1. Introduction
Surveys of existing biodiversity play a vital role in the formulation of conservation policies, especially in countries such as Myanmar, with its high levels of species richness, rapidly changing socio-political climate and issues of environmental degradation [
1]. Over the last decade, citizen science programs have been hailed as an inclusive, community-oriented means of quickly and inexpensively generating large amounts of raw species-occurrence data, on the basis of which a high-resolution estimate of biodiversity can be generated over a wide geographical area [
2,
3,
4,
5]. However, the path from data collection to habitat conservation is a convoluted one, and it has been recognised that the spatial scale at which species occurrence data are collected or analysed can have a profound effect on estimates of species richness [
6,
7]. This phenomenon can have potentially dramatic implications for conservation policy and practice [
8,
9].
The goals of the field of language documentation, with its increasing emphasis on ethnobiological issues [
10,
11,
12], seem complementary to the goals of citizen-science-based biodiversity research, as described above. The native speakers of the language(s) being documented will often happily play the role of citizen scientist, thereby ensuring that the indigenous knowledge being recorded on biological topics is indeed local. Word lists of plant and animal names documented at the village level (or in ecological terms, the local-scale [
13]) could provide valuable information on local species richness, as long as an adequately rigorous methodology is followed. Word lists collected from a number of communities in a state or district could be pooled to generate a larger (landscape or meso-) scale estimate of the number and types of species present. Thus, it is theoretically possible to collect a substantial amount of ethnobiological data at different spatial scales that will be of value and relevance not only to the language communities concerned, but also to field biologists, ecologists and conservation policy-makers. While there will likely be some inaccuracies and imprecisions in the data collected, the advantage of this methodology is that a preliminary survey can be carried out at a fraction of the cost, and in a fraction of the time that it would take to implement a full study of local biodiversity involving professional biologists.
A case study on the application of a language-documentation-based methodology to a bird species survey in Shan State, Myanmar, is described in this paper. The field site was centred on the town of Aungban, situated in a linguistically diverse region with languages from three families. A recently published report of bird species from the nearby town of Kalaw [
14] was used as a baseline against which to compare the bird names obtained from six language communities for accuracy and consistency.
2. Materials and Methods
2.1. Data Collection
Bird names were recorded in six languages spoken in and around the town of Aungban in southwestern Shan State. The languages were Thanau (or Danau), Palaung (Austroasiatic); Taungyo, Pa’O, Danu (Tibeto-Burman); and Tare Shan (Tai-Kadai). The general methodology described in [
15] was used. Briefly, a stimulus set for eliciting bird names was first assembled, using a species checklist specific to Shan State obtained from the website Avibase (
http://avibase.bsc-eoc.org). Around 250 species were chosen from a wide range of families, for which photographs were sourced from the internet. Bird calls were downloaded from the Xeno-Canto website (
www.xeno-canto.org) for as many species as possible.
In each elicitation session, printouts of the bird photographs were presented one at a time, accompanied by playback of the corresponding birdcall or song on a laptop. Care was taken to ensure that there were at least two participants in each elicitation session, preferably adults of different ages (
Table 1). Participants were encouraged to discuss the identification of any problematic stimuli among themselves. The responses and accompanying discussion were recorded with an audio recorder, and simultaneously transcribed using IPA (International Phonetic Alphabet). Each session lasted between 60 and 90 min. In all cases, oral consent was obtained from each participant before the commencement of data collection. Participants were made aware of their right to withhold any information that they deemed unsuitable for the public domain. Elicitation for all languages except Taungyo took place in the villages where the languages are spoken as L1. A Taungyo village could not be accessed for various reasons, which is why the elicitation was carried out with some Taungyo speakers residing in Aungban. In all cases, the contact language used was Burmese.
2.2. Field Site and Participants
All the villages listed in
Table 1 were involved in agriculture, and were located in areas of different topography, microclimate and vegetation. The Thanau villages were located at an altitude of 1200 to 1250 m above sea level, and the surrounding landscape had been heavily deforested to make way for sesame, peanut, potato and ginger fields. Only one small patch of forest (<1 km
2) remained near Taungbohla village, associated with the nearby monastery and a sacred hill adjacent to it. The Palaung village of Taung Ni, on the other hand, was situated at an elevation of around 1500 m, and was surrounded by far more forest than either of the Thanau villages. Here too, much land had been devoted to cultivation, particularly for tea and orange plantations.
As mentioned above, a Taungyo village could not be reached during the course of fieldwork, and a group of five Taungyo men was instead interviewed in the town of Aungban. The oldest (58 years old) of these men, from the village of Sat Pya (or Sar Pyar), also provided the majority of the bird names recorded in this session. Based on information obtained from this individual, the co-ordinates and elevation of his village (
Table 1) were determined through Google Maps (Google, Inc., Mountain View, CA, USA). Sat Pya was found to lie at an elevation of around 1340 m, surrounded by a patchwork of fields and forest (<50% forest cover, by visual estimate).
