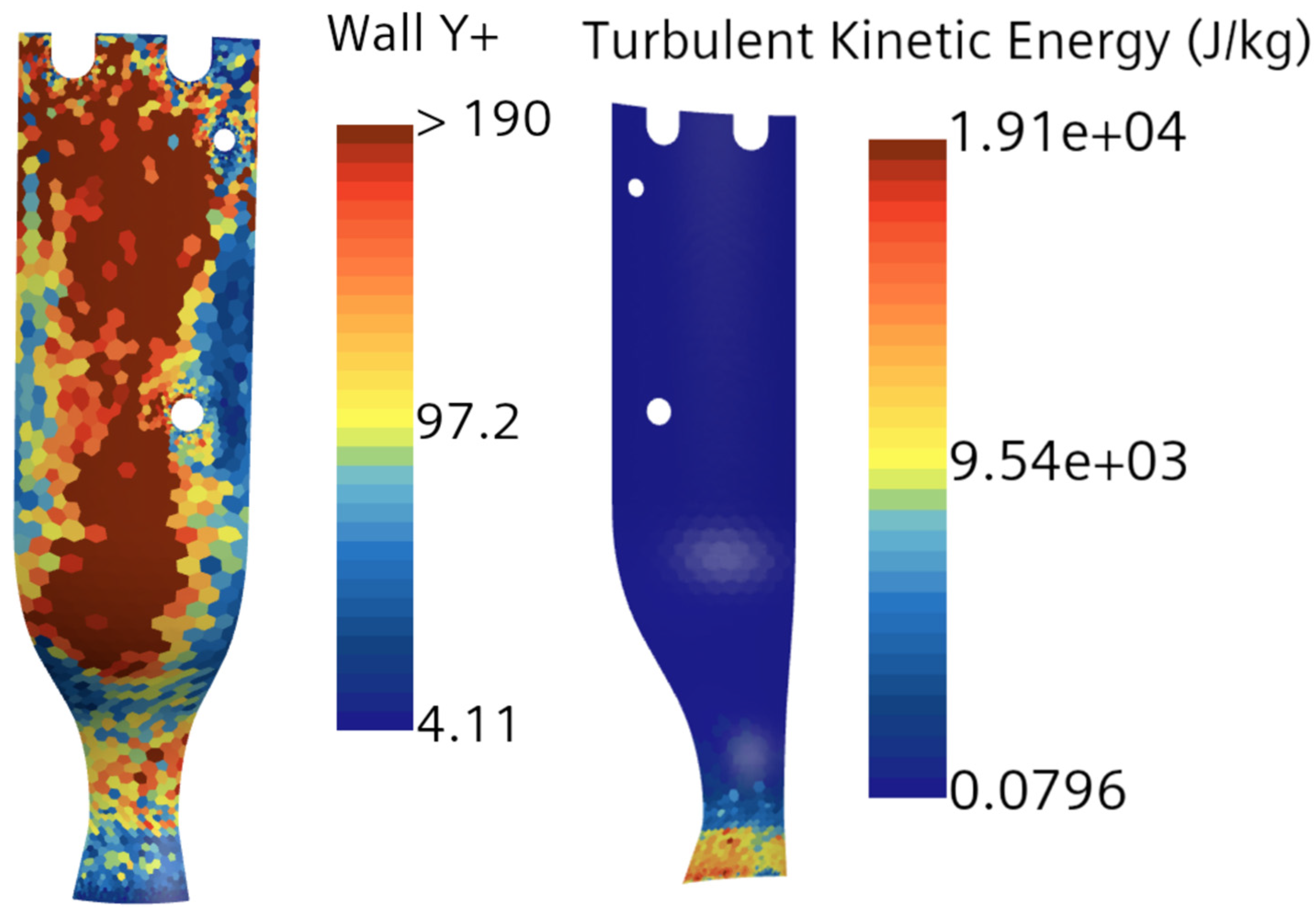
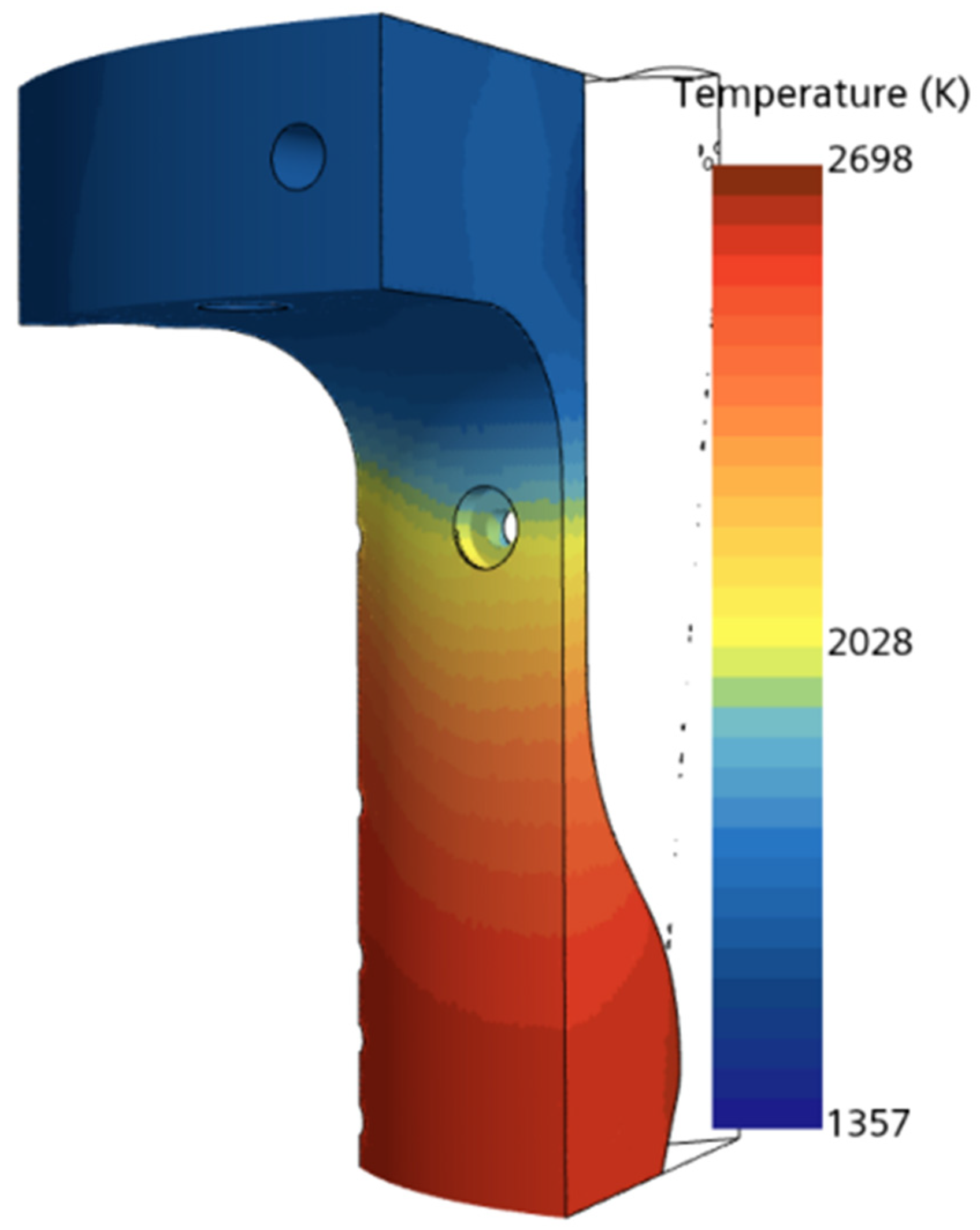
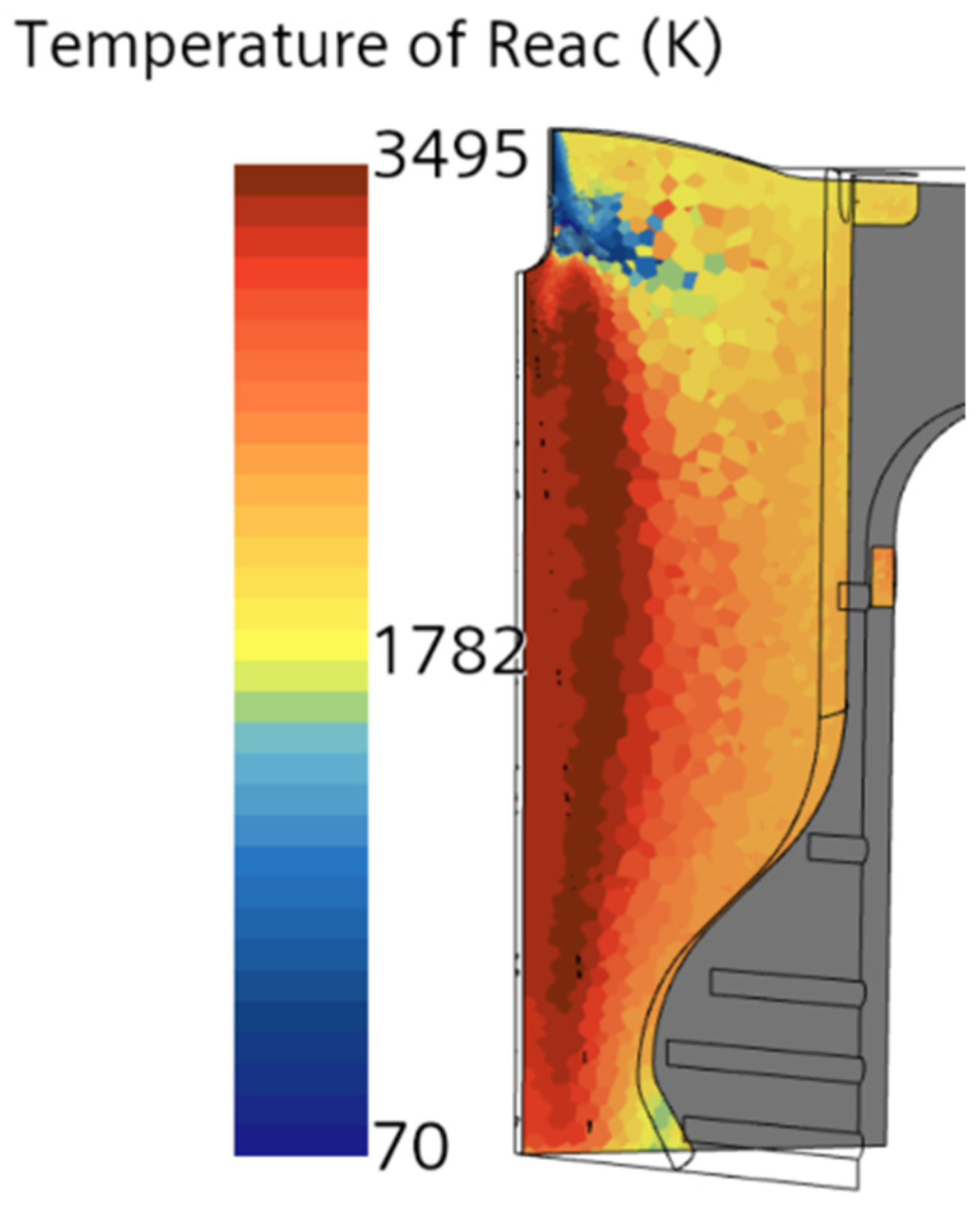
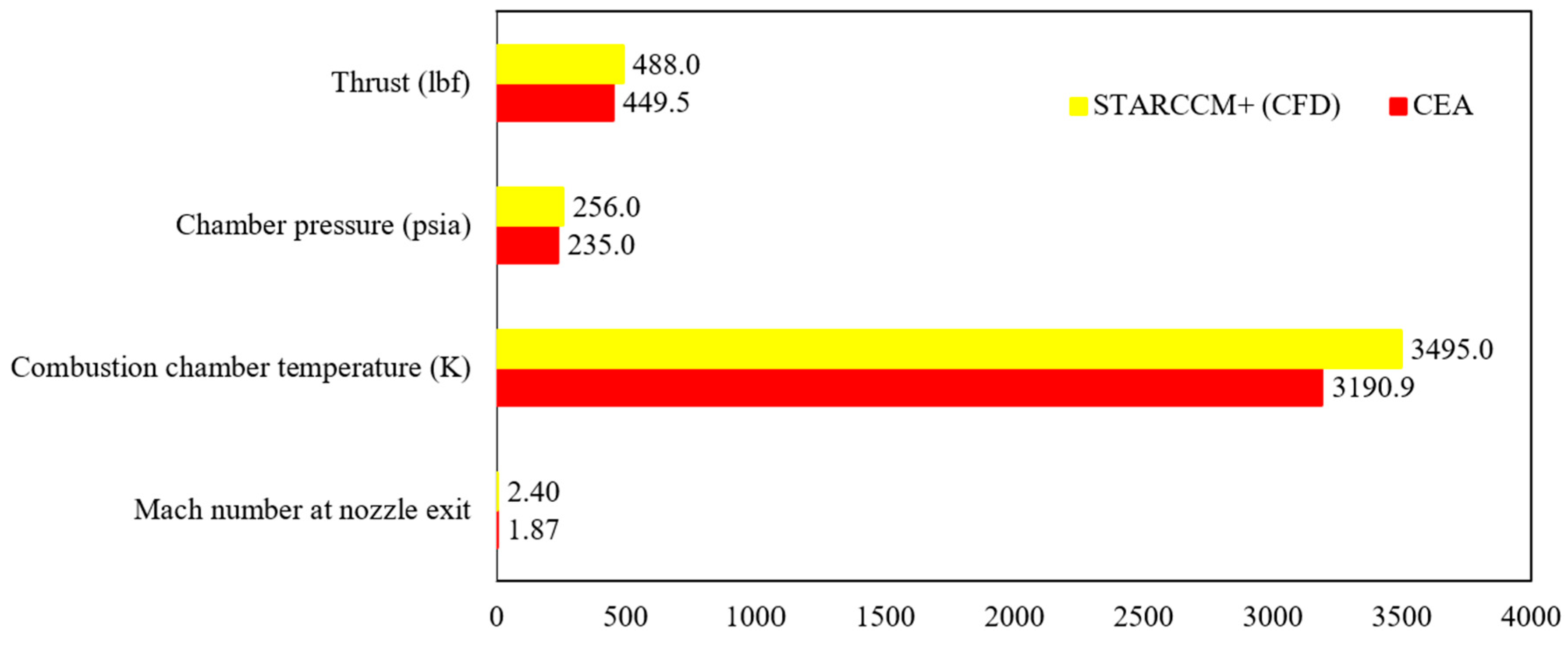
The authors examined the multiphase flow and combustion characteristics of a liquid rocket engine (LOX-LCH4) using insights from theoretical analysis, equilibrium chemical analysis (CEA), and computational modeling with STAR-CCM+. It should be noted that while the theoretical analysis is not discussed in detail in this paper, it was based on established thermodynamic, heat transfer, and propulsion equations, which were employed to predict engine performance parameters such as thrust and impulse for the LOX/LCH4 engine. An equilibrium adiabatic calculation using the major species was also implemented in theoretical analysis. The CEA, a zero-dimensional, steady-state, single-phase, ideal gas, adiabatic analysis method, was used to determine engine performance parameters, including upper-bound temperature, pressure, velocity, Mach at the combustion chamber, nozzle throat, exit, etc.
The authors acknowledge that this approach represents an idealized scenario, and the results are expected to deviate significantly from real-world rocket engine performance. To capture the multiphysics flow properties and engine performance parameters over a wide range of real-world operating conditions, the authors utilized STAR-CCM+, a physics-based modeling approach. Unlike CEA, this method accounts for non-adiabatic, inhomogeneous, non-equilibrium effects and critically includes the complex behavior of two-phase flow during injection, mixing, breakdown, vaporization, interaction of liquid–gas phases, and the impact of wall heat transfer (heat loss) between the combustion chamber and surroundings. As a result, the computational fluid dynamics (CFD) predictions are expected to diverge from the theoretical and CEA results due to the inclusion of real-world physical phenomena. Nevertheless, these different approaches were integrated to provide a comprehensive assessment of engine performance, acknowledging the expected variations between them. The detailed methodologies for combustion modeling, multiphysics flow analysis, and NASA CEA are presented in the following sections.
