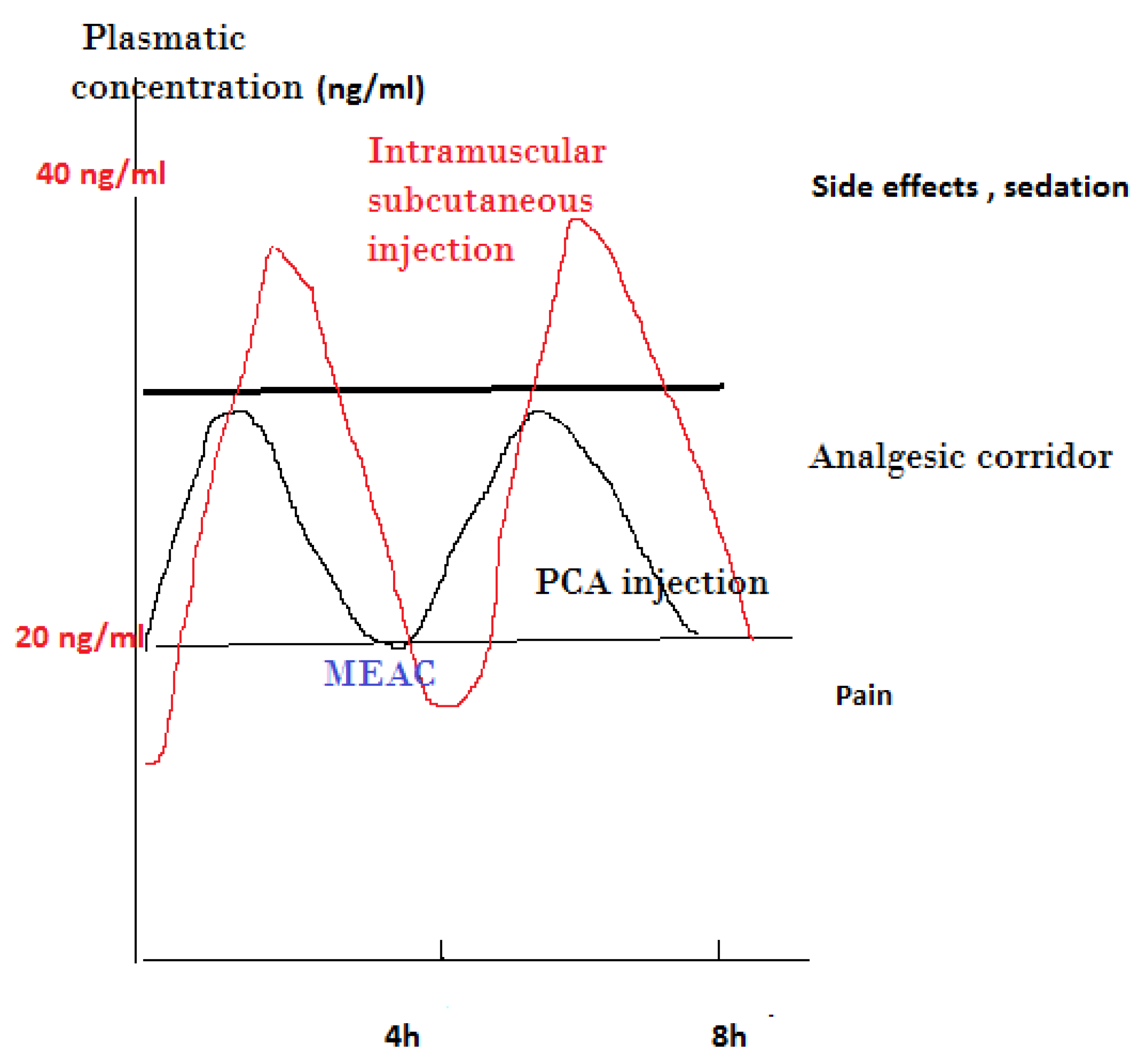
3.4. PCA Concept
3.5. Side Effects of IV-PCA
3.6. Comparison of Different PCA Medications
3.7. Adding Ketamine to PCA Morphine
3.8. Human-Related Issues and Side Effects
3.9. PCA in Children
3.10. PCA for Elderly and Frail Patients
3.11. PCA in Obese Patients
3.12. PCA in Chronic Pain Patients
3.13. Recent Modalities of PCA Administration
3.14. Other Factors Influencing Quality of Pain Management
3.15. Limitation of This Study
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, J.M.; Rosen, M.; MacCarthy, J.; Hogg, M.I. Apparatus for patient-controlled administration of intravenous narcotics during labour. Lancet 1976, 1, 17–18. [Google Scholar] [CrossRef]
- Evans, J.M.; Rosen, M.; McCarthy, J.; Hogg, M.J. Letter: Patient-controlled intravenous narcotic administration during labour. Lancet 1976, 1, 906–907. [Google Scholar] [CrossRef]
- Viscusi, E.R. Patient-controlled drug delivery for acute postoperative pain management: A review of current and emerging technologies. Reg. Anesth. Pain Med. 2008, 33, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Motamed, C.; Salazar, G.; Bourgain, J.L. Incidence of severe postoperative pain after cancer surgery despite intraoperative anticipation: A case controlled study. Bull. Cancer 2010, 97, E37–E41. [Google Scholar] [CrossRef] [PubMed]
- Coppes, O.J.M.; Yong, R.J.; Kaye, A.D.; Urman, R.D. Patient and Surgery-Related Predictors of Acute Postoperative Pain. Curr. Pain Headache Rep. 2020, 24, 12. [Google Scholar] [CrossRef]
- Yang, M.M.H.; Hartley, R.L.; Leung, A.A.; Ronksley, P.E.; Jette, N.; Casha, S.; Riva-Cambrin, J. Preoperative predictors of poor acute postoperative pain control: A systematic review and meta-analysis. BMJ Open 2019, 9, e025091. [Google Scholar] [CrossRef]
- Borges, N.C.; Pereira, L.V.; de Moura, L.A.; Silva, T.C.; Pedroso, C.F. Predictors for Moderate to Severe Acute Postoperative Pain after Cesarean Section. Pain Res. Manag. 2016, 2016, 5783817. [Google Scholar] [CrossRef]
- Charalampidis, A.; Rundberg, L.; Moller, H.; Gerdhem, P. Predictors of persistent postoperative pain after surgery for idiopathic scoliosis. J. Child Orthop. 2021, 15, 458–463. [Google Scholar] [CrossRef]
- Costelloe, C.; Burns, S.; Yong, R.J.; Kaye, A.D.; Urman, R.D. An Analysis of Predictors of Persistent Postoperative Pain in Spine Surgery. Curr. Pain Headache Rep. 2020, 24, 11. [Google Scholar] [CrossRef]
- Joo, J.; Moon, H.K.; Moon, Y.E. Identification of predictors for acute postoperative pain after gynecological laparoscopy (STROBE-compliant article). Medicine 2019, 98, e17621. [Google Scholar] [CrossRef] [PubMed]
- Luedi, M.M.; Schober, P.; Hammoud, B.; Andereggen, L.; Hoenemann, C.; Doll, D. Preoperative Pressure Pain Threshold Is Associated With Postoperative Pain in Short-Stay Anorectal Surgery: A Prospective Observational Study. Anesth. Analg. 2021, 132, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.R.; Gan, T.J.; Urman, R.D. Predicting Postoperative Pain: A Complex Interplay of Multiple Factors. Anesth. Analg. 2021, 132, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Persson, A.K.M.; Akeson, J. Prediction of Acute Postoperative Pain from Assessment of Pain Associated With Venous Cannulation. Pain Pract. 2019, 19, 158–167. [Google Scholar] [CrossRef]