The Pa’O and Danu villages were situated at a similar elevation to Aungban, and were surrounded by a patchwork of forest fragments and agricultural plots. The Danu village of Let Pan Bin was also situated next to a highway which carried moderate amounts of traffic to and from the nearby town of Ywangan. However, approximately 5–6 km to the west of Let Pan Bin lies a range of forested hills with little human settlement. Finally, the Tare Shan village lay near a major highway that connects the urban centres of Aungban and Pindaya, and was almost completely surrounded by farmland. Although the exact dates of the founding of the above villages were not recorded, at least some have been in existence for two or more generations. The two eighty-year-old participants from the Thanau village of Taungbohla were young men during the Japanese occupation of 1942–1945, and living in the same area. The Palaung and Taungyo villages are currently associated with extensive tracts of cultivated land (such as the tea and orange orchards of the Palaung village), and are likely to have existed for at least a few generations. The most likely candidates for recently founded villages are the Pa’O, Danu and Tare Shan villages, as a result of groups of families moving to a new location to avoid overcrowding in their ancestral villages. The Pa’O and Danu, in particular, have the largest populations in the region, and may need to found new villages with a greater frequency. The area around Aungban has largely been unaffected by armed ethnic conflict in recent decades (unlike parts of Shan State further to the east), and it is unlikely that there would have been any significant displacement of populations due to this reason.
2.3. Comparison with Bezuijen et al.
A total of 115 bird species mentioned in Bezuijen et al. [
14] were also included in the stimulus set used in the elicitation sessions. Counts of sightings of these bird species during field observations made by Bezuijen et al. [
14] were compared with the total number of bird names recorded for each species across all six languages in the present study. Testing for a possible correlation between the number of field sightings and the number of names recorded for each species was carried out by means of a Spearman’s Ranked Correlation test using the statistics package IBM SPSS Statistics V. 23 (IBM Corporation, Armonk, NY, USA). Two modifications were made to the raw data to remove outliers arising from known causes: the Bezuijen et al. [
14] count for the Oriental Honey Buzzard (
n = 1) was replaced by that for the Common Buzzard (
n = 11), and the Red-headed Vulture was excluded from analyses (see
Section 4.4 for more details). Moreover, care was taken to ensure that names accompanying imprecise or doubtful identifications were not included in the analyses. Thus, if participants pointed to a page showing pictures of several woodpecker species, and said, “These are all called X”, without being able to confidently state which species were present near their village, then those species would be excluded from the name counts. If, on the other hand, participants were able to confidently point out which woodpeckers they had actually seen, and which they had not, then the name X would be counted for that language for each of the woodpecker species identified.
3. Results
The minimum number of bird names recorded was recorded in the Tare Shan elicitation session (28), with the maximum being 81 for Taungyo (
Table 2). Of the 250 or so stimulus pictures used in the elicitations (i.e., biological species), approximately 44% were not identified by any language group, 32% were named by 1–2 groups, while 24% were named by three or more groups. There are two possible reasons for the large number of unidentified stimuli: the first is that the stimulus set was compiled on the basis of a species checklist of Shan State in its entirety. As a result, many of the stimulus pictures would have been of birds that are not present in the vicinity of the field site, and therefore unknown to the participants. The second possible reason is that many of the birds that were once present at the field site have become rare or locally extinct due to habitat loss through deforestation.
3.1. Naming Patterns
Some broad patterns in the naming of birds could be identified for all languages, although there was also a great deal of language-internal variation. Similar to a pattern that is prevalent in Burmese, all languages investigated had a tendency to include the superordinate category label (i.e., the word/morpheme ‘bird’) in a number of bird names (
Table 3). The Tibeto-Burman languages Taungyo, Pa’O and Danu only formed binomial compounds with the morpheme ‘bird’, although the proportion of such compounds among the bird names recorded for each language varied greatly—from 13.5% for Taungyo to 68% for Pa’O. The Palaung and Thanau ‘bird’ morphemes have been reconstructed back to Proto-Austroasiatic
*ciim ‘bird’ [
16], while the Tibeto-Burman ‘bird’ morphemes are descended from Proto-Tibeto Burman
*s-ŋak ‘bird’ [
17].