2.1. Combustion Modeling and Associated Governing Equations
To accurately determine conditions within the combustion chamber, a method of calculating the properties of combustion within the combustion chamber is needed. To resolve this with the accuracy desired of a digital twin simulation, a form of combustion chemical analysis must be integrated with the simulation. Fortunately for this purpose, the chemistry of methane and oxygen has been well studied in recent years due to the fuel’s popularity for both legacy applications such as heating and newer applications in aerospace as a high-performance fuel mixture. While maximum performance may be acquired by combusting the propellants at or near the stochiometric oxygen/fuel (OF) ratio, to protect the engine during operation from the maximum flame temperature or oxidation from remaining oxygen radicals, the engine core combustion is fuel-rich at an OF ratio of 2.7 compared to the stochiometric OF ratio of 4 [
27]. Using Cantera and the GRI 3.0 mechanism to evaluate the combustion temperature at the given OF ratio, the predicted flame temperatures for each pressure case are given in
Table 1 [
28].
One assumption made for the purpose of this simulation is the assumption of the fuel as pure methane (LCH4), as opposed to the liquid natural gas (LNG) the CROME is slated to use. However, this assumption is not always shown to be valid, as the presence of pollutants can noticeably alter the transport and evaporative characteristics of the liquid natural gas fuel in the high-temperature, high-pressure regime typical of rocket engines [
29]. To analyze the impacts of typical pollutants on combustion, a Monte Carlo (MC) simulation was set up and run to find the impact of differing pollutant fractions on the temperature and ratio of specific heats of the post-combustion mixture. This simulation was programmed within MATLAB, with Cantera used for CEA and GRI-3.0 reaction mechanism files imported into the STAR-CCM+ for combustion modeling of methane along with pollutant compounds.