- Choi, S.W.; Cho, H.K.; Park, S.; Yoo, J.H.; Lee, J.C.; Baek, M.J.; Jang, H.D.; Cha, J.S.; Shin, B.J. Multimodal Analgesia (MMA) Versus Patient-Controlled Analgesia (PCA) for One or Two-Level Posterior Lumbar Fusion Surgery. J. Clin. Med. 2020, 9, 1087. [Google Scholar] [CrossRef]
- Akter, N.; Ratnayake, B.; Joh, D.B.; Chan, S.J.; Bonner, E.; Pandanaboyana, S. Postoperative Pain Relief after Pancreatic Resection: Systematic Review and Meta-Analysis of Analgesic Modalities. World J. Surg. 2021, 45, 3165–3173. [Google Scholar] [CrossRef]
- Niu, L.; Chen, L.; Luo, Y.; Huang, W.; Li, Y. Oxycodone versus morphine for analgesia after laparoscopic endometriosis resection. BMC Anesthesiol. 2021, 21, 194. [Google Scholar] [CrossRef]
- Sun, K.; Liu, D.; Chen, J.; Yu, S.; Bai, Y.; Chen, C.; Yao, Y.; Yu, L.; Yan, M. Moderate-severe postoperative pain in patients undergoing video-assisted thoracoscopic surgery: A retrospective study. Sci. Rep. 2020, 10, 795. [Google Scholar] [CrossRef]
- Qin, M.; Chen, K.; Liu, T.; Shen, X. Dexmedetomidine in combination with sufentanil for postoperative analgesia after partial laryngectomy. BMC Anesthesiol. 2017, 17, 66. [Google Scholar] [CrossRef]
- Vanni, G.; Caiazza, G.; Materazzo, M.; Storti, G.; Pellicciaro, M.; Buonomo, C.; Natoli, S.; Fabbi, E.; Dauri, M. Erector Spinae Plane Block Versus Serratus Plane Block in Breast Conserving Surgery: Alpha Randomized Controlled Trial. Anticancer Res. 2021, 41, 5667–5676. [Google Scholar] [CrossRef]
- Bonnesen, K.; Nikolajsen, L.; Boggild, H.; Hostrup Nielsen, P.; Jacobsen, C.J.; Viemose Nielsen, D. Chronic post-operative opioid use after open cardiac surgery: A Danish population-based cohort study. Acta Anaesthesiol. Scand. 2021, 65, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.; Fiasconaro, M.; Liu, J.; Poeran, J.; Poultsides, L.; Memtsoudis, S.G. Risk of chronic opioid use after simultaneous versus staged bilateral knee arthroplasty. Reg. Anesth. Pain Med. 2021, 46, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G.M.; Tierney, S.; Ramlogan, R.; McCartney, C.J.L.; Bromley, L.A.; McIsaac, D.I. Persistent Postoperative Opioid Prescription Fulfillment and Peripheral Nerve Blocks for Ambulatory Shoulder Surgery: A Retrospective Cohort Study. Anesthesiology 2021, 135, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Anoushiravani, A.A.; Kim, K.Y.; Roof, M.; Chen, K.; O’Connor, C.M.; Vigdorchik, J.; Schwarzkopf, R. Risk factors associated with persistent chronic opioid use following THA. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 681–688. [Google Scholar] [CrossRef]
- Motamed, C.; Spencer, A.; Farhat, F.; Bourgain, J.L.; Lasser, P.; Jayr, C. Postoperative hypoxaemia: Continuous extradural infusion of bupivacaine and morphine vs patient-controlled analgesia with intravenous morphine. Br. J. Anaesth. 1998, 80, 742–747. [Google Scholar] [CrossRef][Green Version]