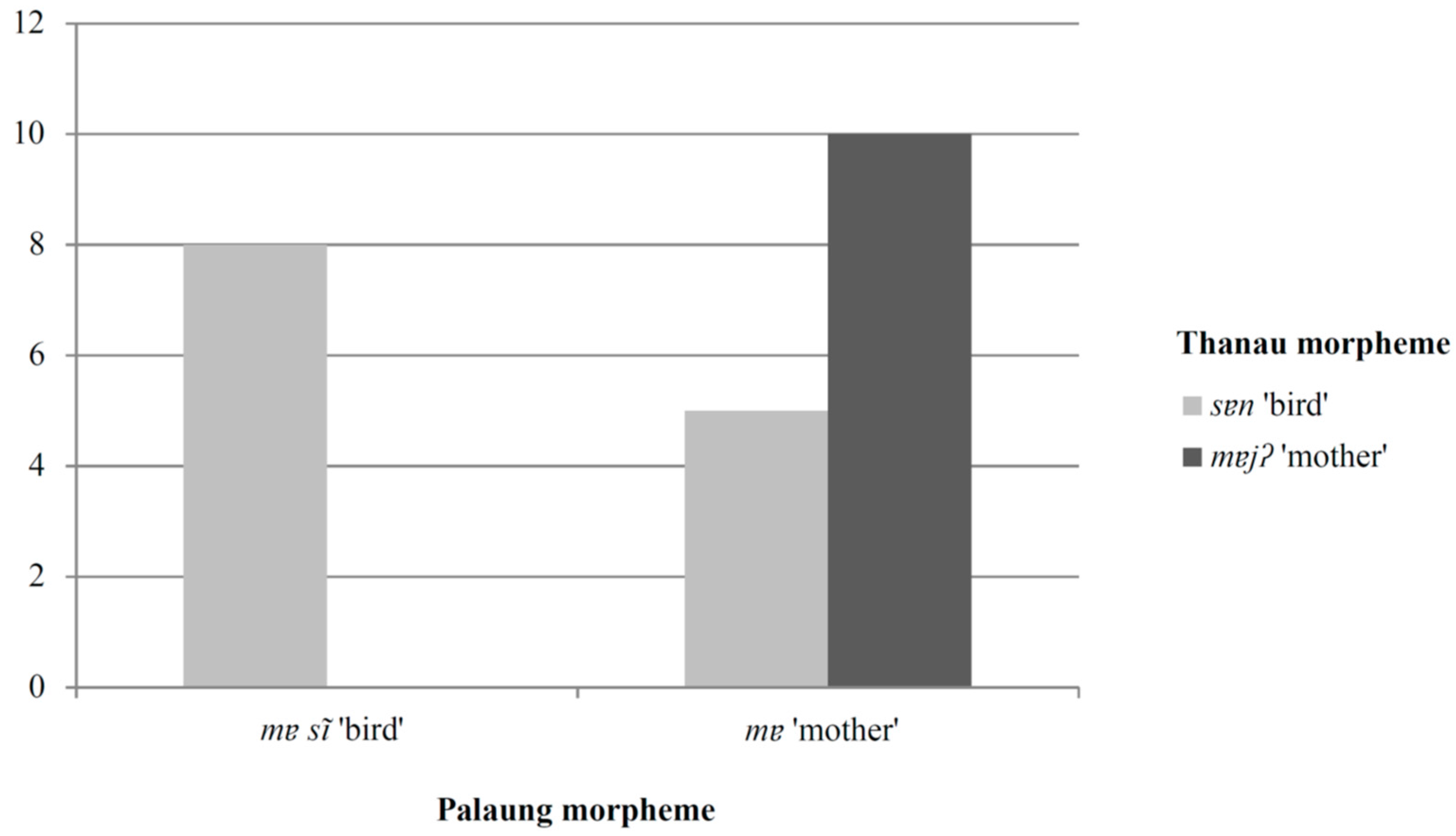
Interestingly, the Austroasiatic languages Thanau and Palaung showed a slightly different pattern from the Tibeto-Burman and Tai-Kadai languages, in having an additional morpheme, possibly meaning ‘mother’, that also formed binomial compounds in bird names. While Palaung had a greater proportion of binomial compounds with the morpheme ‘mother’ (61%) compared to ‘bird’ (27%), the reverse trend was observed for Thanau, with the ‘bird’ morpheme being more common (
Table 3). However, analysis of the mapping of the ‘bird’ and ‘mother’ morphemes between Thanau and Palaung revealed a somewhat overlapping distribution.
Figure 1 shows that, among the birds named in both languages, those that have binomial Palaung names incorporating the morpheme
mɐsĩ ‘bird’ exclusively have Thanau binomial names incorporating the morpheme
sɐn ‘bird’. The relationship between the Palaung and Thanau binomial names incorporating the morpheme ‘mother’ is weaker, but the general trend is for the same morpheme to be used for a particular bird in both languages.
Table 4 includes some examples of bird nomenclature in the six languages investigated, including binomial and mononomial names. The superordinate category labels incorporated into binomial names are shown in bold.
In addition to the binomial compounds discussed above, trinomial bird names (including the superordinate category label plus a descriptive epithet) are also to be found in all six languages. Examples are shown in
Table 5:
3.2. Cross-Linguistic Patterns in Bird Knowledge
Thanau is the only language in the current study for which elicitation sessions were carried out in two different villages. The villages of Taungbohla (TBL) and Thae’thit (TIT) are only 2 km away from each other, and yet differences were observed in not only the birds identified by participants in the two groups, but also in the names assigned to certain birds. Notable species that were identified and named by participants from TBL but not TIT, include the Silver Pheasant, the Red-headed Vulture, the Asian Koel, the Emerald Dove, the Bronzed Drongo, the White-throated Fantail and the Puff-throated Babbler (
Table 6 and
Table 7). The opposite was observed for the Orange-breasted Pigeon, the Chestnut-winged Cuckoo, the Barn Owl, the Red-billed Blue Magpie, the Plaintive Cuckoo and the Common Tailorbird. Differences in naming range from minor lexical/phonological variation, as in the case of the Coppersmith Barbet (TBL:
mɐjʔ ku pɛt, TIT:
mɐjʔ ko ku peʔ) to the existence of completely unrelated names, as in the case of the Little Cormorant (TBL:
pɔŋ pɐj un, TIT:
sɐn mɐj).