To perform such an analysis, the first thing needed is a list of pollutants considered. According to data by Dr. Foss of the University of Texas in Austin, the most common pollutants in a standard liquid natural gas mixture are the larger hydrocarbons ethane, propane, and butane, along with nitrogen and carbon dioxide dilutants [
21]. For the purposes of this analysis, butane has been omitted, as the GRI-3.0 mechanism does not include C4 hydrocarbon reactions, while the typical butane mole fraction of liquid natural gas is >1%, making it relatively insignificant in the combustion process. For each pollutant, bounds of the max and minimum mole fraction in the liquid natural gas mixture are given in
Table 2, with max bounds chosen to be on the high end typical of other reported liquid natural gas compositions [
30,
31].
To provide reference values to calculate relative errors against, Cantera was used to calculate the combustion temperature of pure methane and oxygen at an OF ratio of 2.7 and pressure of 235 psia, with an initial gas temperature of 300 K. This yields an adiabatic flame temperature of 3289.7 K and ratio of specific heat values of 1.2142. Then, to calculate the properties of the flame for a randomized composition, the mole fraction of each pollutant is calculated by Equation (1), where y is the mole fraction of compound
i, with
rng being a randomly generated value between 0 and 1. Minimum and maximum mole fractions are taken per substance from
Table 2.
After the generation of each mole fraction, the mole fraction of CH
4 is calculated to be the value needed to sum the mole fractions to 100%; then, the mole fractions for each component are converted to a mass fraction via Equation (2).
Finally, this mixture is entered into a Cantera gas object with O
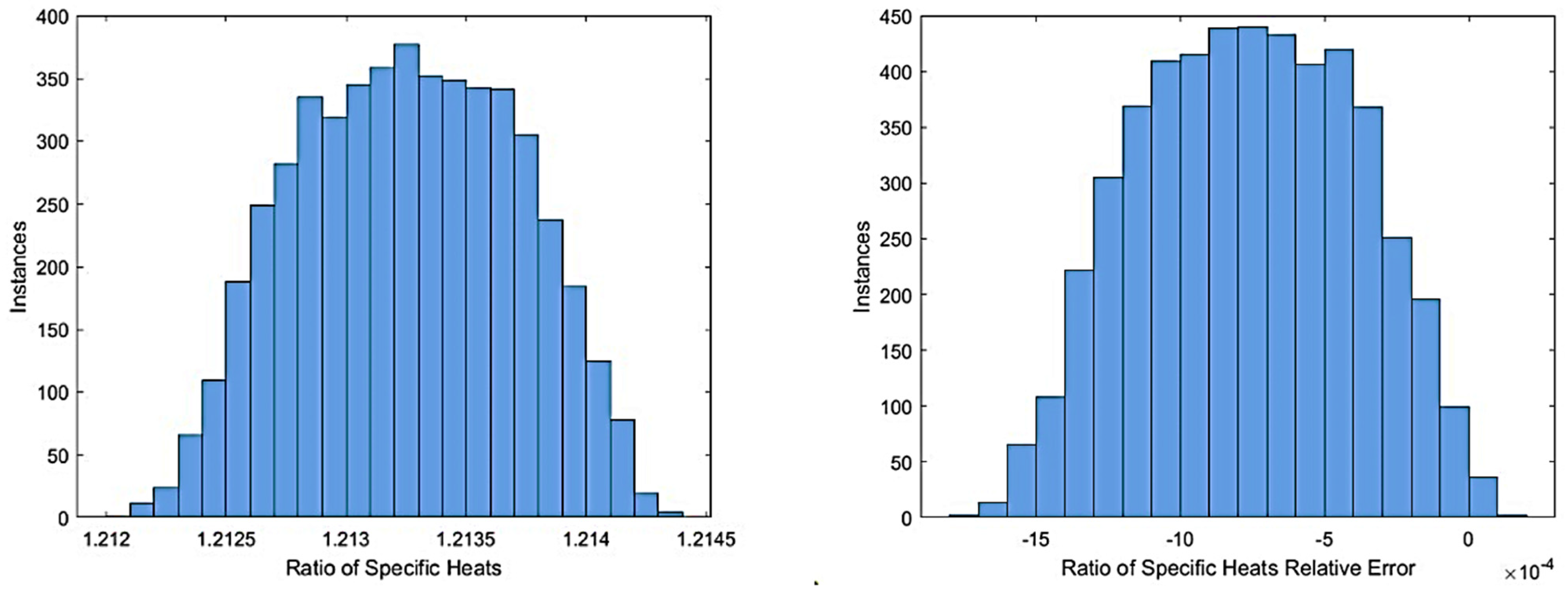
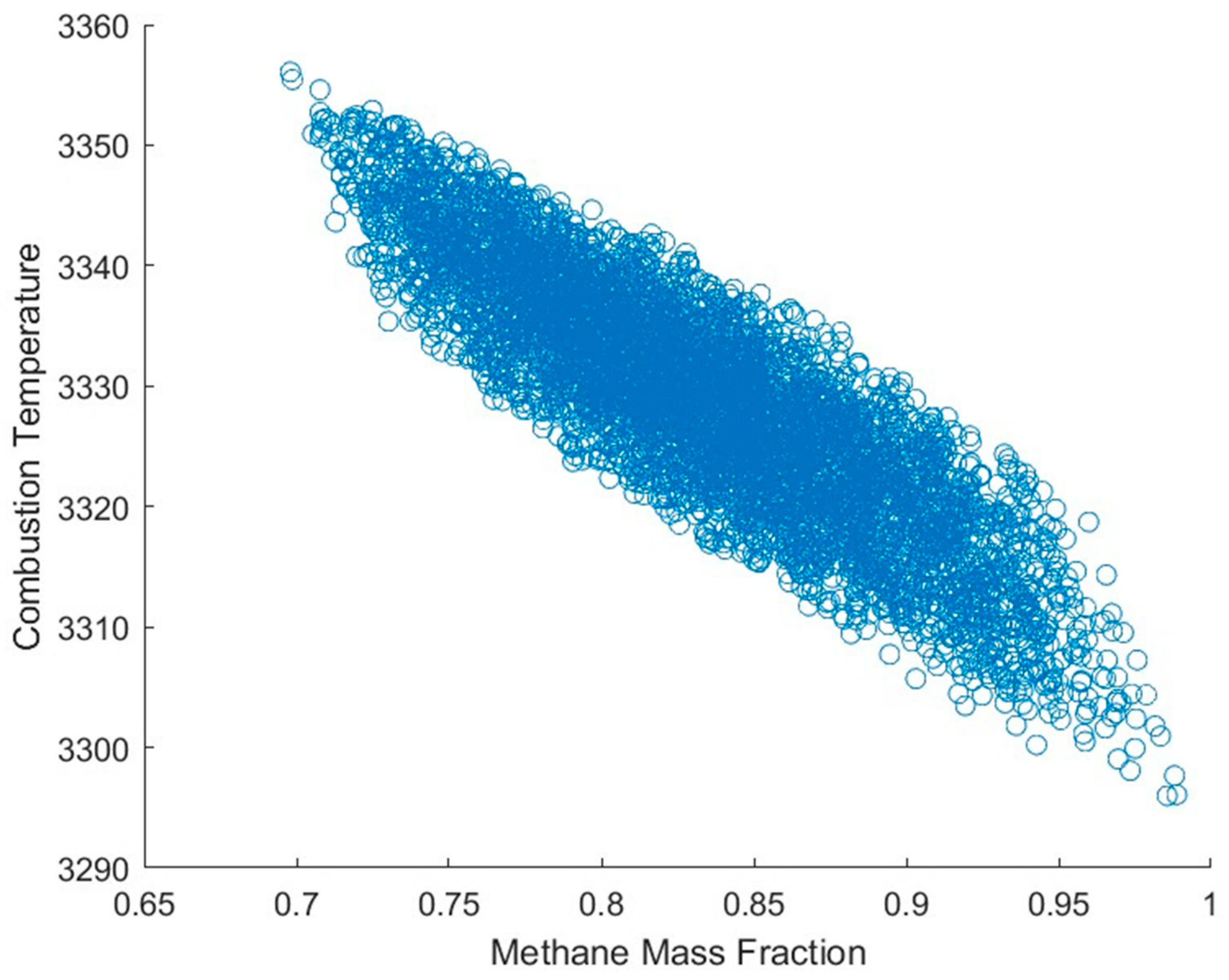
2 at a 2.7 OF ratio, a pressure of 235 psia, and a temperature of 300 K and calculated to chemical equilibrium at constant pressure and enthalpy to simulate combustion. This analysis was run for 5000 iterations, and the final temperature, specific heat results with relative errors, and combustion temperature compared with the methane mass fraction are shown in
Figure 1,
Figure 2 and
Figure 3.
From these results (
Figure 1,
Figure 2 and
Figure 3), it is shown that while fuel mixture has a noticeable impact on equilibrium combustion, the effect for combustion temperature and ratio of specific heats are within 2% and −0.2%, respectively; thus, for the initial iterations of a performance simulation, the assumption of pure methane is accurate within reasonable bounds while reducing computational complexity. Additionally, the simulation of a compositionally accurate fuel mixture requires knowledge of the fuel’s blend; as the CROME team has not finalized the vendor for the LNG, the exact composition used for testing is not known, and thus there is no way to be certain that the simulated fuel blend is realistic enough to justify it over a fully methane simulation. However, for further simulations, the accurate representation of liquid natural gas as the fuel will become more important, as the difference in saturation pressures and transport properties between constituent species can change the behavior of the fuel within the fluid feed systems, which a digital twin model would have to accurately represent [
29].