- Benedetti, M.G.; De Santis, L.; Mariani, G.; Donati, D.; Bardelli, R.; Perrone, M.; Brunelli, S. Chronic pain in lower limb amputees: Is there a correlation with the use of perioperative epidural or perineural analgesia? NeuroRehabilitation 2021, 49, 129–138. [Google Scholar] [CrossRef]
- Chappell, A.G.; Yuksel, S.; Sasson, D.C.; Wescott, A.B.; Connor, L.M.; Ellis, M.F. Post-Mastectomy Pain Syndrome: An Up-to-Date Review of Treatment Outcomes. JPRAS Open 2021, 30, 97–109. [Google Scholar] [CrossRef]
- Clephas, P.R.D.; Hoeks, S.E.; Trivella, M.; Guay, C.S.; Singh, P.M.; Klimek, M.; Heesen, M. Prognostic factors for chronic post-surgical pain after lung or pleural surgery: A protocol for a systematic review and meta-analysis. BMJ Open 2021, 11, e051554. [Google Scholar] [CrossRef]
- Liu, C.W.; Page, M.G.; Weinrib, A.; Wong, D.; Huang, A.; McRae, K.; Fiorellino, J.; Tamir, D.; Kahn, M.; Katznelson, R.; et al. Predictors of one year chronic post-surgical pain trajectories following thoracic surgery. J. Anesth. 2021, 35, 505–514. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, C.K.; Kim, S.H.; Kim, Y.; Kim, J.E.; Shin, Y.K.; Seok, J.; Cho, H.M. Prevalence of chronic post-thoracotomy pain in patients with traumatic multiple rib fractures in South Korea: A cross-sectional study. Sci. Rep. 2021, 11, 2615. [Google Scholar] [CrossRef]
- Samara, E.; Stamatiou, K.; Economou, S.; Tzimas, P. Post Laparoscopy Neuropathic Pain Treated by Capsaicin 8% Dermal Patch. J. Pain Palliat. Care Pharmacother 2021, 35, 123–125. [Google Scholar] [CrossRef]
- Zinboonyahgoon, N.; Patton, M.E.; Chen, Y.K.; Edwards, R.R.; Schreiber, K.L. Persistent Post-Mastectomy Pain: The Impact of Regional Anesthesia Among Patients with High vs Low Baseline Catastrophizing. Pain Med. 2021, 22, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Hudcova, J.; McNicol, E.; Quah, C.; Lau, J.; Carr, D.B. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database Syst. Rev. 2006, 4, CD003348. [Google Scholar] [CrossRef]
- McNicol, E.D.; Ferguson, M.C.; Hudcova, J. Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Syst. Rev. 2015, 6, CD003348. [Google Scholar] [CrossRef]
- Tyler, D.C.; Pomietto, M.; Womack, W. Variation in opioid use during PCA in adolescents. Paediatr. Anaesth. 1996, 6, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Upton, R.N.; Semple, T.J.; Macintyre, P.E. Pharmacokinetic optimisation of opioid treatment in acute pain therapy. Clin. Pharmacokinet. 1997, 33, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Austin, K.L.; Stapleton, J.V.; Mather, L.E. Relationship between blood meperidine concentrations and analgesic response: A preliminary report. Anesthesiology 1980, 53, 460–466. [Google Scholar] [CrossRef]
- Aubrun, F.; Mazoit, J.X.; Riou, B. Postoperative intravenous morphine titration. Br. J. Anaesth. 2012, 108, 193–201. [Google Scholar] [CrossRef]