Birds for which names were recorded in at least half (three to six) of the languages are shown in
Table 6. These include birds that are considered edible (
Phasianidae, such as the francolins, partridges, pheasants and junglefowl), those that are easily recognised by their calls (such as the bulbuls, cuckoos, lapwing, barbets and woodpeckers) and those that have folklore associated with them (such as the Indian Cuckoo and the Bronzed Drongo). As these bird species were recognised and identified by a majority of elicitation groups, it can be concluded that the names recorded for these species are more precise, with respect to their biological referent(s). Still, one needs to be careful when dealing with groups of similar-looking birds (see
Section 4.1) that can only be observed from a distance: birds of prey, swifts and swallows and owls, for example.
A higher level of caution is advisable when dealing with the birds for which only one or two names, out of a total of six languages, were recorded (
Table 7). There is a greater likelihood, in such cases, that the participants were guessing the identity of the bird upon seeing a stimulus picture and/or hearing the birdcall, because of a lack of clarity in the picture, or because it visibly or aurally resembled another bird that they were familiar with. Nevertheless, a number of species with distinctive appearance, behaviours or calls were probably identified accurately. These include the Large-Tailed Nightjar, the Common Kingfisher, the Green Bee-eater, the hornbills, the barbets, the Greater Racket-tailed Drongo, the babblers and scimitar-babblers and the mynas.
3.3. Shared Vocabulary
Unsurprisingly for a highly multilingual region, numerous bird names are shared across two or more languages. Most of these shared names are to be found in
Table 6, although
Table 7 also contains a handful of examples. Most instances of shared vocabulary can be attributed to lexical borrowing, either from the national language Burmese, or amongst the target languages themselves. The most obvious examples of the former phenomenon are the Burmese words
daũ ‘peafowl’ and
ləda ‘vulture’, which have been borrowed by the majority of the target languages (see
Section 4.4.1 for further discussion). Other examples, which are less widespread, include
tçɛʔ tu jwe ‘parrot/parakeet’ in Thanau,
khɐ ‘partridge’ in Palaung,
kho ‘pigeon/dove’ in Thanau. Naturally, shared vocabulary may also be indicative of shared ancestry. For the Tibeto-Burman languages, this can be seen in the Taungyo, Danu and Pa’O names of the Red Junglefowl (Burmese
tçɛʔ), the Taungyo and Danu names for quails and partridges (Burmese
ŋoũ), and the Taungyo and Danu names for swifts and swallows.
Borrowing from one language family to another can be most readily observed in Thanau and Pa’O, as the speakers of the two languages live in close proximity to each other, at least within the study area. Some owls share the name
u khɹɔŋ and
t̪ɔk t̪u is an acceptable name in both languages for the Spotted Dove, while the Thanau variants
klitklɔt and
tçi kloɐt for the bulbuls may originate in the Pa’O name
t̪i thɔt. There are also some intriguing correspondences, such as the Thanau
(sɐn) pəti and the Pa’O
(wɐ) pe di tit, which unfortunately, were associated with different species by speakers of the two languages. Insufficient data preclude a more thorough investigation of loanwords among the bird names shown in
Table 6 and
Table 7, but this topic (along with related issues such as the motivation for borrowing, phonological changes and semantic shifts) will be dealt with in detail in a future publication.
3.4. Comparison with a Biodiversity Survey
An analysis of the correspondences between the number of sightings for individual bird species by Bezuijen et al. [
14] and the number of names recorded for those species across the six languages indicated a positive correlation.
Figure 2 shows that the bird species that were not named, or named in only one session (0–1) tended to have fewer sightings in the Bezuijen et al. study [
14], compared to bird species for which 4–6 names were obtained. Tests of normality on the raw data for both variables revealed them to be highly skewed, due to the large number of low values, particularly 1 and 0. Consequently, the non-parametric Spearman’s Ranked Correlation test was carried out on the raw data to check for a potential relationship between the two variables. The test revealed a small, but statistically significant positive correlation between the number of names recorded for each bird, and the number of sightings of that bird in the field by birdwatchers over the duration of the Bezuijen et al. study [
14] (Spearman’s
ρ = 0.292, degrees of freedom (d.f.) = 112,
p < 0.01 (two-tailed)). In other words, birds that are rarely seen in the wild are infrequently mentioned by speakers of the languages of the region in elicitation sessions, whereas common birds are more likely to be recognised and named. This suggests that the wordlists obtained from elicitation sessions with visual and audio stimuli can, in part, be treated as proxies for field observations by birdwatchers.
Examples of good matches between the two studies include the Scarlet Minivet, Oriental Turtle-Dove, Spotted Turtle-Dove, Bronzed Drongo, White-throated Fantail, Red-billed Blue Magpie and various bulbuls. In all these cases, names were obtained in at least four out of the six languages investigated, and the birds were sighted at least 10 different times in the Bezuijen et al. study [
14] (
Table 8). There are also numerous examples of infrequently-sighted birds being named in only one or two languages: these include the Oriental Pied Hornbill, the Common Hill Myna, the Lineated Barbet, the White-rumped Shama and the Greater Racket-tailed Drongo.