For accurate modeling of combustion within the chamber, some methods of evaluating finite-rate chemistry effects within the chamber are required. For this, Star-CCM+ offers a few simulation methods, divided into two primary categories: Reacting Species Transport models (RSTMs), in which the conservation equations for each chemical species are calculated individually, and flamelet models (FLMs), in which the conservation equations are calculated for a reduced number of general chemical reaction factors, primarily the mixture fraction and mixture fraction variance [
32,
33,
34]. Generally, flamelet models are generally less computationally intensive than the Reacting Species Transport models due to this simplification of conservation. However, flamelet models are also less accurate, especially for simulations where the mixing timescale is significant in relations to the reaction timescale, as is generally the case within rocket engine combustion, where the residence time of the reacting fluids is small [
35]. Also, RSTM features a comprehensive chemistry model, allowing it to be used in various combustion scenarios (both premixed and non-premixed). It has greater dimensionality, can adapt to dynamic changes in flow conditions, fuel characteristics, and temperature, and, importantly, it incorporates transport phenomena like species diffusion and convection. This results in enhanced accuracy (higher fidelity) in predicting species distributions and flame structures compared to a flamelet model [
36,
37]. Thus, for the engine simulation, Reacting Species Transport is deemed more accurate and is the selected overarching model for this application.
Within Reacting Species Transport are two primary reaction models: complex chemistry (CC) and Eddy Break Up (EBU). The complex chemistry model is considered the most computationally accurate and complex model, as it uses a stiff ODE solver to solve for the chemical source terms [
38,
39,
40,
41]. Through this robust solver, this model can calculate the chemical source terms for equations comprising hundreds of reactions over varying timescales for dozens or more species, making it the best candidate for simulating complex chemical models. The other option, Eddy Break Up, is more optimized for simpler mechanisms. Eddy Break Up (EBU) computes reaction rates based on turbulent mixing and kinetic rates or their combination [
42,