- Motamed, C.; Weil, G.; Deschamps, F.; Billard, V. Remifentanil target-controlled infusion: A safe rescue protocol for unexpected severe postoperative pain. J. Opioid. Manag. 2014, 10, 284–288. [Google Scholar] [CrossRef]
- Semple, T.J.; Upton, R.N.; Macintyre, P.E.; Runciman, W.B.; Mather, L.E. Morphine blood concentrations in elderly postoperative patients following administration via an indwelling subcutaneous cannula. Anaesthesia 1997, 52, 318–323. [Google Scholar] [CrossRef]
- Dahmani, S.; Dupont, H.; Mantz, J.; Desmonts, J.M.; Keita, H. Predictive factors of early morphine requirements in the post-anaesthesia care unit (PACU). Br. J. Anaesth. 2001, 87, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Dinges, H.C.; Otto, S.; Stay, D.K.; Baumlein, S.; Waldmann, S.; Kranke, P.; Wulf, H.F.; Eberhart, L.H. Side Effect Rates of Opioids in Equianalgesic Doses via Intravenous Patient-Controlled Analgesia: A Systematic Review and Network Meta-analysis. Anesth. Analg. 2019, 129, 1153–1162. [Google Scholar] [CrossRef]
- Miyoshi, H.; Nakamura, R.; Noda, Y.; Yokomi, H.; Kamiya, S.; Morio, A.; Watanabe, T.; Narasaki, S.; Toyota, Y.; Saeki, N.; et al. Intravenous patient-controlled analgesia does not increase the risk of postoperative delirium compared to patient-controlled epidural analgesia: A propensity score-matched retrospective cohort study. Ann. Palliat. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.D.; Gelfand, H.J.; Bicket, M.C.; Ouanes, J.P.; Kumar, K.K.; Isaac, G.R.; Wu, C.L. Analgesic efficacy of intravenous naloxone for the treatment of postoperative pruritus: A meta-analysis. J. Opioid. Manag. 2011, 7, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Barrons, R.W.; Woods, J.A. Low-Dose Naloxone for Prophylaxis of Postoperative Nausea and Vomiting: A Systematic Review and Meta-analysis. Pharmacotherapy 2017, 37, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Firouzian, A.; Gholipour Baradari, A.; Alipour, A.; Emami Zeydi, A.; Zamani Kiasari, A.; Emadi, S.A.; Kheradmand, B.; Hadadi, K. Ultra-low-dose Naloxone as an Adjuvant to Patient Controlled Analgesia (PCA) With Morphine for Postoperative Pain Relief Following Lumber Discectomy: A Double-blind, Randomized, Placebo-controlled Trial. J. Neurosurg. Anesthesiol. 2018, 30, 26–31. [Google Scholar] [CrossRef]
- Pieters, B.J.; Anderson, J.T.; Price, N.; Anson, L.M.; Schwend, R.M. Low-Dose Versus High-Dose Postoperative Naloxone Infusion Combined With Patient-Controlled Analgesia for Adolescent Idiopathic Scoliosis Surgery: A Randomized Controlled Trial. Spine Deform. 2018, 6, 430–434. [Google Scholar] [CrossRef]
- Wagemans, M.F.; Scholten, W.K.; Hollmann, M.W.; Kuipers, A.H. Epidural anesthesia is no longer the standard of care in abdominal surgery with ERAS. What are the alternatives? Minerva. Anestesiol. 2020, 86, 1079–1088. [Google Scholar] [CrossRef]