Finally, there were a number of species for which a substantial mismatch was detected between the number of names recorded and the number of field sightings. Highlighted in grey in
Table 8 are (1) birds very common to the region, for which no or few names were recorded; and (2) birds sighted infrequently or not at all by birdwatchers, for which names were recorded in several languages. Four broad categories of mismatch can be identified, based on certain characteristics of the birds involved, and these are elaborated upon in the
Section 4.4.
4. Discussion
This study has demonstrated that a targeted audiovisual stimulus set can be used to successfully elicit a great deal of ethnobiological vocabulary, which is a valuable component of any language documentation project. In linguistically diverse parts of the world, such data can also be readily used to examine issues such as language contact (substrate influences, loanwords), dialectal differences, semantic shifts and the genetic relatedness of languages. Moreover, a comparison with an ornithological report from an area close to the linguistic field site revealed a significant degree of correlation between birdwatchers’ sightings and the responses of participants in language elicitation sessions. This suggests that field linguistic data in the form of wordlists of plants and animals have some utility in informing biodiversity studies, and can be regarded as complementary to the observations of professional naturalists.
Soberón and Peterson [
18] have argued that primary species-occurrence data can be modelled, along with information on change in land-use patterns, to provide useful indices of biodiversity loss in a region. The authors characterised such data as “the key infrastructural element in biodiversity informatics”, adding that the advantages of observation-based biodiversity surveys included “the force of numbers, the possibility of direct use in time series, and the potential for broad public participation” [
18] (p. 30). However, primary data are expensive and time-consuming to obtain, and are frequently unavailable in developing countries. The data in the present study can be considered primary species-occurrence data, with the added advantage that they address two key spatial scales—the local scale and the landscape scale—required in developing a more sophisticated, hierarchical model that accounts for patterns of biodiversity [
13]. However, linguistic data collected through stimulus-based elicitation sessions have certain limitations, which are discussed in the following section.
4.1. Limitations of the Current Study
Ideally, an ethno-ornithological study would involve the sighting or collection of living birds in situ, in the company of an ornithologist and expert consultants from the target language community (as in [
19]), or an elicitation session led by an ornithologist familiar with the birds found in the area where the language is spoken (as in [
20]). Such an enterprise, while undoubtedly rewarding, is also time- and labour-intensive, especially when the intention is to document bird knowledge in several languages at once. An alternative methodology was described in [
15], wherein targeted stimulus sets containing pictures and recordings of the species present in the study area may serve as a reasonable substitute for field observations of the species, and/or the presence of a professional naturalist with expertise in the taxon under investigation. While this procedure was successfully used to generate the linguistic data presented in the current paper, certain caveats should be borne in mind when analysing those data, and making generalisations about the languages involved.
The most obvious issue is the potential for incorrect identifications (i.e., incorrect matches between a name and its biological referent(s)) resulting from an imperfect stimulus set.
Table 5 and
Table 6 likely contain numerous examples of incorrect or imprecise identifications, either because the photographs and accompanying sound files were unclear or misleading, or because they were of similar-looking species that were not normally to be found in the vicinity of the participants’ villages. This is often the case for groups of similar-looking birds that are usually only observed from a distance, such as swifts and swallows, birds of prey, owls, some woodpeckers, some kingfishers and assorted small passerines. Note that among these birds are many that also cannot be easily distinguished by their calls. Static images also have the disadvantage of forcing participants to make identifications without the benefit of salient cues such as relative size, characteristic movement patterns, natural appearance when partially obscured by vegetation, and so on. The playback of songs and calls alleviates this problem somewhat, but the task given to participants remains an artificial one.
Another potential complication that could arise from the procedure used here is the inclusion, in the final wordlists, of birds that are now locally extinct in the area where the target languages are spoken. Such names would make their way into the wordlists because of the presence of older participants in the elicitation sessions, who may remember seeing certain locally-extinct birds during their youth. Naturally, this is a definite advantage if the intention of the researcher is also to document traditional knowledge and vocabulary that is on the verge of disappearing, due to changing local environmental conditions. However, this poses a problem when the aim is to use the wordlists as a proxy for a biological survey, as it will have the effect of artificially inflating current local species diversity.
4.2. Cross-Linguistic Differences
Differences in the ability of the language groups to name certain bird species can be taken as evidence of differences in the distribution of these birds at the small to medium spatial scale. This is particularly true of large, conspicuous birds, or those with memorable or evocative calls. For such birds, the absence of a name in the language of a particular community is a good indication that that species is currently absent from the region where that community resides. Among the birds presented in
Table 6, examples of visually distinctive birds lacking names include the Silver Pheasant and the Grey Peacock Pheasant for the Thanau-speaking community at Tha’ethit village and the Danu-speaking community at Let Pan Bin village, and the Eurasian Hoopoe for Tare Shan and the Taungyo speakers living in Aungban. Similarly, the Indian Cuckoo, whose easily recognisable four-note call is the source of its name in Taungyo and Palaung, is not named by Thanau and Danu speakers.