43]. It implements a straightforward equation for species source terms, avoiding the need for stiff ODE solving used in complex chemistry. However, this restriction confines the model to simpler reactions, typically one or two steps, making it unsuitable for the complex multi-stage mechanisms being studied [
44,
45]. Therefore, complex chemistry is preferred as the solver [
46]. In addition, the authors considered using the eddy dissipation concept (EDC) along with the complex chemistry model so that the turbulent mixing component could be precisely combined with the chemical reactions [
47]. The EDC has improved predictions for reaction turbulence rates and provides a more realistic representation of turbulent flow and combustion efficiency. It simplifies computations by separating turbulence and chemistry calculations, leading to better predictions of combustion characteristics such as flame stability, ignition behavior, and pollutant formation [
48,
49]. The governing equations considered for complex chemistry (CC) with the eddy dissipation concept (EDC) are shown in Equations (3) and (4) below [
50,
51]:
Species Transport Equation:
where
Yi is the mass fraction of species
i,
Di is the diffusion coefficient of species
i, and
i is the reaction rate of species
i.
Eddy Dissipation Concept (EDC): where
i is the reaction rate,
EDCi,chem is the chemical reaction rate,
EDCi,turb is the turbulent reaction rate,
τeddy is the timescale for eddy dissipation, which is modeled based on the turbulence characteristics (from the k − ω or SST turbulence models), and
A is a constant.
The Gas Research Institute developed the GRI 3.0 mechanism for complex chemistry (CC) analysis [
52]. Later on, this was also embedded in Berkeley’s depository [
52]. Initially, the combustion of vaporized propellants was modeled using the GRI-3.0 mechanism for methane–air combustion, which is widely recognized for its comprehensive nature [
28]. However, due to its complexity, involving 325 reactions across 53 species, it significantly strains the chemical solver during high-computation tasks. This complexity arises from the inclusion of detailed nitrogen chemistry for air combustion, along with mechanisms irrelevant to the specific use-case, such as those related to flame radiation. As a result, while GRI-3.0 served as a computational benchmark, a reduced model for methane combustion was prioritized to improve efficiency.