- Morlion, B.; Schafer, M.; Betteridge, N.; Kalso, E. Non-invasive patient-controlled analgesia in the management of acute postoperative pain in the hospital setting. Curr. Med. Res. Opin. 2018, 34, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, F.; Doronzio, A.; Brambilla, R.; Melis, M.; Langer, M. Integration of pain scores, morphine consumption and demand/delivery ratio to evaluate patient-controlled analgesia: The C-SIA score. Korean J. Anesthesiol. 2017, 70, 311–317. [Google Scholar] [CrossRef][Green Version]
- Engsusophon, P.; Laosuwan, P.; Songthamwat, B.; Wattanachai, P.; Ussawanopkiat, M.; Charuluxananan, S. Factors Influencing Patient Satisfaction on Patient-Controlled Analgesia (PCA) for Postoperative Pain Management. Thai J. Anesthesiol. 2019, 45, 15–19. [Google Scholar]
- Kim, K.T.; Kim, C.K.; Kim, Y.C.; Juh, H.S.; Kim, H.J.; Kim, H.S.; Hong, S.J.; Hey, H.W.D. The effectiveness of low-dose and high-dose tranexamic acid in posterior lumbar interbody fusion: A double-blinded, placebo-controlled randomized study. Eur. Spine J. 2017, 26, 2851–2857. [Google Scholar] [CrossRef]
- Lu, G.; Yao, W.; Chen, X.; Zhang, S.; Zhou, M. Remifentanil patient-controlled versus epidural analgesia on intrapartum maternal fever: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2020, 20, 151. [Google Scholar] [CrossRef]
- Lee, W.; Gao, X.; Tang, J.; Li, A.; Zhu, Y.; Ling, X.; Cang, J.; Fang, F. Postoperative sufentanil intravenous patient-controlled analgesia within the first 24 h: A retrospective study. Ann. Palliat. Med. 2020, 9, 3932–3937. [Google Scholar] [CrossRef]
- Brinck, E.C.V.; Virtanen, T.; Makela, S.; Soini, V.; Hynninen, V.V.; Mulo, J.; Savolainen, U.; Rantakokko, J.; Maisniemi, K.; Liukas, A.; et al. S-ketamine in patient-controlled analgesia reduces opioid consumption in a dose-dependent manner after major lumbar fusion surgery: A randomized, double-blind, placebo-controlled clinical trial. PLoS ONE 2021, 16, e0252626. [Google Scholar] [CrossRef]
- Matsota, P.K.; Koukopoulou, I.C.; Kalimeris, K.A.; Kyttari, A.C.; Drachtidi, K.H.; Kostopanagiotou, G.G. Ketamine Versus Tramadol As an Adjunct To PCA Morphine for Postoperative Analgesia After Major Upper Abdominal Surgery: A Prospective, Comparative, Randomized Trial. Rom. J. Anaesth. Intensive Care 2020, 27, 43–51. [Google Scholar] [CrossRef]
- van Heijster, S.; Janssen, J.; Sarton, E.; Niesters, M.; Dahan, A. Postoperative opioid overdose due to patient-controlled analgesia by proxy. Ned. Tijdschr. Geneeskd. 2020, 164, D5084. [Google Scholar]
- Vicente, K.J.; Kada-Bekhaled, K.; Hillel, G.; Cassano, A.; Orser, B.A. Programming errors contribute to death from patient-controlled analgesia: Case report and estimate of probability. Can. J. Anaesth. 2003, 50, 328–332. [Google Scholar] [CrossRef][Green Version]
- Flynn, F.; Mohr, L.; Lawlor-Klean, P. Right programming of pumps to prevent errors in the infusion process. Jt. Comm. J. Qual. Saf. 2003, 29, 37–40. [Google Scholar] [CrossRef]
- Rashed, A.N.; Tomlin, S.; Aguado, V.; Forbes, B.; Whittlesea, C. Sources and magnitude of error in preparing morphine infusions for nurse-patient controlled analgesia in a UK paediatric hospital. Int. J. Clin. Pharm. 2016, 38, 1069–1074. [Google Scholar] [CrossRef]