Such differences are exaggerated when examining the bird species that were only named in one or two of the languages sampled (
Table 7). Those birds which were accurately identified by participants in the elicitation sessions (see Results for the birds whose identification is considered reliable) are likely to have narrow ranges due to their being restricted to a particular habitat type. As mentioned earlier, the villages where the six target languages are spoken are situated in a range of habitat types, which will undoubtedly have a bearing on local species composition. The moderate to severe deforestation that has occurred around Aungban and nearby villages, the differences in elevation and the closeness to urban centres and highways could have an effect on which birds are commonly encountered and named by the residents of different villages.
Another important factor that could have a large effect on the birds that are identified and named is the age of individual participants. Older participants are likely to have grown up in a time when the forest cover around their villages was more intact, and the species diversity and abundance of plants and animals was higher than it is now. The Palaung and Pa’O elicitation groups each included at least one participant older than 60 years of age; the TBL Thanau group included two participants in their eighties, while the Taungyo group included one participant who was 58 years old (
Table 1). The Tare Shan and Danu groups, however, consisted entirely of people no older than 50 years of age, and it is conceivable that the inclusion of older participants could increase the number of bird names recorded for these languages. It is interesting that these two groups, along with the TIT Thanau group (also composed of younger participants), were unable to identify the Silver Pheasant. This large, distinctive but now-rare bird was easily identified and named by the other groups which contained at least one senior individual. Having speakers of very different ages in the same elicitation session can also result in different names being provided for the same bird (e.g., TBL in
Table 6 and
Table 7). This phenomenon will be investigated in a future paper.
Finally, the age of a particular community (i.e., the time since the founding of a village) could affect the number of birds known to that community. A young community that has been living at a particular location for, say, less than two generations, may have yet to become fully aware of the flora and fauna at that location. This is particularly true for populations that have been displaced over long distances. While the dates of the founding of the villages associated with the current study were not recorded, there is no a priori reason to expect that any of the target communities are particularly young. However, the period of residence of a particular community at its present site may have a bearing on people’s knowledge of local ecology, and should be considered to be an important variable in future studies.
4.3. Correspondence with Birdwatchers’ Reports
While there was overall a statistically significant correlation between the results of the elicitation sessions and the sightings reported in Bezuijen et al. [
14], a particularly high degree of correspondence was noted for some birds. These included the Scarlet Minivet, Oriental Turtle-Dove, Spotted Dove, Bronzed Drongo, White-throated Fantail, Red-billed Blue-Magpie, Black-crested Bulbul, Red-vented Bulbul and Red-whiskered Bulbul, all of which were sighted 10 or more times in the field, and were named in at least four languages. The Brown Shrike, Crested Finchbill, Gray-capped Woodpecker, Indian Roller and Coppersmith Barbet were recorded a moderate number of times in both studies, while numerous other birds in
Table 8 were recorded infrequently or not at all. This shows that the statistical correlation reported earlier is driven by birds at all levels of diversity.
Note here that many other bird species, which were not mentioned in the Bezuijen et al. study [
14], were identified and named in the elicitation sessions. The Silver Pheasant, Little Cormorant, Eurasian Collared Dove and Jungle Myna were named in a number of languages, as were the Rain Quail and numerous partridges. The Silver Pheasant is likely to have been locally extirpated, or nearly so, as some consultants stated that this bird was now rare in the vicinity of their villages. The other birds are likely to have ranges that do not overlap with the site of the Bezuijen et al. study [
14].
No systematic attempts were made in this study to record participants’ perceptions on changes in the abundance or diversity of local bird species. However, this additional line of questioning could, in theory, be incorporated into the elicitation sessions in order to assess potential declines in biodiversity due to anthropogenic or climatic factors. With the Bezuijen et al. study [
14] as a baseline study carried out in a relatively undisturbed forest habitat, it should be possible to determine the differences in the avifauna local to each village, and correlate those differences to e.g., human activities, such as agriculture and forest clearance. Even in the data presented in
Table 6 and
Table 7 above, some instances of deviation from Bezuijen et al.’s [
14] findings could possibly be attributed to such factors (See ‘Birds with limited or reduced ranges’ in the following section). On the other hand, there are indications that the total number of bird names recorded in a community may be influenced by the remaining forest cover around that community. The most names were recorded from a Taungyo speaker (
Table 2), who had lived in possibly the least accessible village with arguably the highest amount of forest cover. On the other and, the lowest number of names was recorded in Tare Shan, in a village close to a highway and with almost no remaining forest cover.
4.4. Cases of Mismatch
A number of birds sighted frequently by birdwatchers were named in only a few languages, and vice versa. These can be divided into four categories, which are discussed below.