The initial candidate for this reduced model became the methane–air combustion model developed by Lu et al. [
53]. Lu’s model operates under similar conditions and with similar reactions to the GRI-3.0 model, as it was originally reduced from the GRI’s original model. Thus, it was predicted that its behavior would imitate the GRI model suitably to become a replacement [
54]. With 184 reactions between 30 tracked species, this model features approximately half the complexity of the GRI model. This was accomplished through the application of a directed relation graph, in which the coupling between each species in the reaction is analyzed on a directed graph, with reactions with weak couplings, as determined by a user-defined error tolerance, being eliminated. Under an error tolerance of 0.13, and with the elimination of species related to NO formation, the removal of 23 species and 141 reactions was accomplished with minimal change in combustion characteristics.
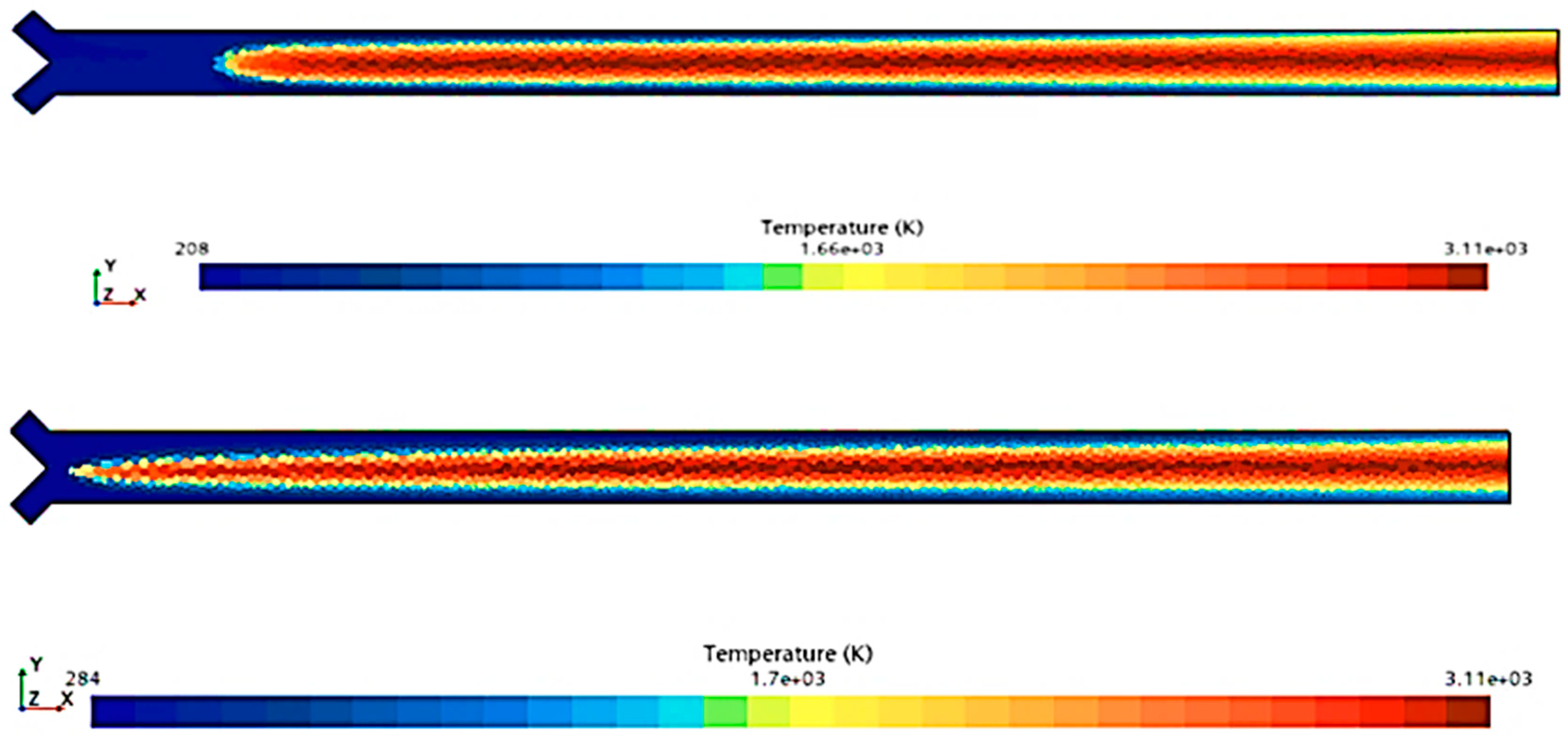
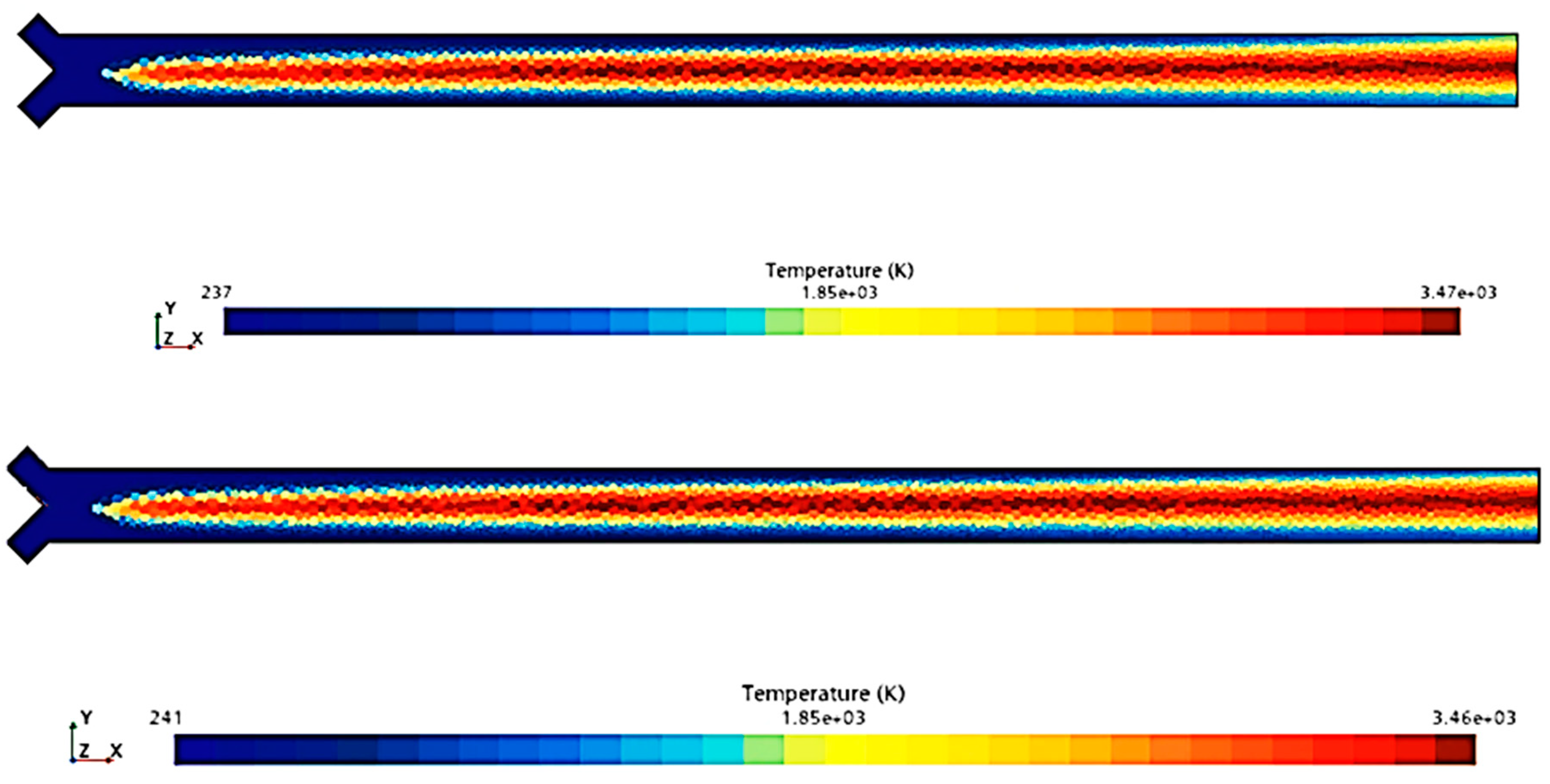
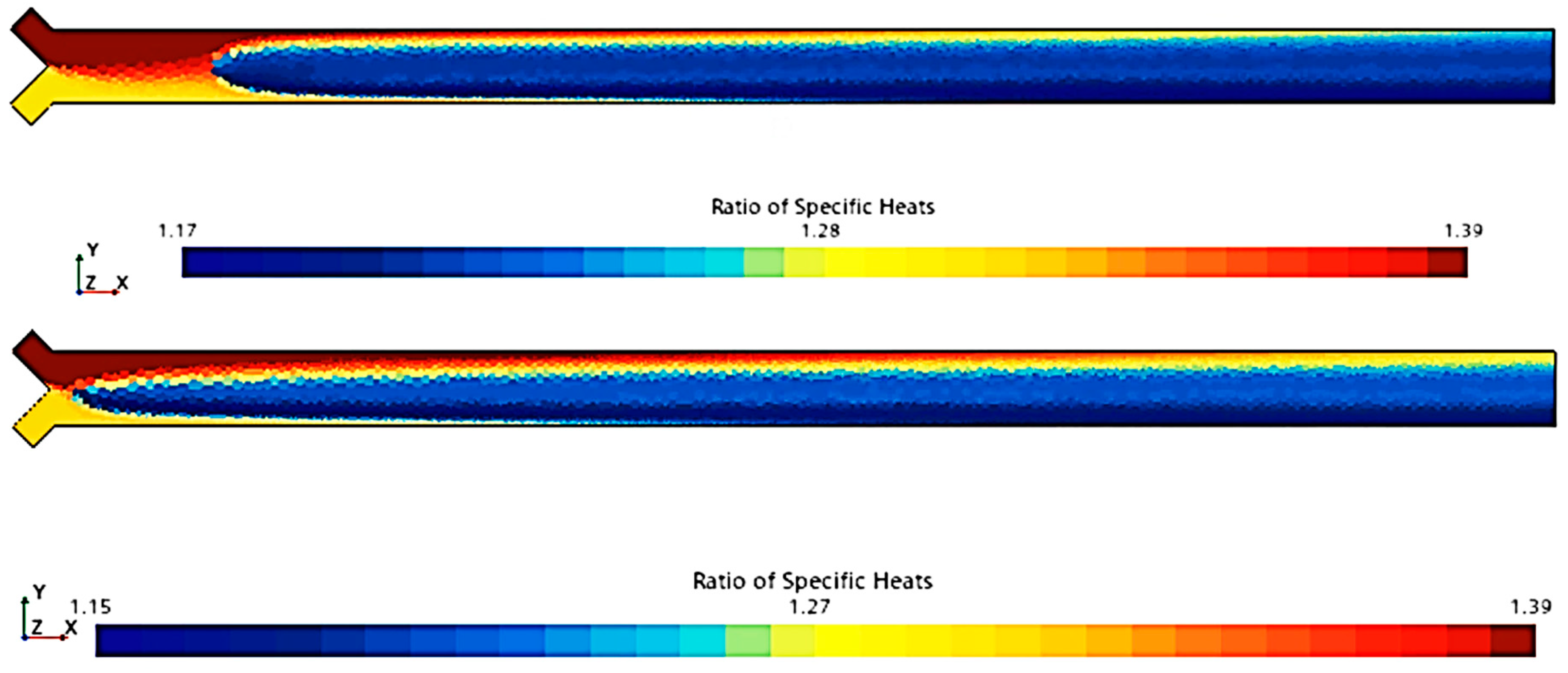
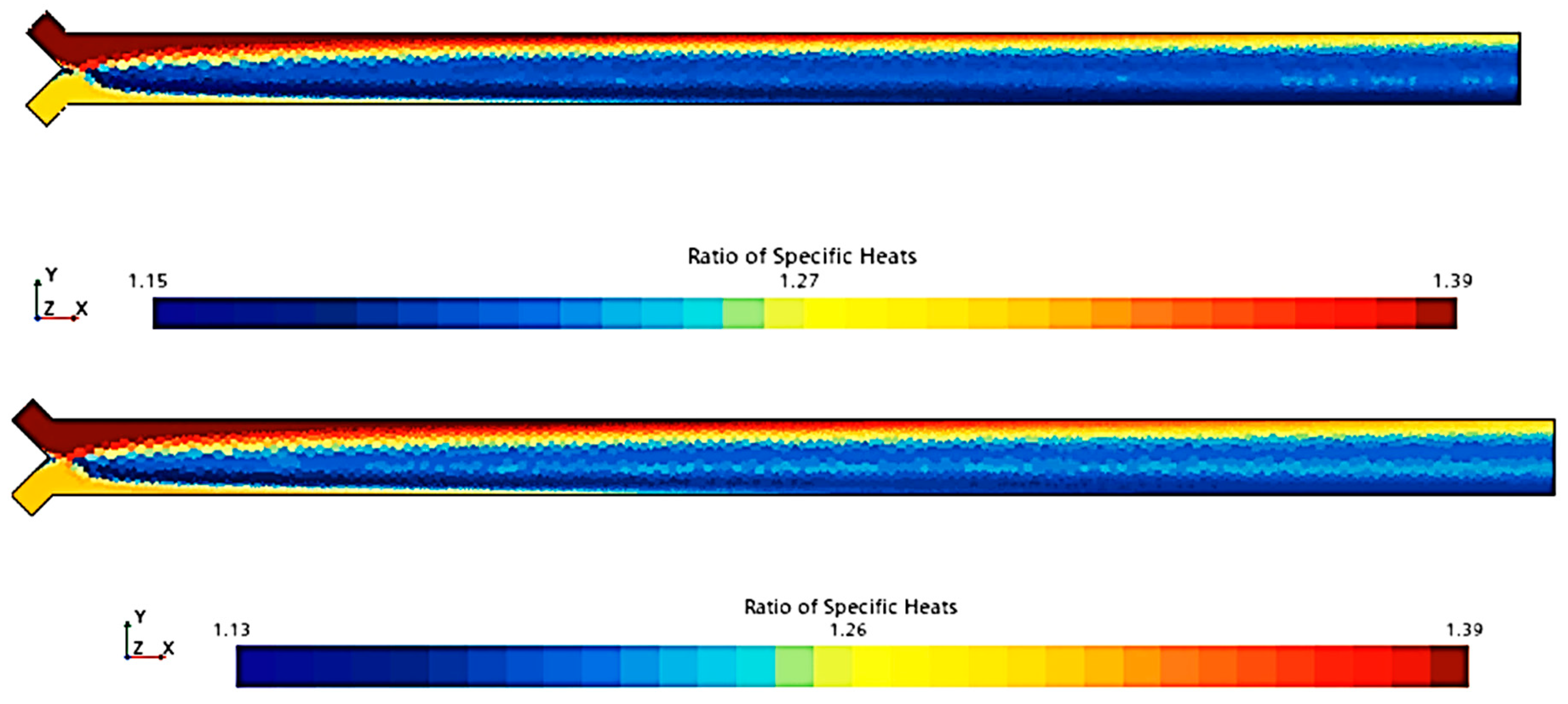
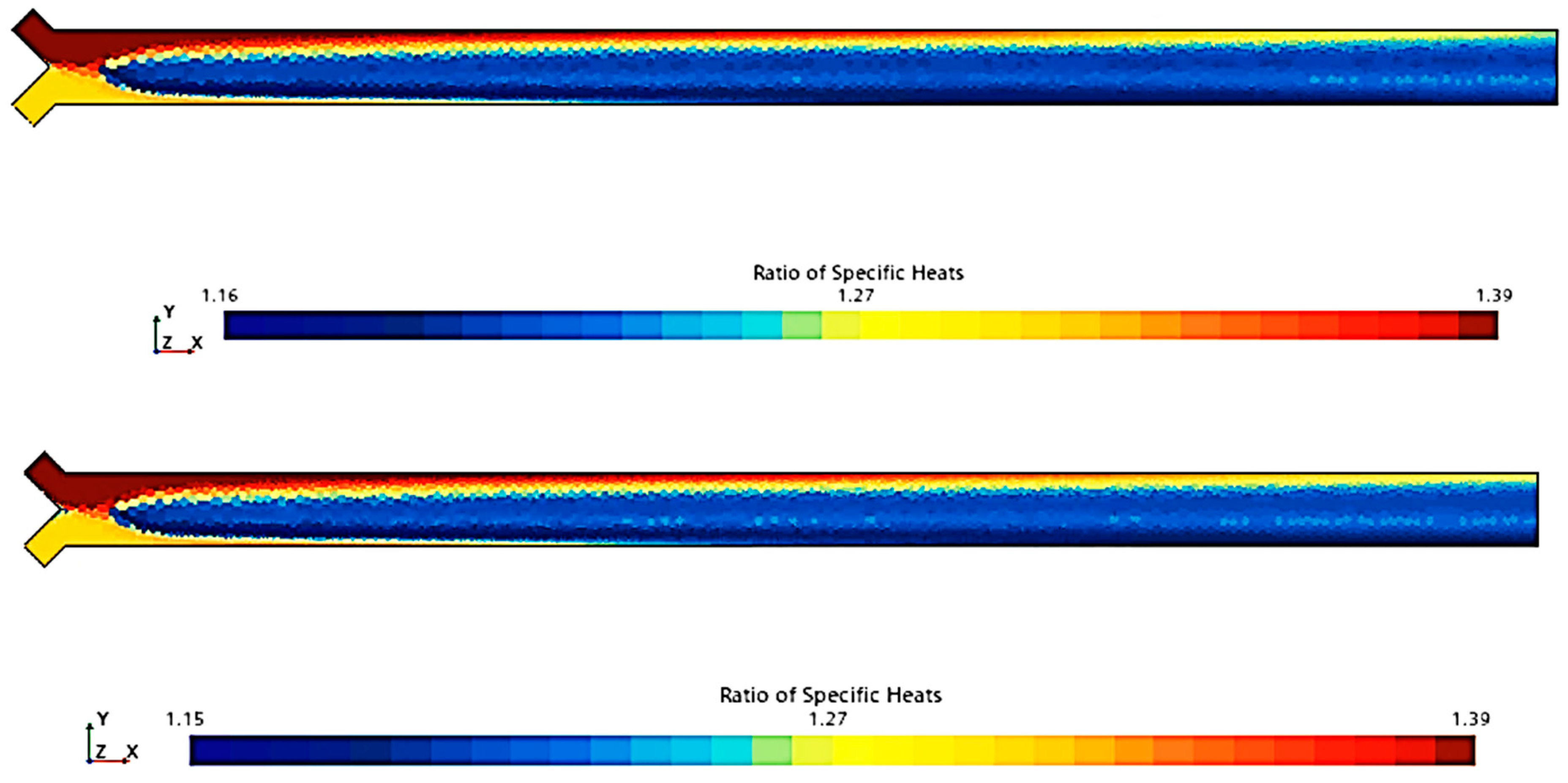
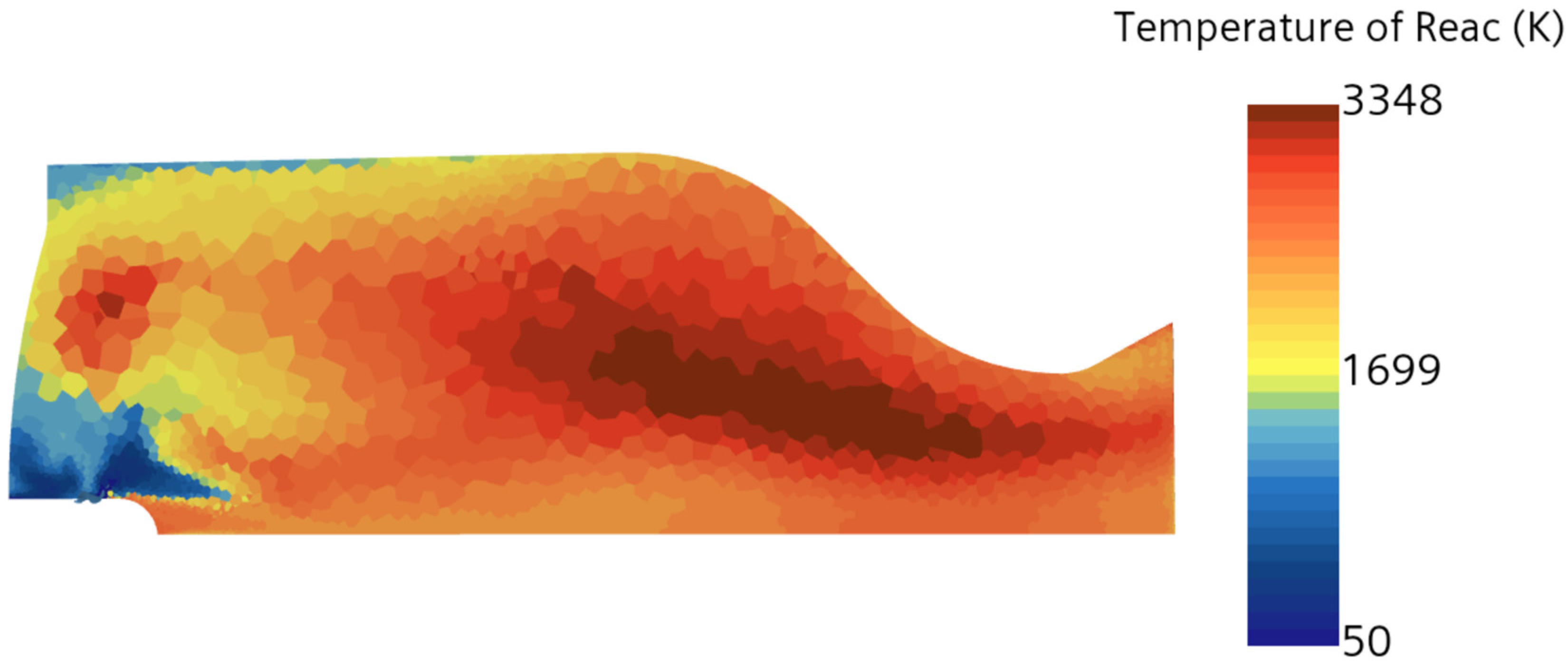
To compare the combustion characteristics of the two models, a 2D test case was set up in Star-CCM+. The geometry consisted of a converging “tube” where fuel and oxidizer paths intersected at a 90-degree angle before merging into a straight section angled at 45 degrees to both paths. Fuel and oxidizer were injected at an OF ratio of 2.7 and pressures of 0, 125, and 250 psig were used to simulate expected rocket chamber conditions. A Coupled Flow solver was used for the flow model, enhancing stability by controlling the CFL number, along with a standard k-omega SST model for turbulence. Each pressure scenario was tested over roughly 8000 iterations, following a staged CFL progression: 0.1 for the first 1000 iterations, 1 for the next 1000, 10 for 2000 iterations, 100 for another 2000, and 1000 for the final 2000 iterations. This approach achieved satisfactory convergence, confirmed by a three-order reduction in continuity residuals and no visual changes in the temperature field during the final iterations. Post-convergence, temperature and specific heat ratio fields were compared, as shown in
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8 and
Figure 9.