- Rashed, A.N.; Tomlin, S.; Forbes, B.; Whittlesea, C. Current practice of preparing morphine infusions for nurse/patient-controlled analgesia in a UK paediatric hospital: Healthcare professionals’ views and experiences. Eur. J. Hosp. Pharm. 2018, 25, 327–330. [Google Scholar] [CrossRef]
- Syed, S.; Paul, J.E.; Hueftlein, M.; Kampf, M.; McLean, R.F. Morphine overdose from error propagation on an acute pain service. Can. J. Anaesth. 2006, 53, 586–590. [Google Scholar] [CrossRef]
- Finger, M.J.; McLeod, D.G. Postoperative myocardial infarction after radical cystoprostatectomy masked by patient-controlled analgesia. Urology 1995, 45, 155–157. [Google Scholar] [CrossRef]
- Dinter, K.; Bretschneider, H.; Zwingenberger, S.; Disch, A.; Osmers, A.; Vicent, O.; Thielemann, F.; Seifert, J.; Bernstein, P. Accelerate postoperative management after scoliosis surgery in healthy and impaired children: Intravenous opioid therapy versus epidural therapy. Arch. Orthop. Trauma Surg. 2021. [Google Scholar] [CrossRef]
- Dekonenko, C.; Dorman, R.M.; Duran, Y.; Juang, D.; Aguayo, P.; Fraser, J.D.; Oyetunji, T.A.; Snyder, C.L.; Holcomb, G.W., 3rd; Millspaugh, D.L.; et al. Postoperative pain control modalities for pectus excavatum repair: A prospective observational study of cryoablation compared to results of a randomized trial of epidural vs patient-controlled analgesia. J. Pediatr. Surg. 2020, 55, 1444–1447. [Google Scholar] [CrossRef]
- Sharp, D.; Jaffrani, A. A PRISMA systematic review on the safety and efficacy of patient-controlled analgesia (PCA) in pediatrics. J. Pediatr. Nurs. 2021, 61, 219–223. [Google Scholar] [CrossRef]
- Cravero, J.P.; Agarwal, R.; Berde, C.; Birmingham, P.; Cote, C.J.; Galinkin, J.; Isaac, L.; Kost-Byerly, S.; Krodel, D.; Maxwell, L.; et al. The Society for Pediatric Anesthesia recommendations for the use of opioids in children during the perioperative period. Paediatr. Anaesth. 2019, 29, 547–571. [Google Scholar] [CrossRef]
- Aubrun, F.; Marmion, F. The elderly patient and postoperative pain treatment. Best Pract. Res. Clin. Anaesthesiol. 2007, 21, 109–127. [Google Scholar] [CrossRef]
- Koh, J.C.; Lee, J.; Kim, S.Y.; Choi, S.; Han, D.W. Postoperative Pain and Intravenous Patient-Controlled Analgesia-Related Adverse Effects in Young and Elderly Patients: A Retrospective Analysis of 10,575 Patients. Medicine 2015, 94, e2008. [Google Scholar] [CrossRef]
- Brown, A.; Boshers, B.; Chapman, L.F.; Huckaba, K.; Pangle, M.; Pogue, L.C.; Potts, M.; Ray, E.; Thomason, N.; Poynter, A.; et al. Do Elderly Patients Use Patient-Controlled Analgesia Medication Delivery Systems Correctly? Orthop. Nurs. 2015, 34, 203–208. [Google Scholar] [CrossRef]
- de Hoogd, S.; Valitalo, P.A.J.; Dahan, A.; van Kralingen, S.; Coughtrie, M.M.W.; van Dongen, E.P.A.; van Ramshorst, B.; Knibbe, C.A.J. Influence of Morbid Obesity on the Pharmacokinetics of Morphine, Morphine-3-Glucuronide, and Morphine-6-Glucuronide. Clin. Pharmacokinet. 2017, 56, 1577–1587. [Google Scholar] [CrossRef]