4.4.1. Well-Known or ”National” Birds
Large birds which are known due to their cultural importance—such as birds of national significance or cultural icons, birds featuring in folk tales, or birds with value as a common food source—are frequently named in regions where the respective species may not be present. Birds such as the Green Peafowl
Pavo muticus (not mentioned in
Table 8 as this bird has never been observed near Bezuijen et al.’s field site [
14]) and the Red Junglefowl were therefore known to speakers of all six languages, in spite of these species being rare or absent around Aungban. In addition, some birds, known solely by virtue of the fact that they are mentioned during formal schooling, were also named in all languages, despite many participants never having seen those birds near their villages. Prominent among these is the Red-headed Vulture, which Bezuijen et al. [
14] never observed in their field survey, despite historical reports of the presence of this species. Participants generally struggled to identify this bird from its photo, and only provided a name when prompted with the Burmese name for vultures. The Burmese word
lədà ‘vulture’ was known to all participants, with many volunteering that this was the bird that scavenged on the corpses of animals such as cows and buffalos. Even if some of the participants in the elicitation sessions had seen a vulture in the wild, it is more likely to have been a species such as the White-rumped Vulture
Gyps bengalensis, which is said to be more common in this part of Myanmar [
21]. Unfortunately, this species was not included among the stimulus pictures shown during the elicitation sessions, which led to the Red-Headed Vulture being unanimously (and mistakenly) identified.
4.4.2. Inconspicuous or Culturally Unimportant Birds
A handful of small birds, such as the Velvet-fronted Nuthatch, Chestnut-flanked White-eye, probably belong in this category. These birds, being difficult to accurately identify from a distance, may lack names completely in the languages investigated.
4.4.3. Birds Incorrectly Named Due to Similarity with Other Species
Birds such as the Oriental Honey Buzzard, Emerald Dove, Large Hawk-Cuckoo, Indian Cuckoo, Asian Koel, Barn Owl and Chestnut-capped Babbler were all sighted infrequently by Bezuijen et al. [
14] (or only heard, but not sighted), whereas they were all identified and named in at least four languages each. This could be due to overgeneralisation of ethnospecies categories on the part of the participants, where a label that normally would apply to several undifferentiated species is used to name a novel species because of its similar appearance. The case of the Oriental Honey-Buzzard could be one such example, as in some languages, the generic term for ‘raptor’, or possibly a different kind of raptor, was offered as the name of this bird. Bezuijen et al. [
14] report 11 sightings for the similar-looking Common Buzzard
Buteo buteo, and it is very likely that this species, rather than the Oriental Honey-Buzzard, is the one named in four languages (
Table 6 and
Table 8). The Emerald Dove and Barn Owl may also have been named with generic ‘pigeon’ and ‘owl’ labels respectively by some speakers (although, note that for TBL speakers of Thanau, and for Taungyo and Pa’O, the latter bird has a name that is distinct from the other owls). Finally, the cuckoos, the Asian Koel and Chestnut-capped Babbler are more likely to have been correctly identified, by virtue of their distinctive calls. It is possible that these birds were sighted infrequently by the birdwatchers in [
14] due to their ranges not extending to that study’s field site (see below for a related phenomenon).
4.4.4. Birds with Limited or Reduced Ranges
A number of birds were reported as being common in their study area by [
14], but were only named in one or two languages, or not at all, in the elicitation sessions. These include the Black Bulbul, Mountain Bulbul, Common Kingfisher, Blue-throated Barbet, White-browed Scimitar-Babbler, Rusty-cheeked Scimitar-Babbler, Puff-throated Babbler, White-browed Laughingthrush, Common Myna and Black-collared Starling. Many of these birds, such as the bulbuls, the Blue-throated Barbet, Black-collared Starling and the babblers may be restricted to higher elevations or more forested areas, while the Common Kingfisher would usually be found close to permanent water bodies—all features that are lacking in the area where the six languages are spoken. It is surprising that the Common Myna was only named in two languages (and even then, it shared its name with other species such as the Jungle Myna), in spite of it having been sighted on 10 different occasions by Bezuijen et al. [
14]. Possibly, this is also an accurate reflection of the range of this bird, with the Jungle Myna being more prevalent in the study area (
Table 6).
5. Conclusions
The aims of this study have been twofold: to present a preliminary documentation of ornithological knowledge among six language communities in a linguistically diverse part of Myanmar, and to determine the utility of such a documentation in supplementing a checklist-based biodiversity survey. The elicitation method used here proved to be a quick and effective way of recording a substantial amount of ethnobiological vocabulary. While some birds may have been misidentified due to the artificial nature of the task provided, bird species which were named in three or more languages may be regarded with confidence. A statistically significant correlation was obtained between the frequency with which birds were sighted by ornithologists in a previously published report, and the number of languages in which those birds were named. There are some noteworthy mismatches between the two metrics, and the general trends evident in these mismatches should be regarded as caveats when carrying out a study of this nature. Nevertheless, the results of stimulus-based linguistic elicitation sessions can be of great value in providing information of local biodiversity at a fine spatial scale. They can not only provide novel useful data that can inform and increase the local relevance and utility of biodiversity surveys, but also serve as a rapid, first-pass assessment of biodiversity in places where surveys by professional biologists are lacking.
The results presented in this paper highlight the need for close cooperation between biologists and linguists, in order to rapidly achieve data of reasonable quality. The involvement of an ornithologist familiar with local bird species should ensure that the mis-identification of bird species (e.g., those that are never found in a particular habitat, or those that are endemic to another region) is kept to a minimum. The ornithologist will also be able to prompt participants with relevant information on the behaviour and ecology of local species, thereby facilitating their identification from static images. Linguistic expertise is also an essential requirement in a study of this nature. A linguist will be able to identify potential issues associated with sociolinguistic variables, such as the age of participants, and identify cases of lexical borrowing and semantic shift. A linguist will also ensure that bird names are accurately transcribed. The present study therefore strongly supports the inclusion of linguistic data, where possible, in rapid biodiversity surveys, and a close collaboration between biologists and linguists, especially in areas of high language diversity.