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8 and
Figure 9 show that there is a minimal difference in flame characteristics and shape between the two mechanisms tested. Across all conditions, the core flame temperature and ratio of specific heats for the products are nearly identical. The main difference observed is a longer ignition delay in the GRI-3.0 mechanism at 0 psig, where the flame shape significantly deviates from other conditions, suggesting that the Lu mechanism’s flame behavior is more accurate. Additionally, the Lu mechanism closely matches flame temperatures and exhibits similar specific heats profiles for post-combustion products (see
Table 3), supporting its use for nozzle flow modeling.
Thus, according to the tests conducted (
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8 and
Figure 9 and
Table 3), the Lu model maintains most of the accuracy afforded by the GRI-3.0 model at a considerably lower runtime of approximately half that of the GRI-3.0 model due to the reduction in tracked species and reactions. For these reasons, the Lu mechanism was chosen for usage in the final combustion model, as also reported in [
55].
2.2. Multiphase Flow Modeling and Associated Governing Equations
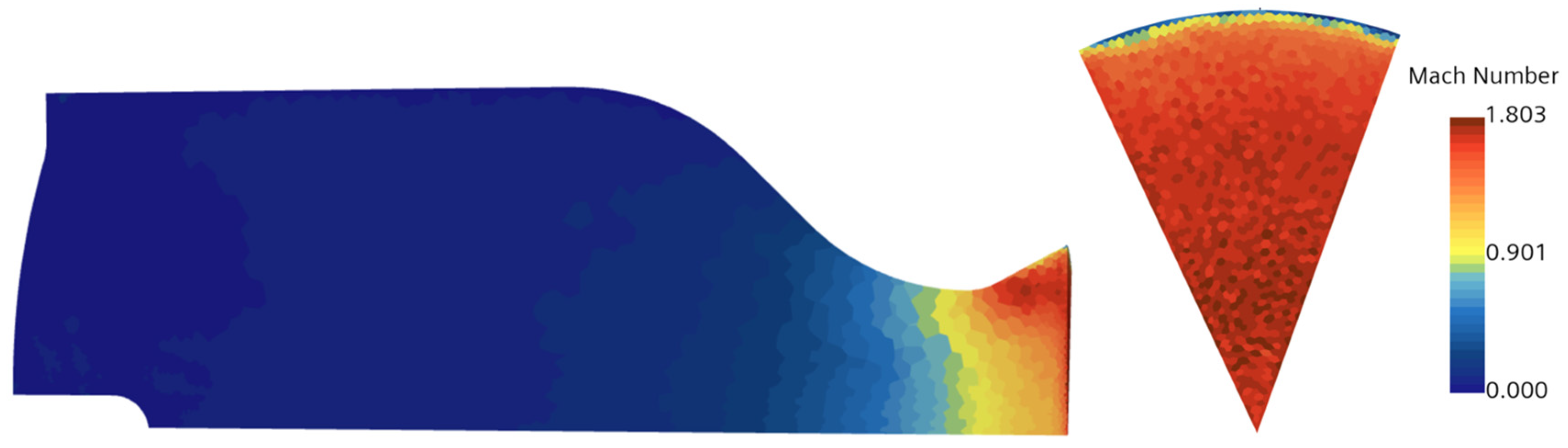
The compressible flow within the nozzle was modeled using methods that balance accuracy and computational efficiency. The ideal gas model was selected for the gaseous equation of state due to its simplicity and sufficient accuracy within the engine’s operating range, compared to the more complex Real Gas model. The Reynolds-Averaged Navier–Stokes (RANS) approach, combined with the K-Omega turbulence model, was applied to solve the Navier–Stokes equations for chamber flow, chosen for its ability to accurately predict near-wall turbulence [
56], which influences boundary layer effects and fuel film cooling. Similarly, Wilcox [
57] numerically validated that the k-Omega model is quite accurate for the attached boundary layer in an adverse pressure gradient, compressible boundary layer, and free shear flow. Likewise, Menter [
58] confirmed that integrating RANS with the k-omega model enhances the understanding of near-wall behavior compared to other models. They also noted that the k-omega model avoids the use of damping functions, relying instead on straightforward Dirichlet boundary conditions. This simplicity makes the k-omega model more stable numerically, while still maintaining accuracy in predicting mean flow profiles, comparable to other models. Also, they suggested using SST k-Omega, which is like a hybrid approach and helps in resolving both the near-wall performance and free-stream stability. Because of the aforementioned reasons, the authors in this paper combined RANS with k-omega (SST). A segregated solver was employed for the multiphase flow model, offering faster computation by solving mass and momentum conservation equations sequentially, though it is less robust than the coupled solver, particularly for supersonic flow [
56,
59,
60]. This method, supported by fine-tuning of under-relaxation parameters, was adopted to ensure stable convergence for the multiphase flow.
To accurately model the injection of liquid propellants, it is essential to represent each phase along with the associated mass and heat transfer properties. In Star-CCM+, two major methods are available for simulating multiphase flows: the Lagrangian model [
61,
62], where phases are treated as discrete particles moving through the primary phase within a resolved mesh, and the Eulerian model [
63,
64], which solves the conservation equations for all phases on the same mesh grid. Initially, the Lagrangian model was used to simulate particle flows from fuel orifices due to its ease of implementation and its effectiveness in replicating spray injections in rocket engines [
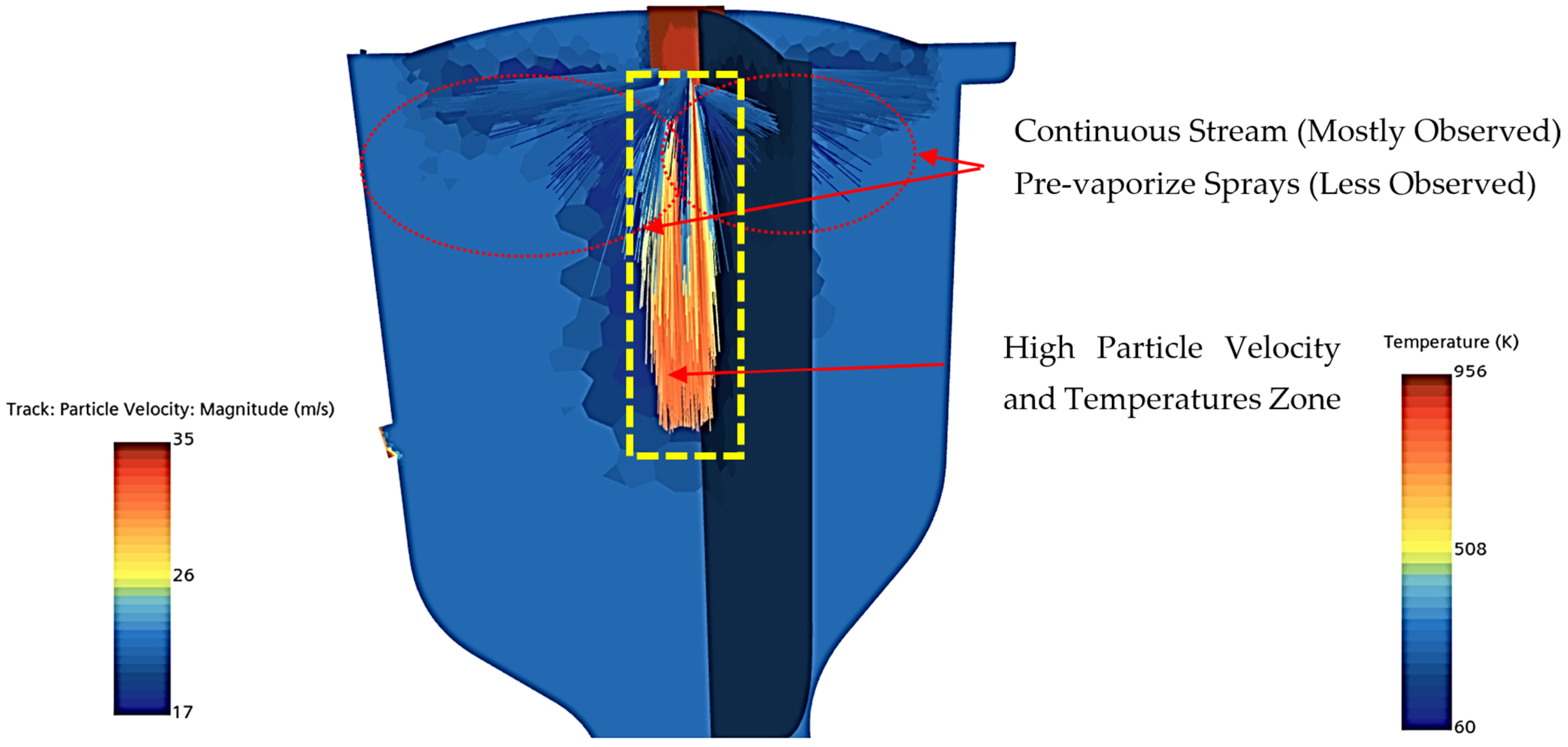
61]. However, the CROME’s injection elements produce continuous (intact) streams rather than pre-vaporized sprays; thus, the driving force for mixing is impingement rather than atomized spray, as demonstrated in initial cold flow tests [
27]. As depicted in
Figure 10, the Lagrangian model’s probabilistic NTC particle impingement did not achieve adequate impingement, failing to capture the expected dynamics near the injector.