- Levin, A.; Klein, S.L.; Brolin, R.E.; Pitchford, D.E. Patient-controlled analgesia for morbidly obese patients: An effective modality if used correctly. Anesthesiology 1992, 76, 857–858. [Google Scholar] [CrossRef]
- Motamed, C.; Audibert, J.; Albi-Feldzer, A.; Bouroche, G.; Jayr, C. Postoperative pain scores and opioid consumption in opioid-dependent patients with cancer after intraoperative remifentanil analgesia: A prospective case-controlled study. J. Opioid. Manag. 2017, 13, 221–228. [Google Scholar] [CrossRef]
- Lee, S.H.; Baek, C.W.; Kang, H.; Park, Y.H.; Choi, G.J.; Jung, Y.H.; Woo, Y.C. A comparison of 2 intravenous patient-controlled analgesia modes after spinal fusion surgery: Constant-rate background infusion versus variable-rate feedback infusion, a randomized controlled trial. Medicine 2019, 98, e14753. [Google Scholar] [CrossRef]
- Jung, K.T.; So, K.Y.; Kim, S.U.; Kim, S.H. The Optimizing Background Infusion Mode Decreases Intravenous Patient-Controlled Analgesic Volume and Opioid Consumption Compared to Fixed-Rate Background Infusion in Patients Undergoing Laparoscopic Cholecystectomy: A Prospective, Randomized, Controlled, Double-Blind Study. Medicina 2021, 57, 42. [Google Scholar] [CrossRef]
| Analgesic | Bolus Dose | Lockout Period (Minutes) |
|---|---|---|
| Morphine | 1 mg | 5–10 |
| Fentanyl | 10 µg | 5–10 |
| Hydromorphone | 0.25 mg | 5–10 |
| Remifentanil | 0.5 µg/kg | 2 |
| Sufentanil | 5 µg | 5–10 |
| Efficiency | Side Effects | |
|---|---|---|
| Oxycodone | As potent as morphine | May have fewer severe side effects |
| Hydromorphone | Higher incidence of CNS side effects, excitation at higher dose | |
| Fentanyl | High potency +, may require more need for basal infusion rate | Lesser incidence of respiratory depression in comparison to morphine, but more programming errors |
| Sufentanil | High potency ++, high therapeutic index, more predictable profile, more need for basal infusion | Lower incidence of PONV in comparison to fentanyl |
| Tramadol | Ten times less potent than morphine | More PONV in some type of surgeries (e.g., spinal fusion) |
| Remifentanil | Very short duration, studies mainly in labor | Higher respiratory depression, less satisfaction in comparison to epidural analgesia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motamed, C. Clinical Update on Patient-Controlled Analgesia for Acute Postoperative Pain. Pharmacy 2022, 10, 22. https://doi.org/10.3390/pharmacy10010022
Motamed C. Clinical Update on Patient-Controlled Analgesia for Acute Postoperative Pain. Pharmacy. 2022; 10(1):22. https://doi.org/10.3390/pharmacy10010022
Chicago/Turabian StyleMotamed, Cyrus. 2022. "Clinical Update on Patient-Controlled Analgesia for Acute Postoperative Pain" Pharmacy 10, no. 1: 22. https://doi.org/10.3390/pharmacy10010022
APA StyleMotamed, C. (2022). Clinical Update on Patient-Controlled Analgesia for Acute Postoperative Pain. Pharmacy, 10(1), 22. https://doi.org/10.3390/pharmacy10010022