Acknowledgments
This study was partly funded by a Small Grant SG0205 from the Endangered Languages Documentation Programme. The author thanks two anonymous referees for comments which helped improve the manuscript.
Conflicts of Interest
The author declares no conflict of interest.
References
- Sovacool, B.K. Environmental Conservation Problems and Possible Solutions in Myanmar. Contemp. South. Asia 2012, 34, 217–248. [Google Scholar] [CrossRef]
- Dickinson, J.L.; Shirk, J.; Bonter, D.; Bonney, R.; Crain, R.L.; Martin, J.; Phillips, T.; Purcell, K. The current state of citizen science as a tool for ecological research and public engagement. Front. Ecol. Environ. 2012, 10, 291–297. [Google Scholar] [CrossRef]
- Francesco, P. Marine Biodiversity Survey in the Northern Red Sea: A Large-Scale Monitoring Carried Out in Collaboration with Volunteer Divers. Ph.D. Thesis, University of Bologna, Bologna, Italy, 2013. [Google Scholar]
- Kehinde, T.; Amusan, B.; Ayansola, A.; Oyelade, S.; Adu, W. Status of insect diversity conservation in Nigeria: A review. Ife J. Sci. 2014, 16, 319–330. [Google Scholar]
- Wei, J.W.; Lee, B.P.; Wen, L.B. Citizen Science and the Urban Ecology of Birds and Butterflies—A Systematic Review. PLoS ONE 2016, 11, e0156425. [Google Scholar] [CrossRef]
- Crawley, M.J.; Harral, J.E. Scale Dependence in Plant Biodiversity. Science 2001, 291, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Lennon, J.J.; Kolef, P.; Greenwood, J.J.D.; Gaston, K.J. The geographical structure of British bird distributions: Diversity, spatial turnover and scale. J. Anim. Ecol. 2001, 70, 966–979. [Google Scholar] [CrossRef]
- Mac Nally, R. Monitoring forest bird communities for impact assessment: The influence of sampling intensity and spatial scale. Biol. Conserv. 1997, 82, 355–367. [Google Scholar] [CrossRef]
- Hurlbert, A.H.; Jetz, W. Species richness, hotspots, and the scale dependence of range maps in ecology and conservation. Proc. Natl. Acad. Sci. USA 2007, 104, 13384–13389. [Google Scholar] [CrossRef] [PubMed]
- Si, A. Biology in Language Documentation. Lang. Doc. Conserv. 2011, 5, 169–186. [Google Scholar]
- Hyslop, G. Worlds of knowledge in Central Bhutan: Documentation of ‘Olekha. Lang. Doc. Conserv. 2016, 10, 77–106. [Google Scholar]
- Odango, E.L. A discourse-based approach to the language documentation of local ecological knowledge. Lang. Doc. Conserv. 2016, 10, 107–154. [Google Scholar]
- Willis, K.J.; Whittaker, R.J. Species Diversity—Scale matters. Science 2002, 295, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Bezuijen, M.R.; Eaton, J.A.; Gidean; Hutchinson, R.O.; Rheindt, F.E. Recent and historical bird records for Kalaw, eastern Myanmar (Burma), between 1895 and 2009. Forktail 2010, 26, 49–74. [Google Scholar]
- Lahe-Deklin, F.; Si, A. Ex-situ Documentation of Ethnobiology. Lang. Doc. Conserv. 2014, 8, 788–809. [Google Scholar]
- Shorto, H. A Mon-Khmer Comparative Dictionary; Sidwell, P., Ed.; Pacific Linguistics: Canberra, Australia, 2006; p. 363. [Google Scholar]
- Matisoff, J.A. Handbook of Proto-Tibeto-Burman; University of California Press: Berkeley, CA, USA, 2003; pp. 317–318. [Google Scholar]
- Soberón, J.; Peterson, A.T. Monitoring Biodiversity Loss with Primary Species-Occurrence Data: Toward National-Level Indicators for the 2010 Target of the Convention on Biological Diversity. Ambio 2009, 38, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.; Bishop, D. Ethno-ornithology of the Ketengban people, Indonesian New Guinea. In Folk Biology; Medin, D., Atran, S., Eds.; MIT Press: Cambridge, MA, USA; London, UK, 1999; pp. 17–46. [Google Scholar]
- Agnihoti, S.; Si, A. Solega Ethno-Ornithology. J. Ethnobiol. 2012, 32, 185–211. [Google Scholar] [CrossRef]
- Hla, H.; Shwe, N.M.; Htun, T.W.; Zaw, S.M.; Mahood, S.; Eames, J.C.; Pilgrim, J.D. Historical and current status of vultures in Myanmar. Bird Conserv. Int. 2011, 21, 376–387. [Google Scholar] [CrossRef]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).