From there, it was determined that an Eulerian-type model had to be used, of which there are three primary options to choose from: Eulerian Multiphase (EMP), which solves the conservation equations of each phase separately, Mixture Multiphase (MMP), which solves the conservation equations of the phases using a mixture assumption, and Volume of Fluid (VOF), which is similar to MMP with an additional assumption of stratified flow in each phase. Initial impingement tests were carried out with the VOF model, due to its valid assumption of flow stratification and relative ease of computation compared to EMP [
65]. However, for full engine simulation, this model is unsuitable as it calculates the energy of each phase through a temperature-dependent solver, rather than an enthalpy-based solver, and therefore could not simulate the effect on gas temperature from the heat release of combustion [
38]. The only available Eulerian model to feature an enthalpy-based energy solver is EMP and, thus, multiphase modeling had to be switched to the more complex but proven Eulerian Multiphase model [
66]. The governing equations used under EMP are shown in Equations (5)–(7) below [
67,
68]:
Continuity Equation (for each phase α): where
ρα is the density,
ϵα is the volume fraction,
uα is the velocity field,
Gα is the diffusion flux, and
Sα represents source terms.
Momentum Equation (for each phase α): where p is the pressure shared between phases,
is the stress tensor for phase
,
is the interphase interaction forces, and
is the source term (e.g., gravity).
Energy Equation (for each phase α): where
hα is the enthalpy,
kα is the thermal conductivity,
Tα is the temperature,
Φα =
Qα represents heat sources,
Eα is the energy per unit mass of phase
α,
qα is the heat flux in phase
α, and
is the interphase heat transfer from
to phase
.
The saturation pressure of the fuels is modeled using the Antoine equation [
69,
70], as described in Equation (8), where
A,
B, and
C are empirical constants, and
T refers to the liquid temperature, with values for oxygen and methane provided by the NIST [
71]. Heat transfer and evaporation are simulated using the Multicomponent Droplet Evaporation (MDE) model, with heat and mass transfer determined by the Ranz–Marshall correlations for Sherwood and Nusselt numbers. Liquid–vapor equilibrium is modeled using Raoult’s Law [
66,
72]. Droplet atomization and coalescence are modeled without a Lagrangian approach by employing an S-Gamma distribution of droplet size based on Sauter mean diameter, alongside the Luo and Coulaloglou and Eskin models for droplet coalescence and breakup, respectively [
73,
74,
75].
A steady-state simulation for nozzle flow and combustion was performed using these parameters. The model settings for steady-state combustion and nozzle flow are outlined in
Table 4 below.
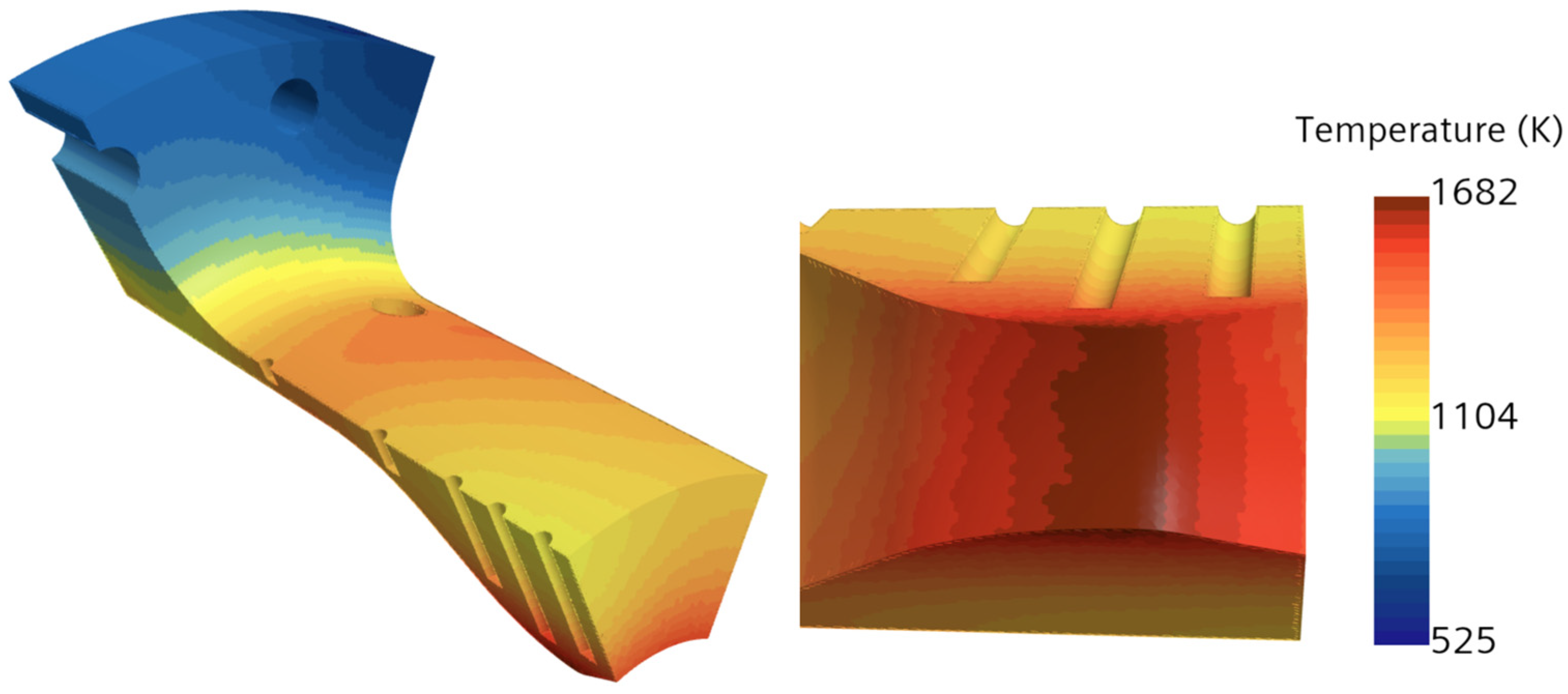
2.3. Conjugate Heat Transfer and Associated Governing Equations
Conjugate heat transfer analysis in a combustion chamber is critical for ensuring the performance, efficiency, and safety of combustion chambers, especially in high-performance aerospace propulsion systems. It is also important because it helps to understand the interaction between fluid dynamics, heat transfer, and structural integrity within the chamber. This analysis combines the heat transfer mechanisms in both the solid walls (conduction) and the fluid flow (convection and radiation). For this, the solid component was added in the simulation alongside the fluid domain. In this simulation, the solid domain is discretized using a polyhedral mesh, which provides improved accuracy in capturing complex geometries. The physics settings include the segregated solid energy model, where heat transfer is analyzed in a steady-state regime with constant density, ensuring that energy conservation is accurately computed within the solid domain. To enable data exchange between the fluid and solid regions, contacts were established between the two domains, particularly in areas like the combustion chamber, nozzle, and internal orifices (e.g., acoustic chambers and injector orifice). This approach is commonly used to study heat exchange between solids and fluids, enabling the design and optimization of thermal management systems. The governing equation for solid energy heat transfer is provided in Equation (9) [
76,
77]. The physics and mesh settings used for the solid component are shown in
Table 5.
Solid energy equation used for heat transfer: where
ρs is the solid density,
Cp,s is the specific heat capacity at constant pressure,
Ts is the solid temperature,
ks is the thermal conductivity of the solid, and
Qs represents internal heat generation or any heat source term. In the modeling, Equation (10) was used to determine the heat flux due to conduction:
where
k is the thermal conductivity of the material and
is the temperature gradient [
38]. Inconel 718 is used to fabricate the engine chamber and nozzle, with a thermal conductivity value of k of 9.94 W/m·K at 300 K. The thermophysical properties of Inconel 718 at various temperatures were taken from literature sources [
78,
79]. This heat flux,
q, is used to determine the
Qs mentioned in the above equation. Further details about the engine requirements are found in a related publication by this team [
27].
The model considers 1/8th of the full engine volume, to reduce the computational expense of the simulation. It utilizes a symmetric boundary condition on the section walls where the model was sliced. Similar strategies are employed by other research dealing with combustion and heat transfer analysis. This is also dependent on the dimensionality of the simulation, as certain approaches consider more simplified assumptions, such as only considering convection from the combustion gases or considering one-dimensional heat transfer. Lv et al. [
18] show how an engine’s one-dimensional model can approach similar results to an experimental set up, when considering all possible uncertainties between the model and the experiment. Munk et al. [
15] use a 2-dimensional model, with two distinct grids. One is used to predict the flow stream, temperature, and mass fractions for the combustion of methane and oxygen, while the second grid uses the boundary values to predict the heat transfer across the solid engine walls.
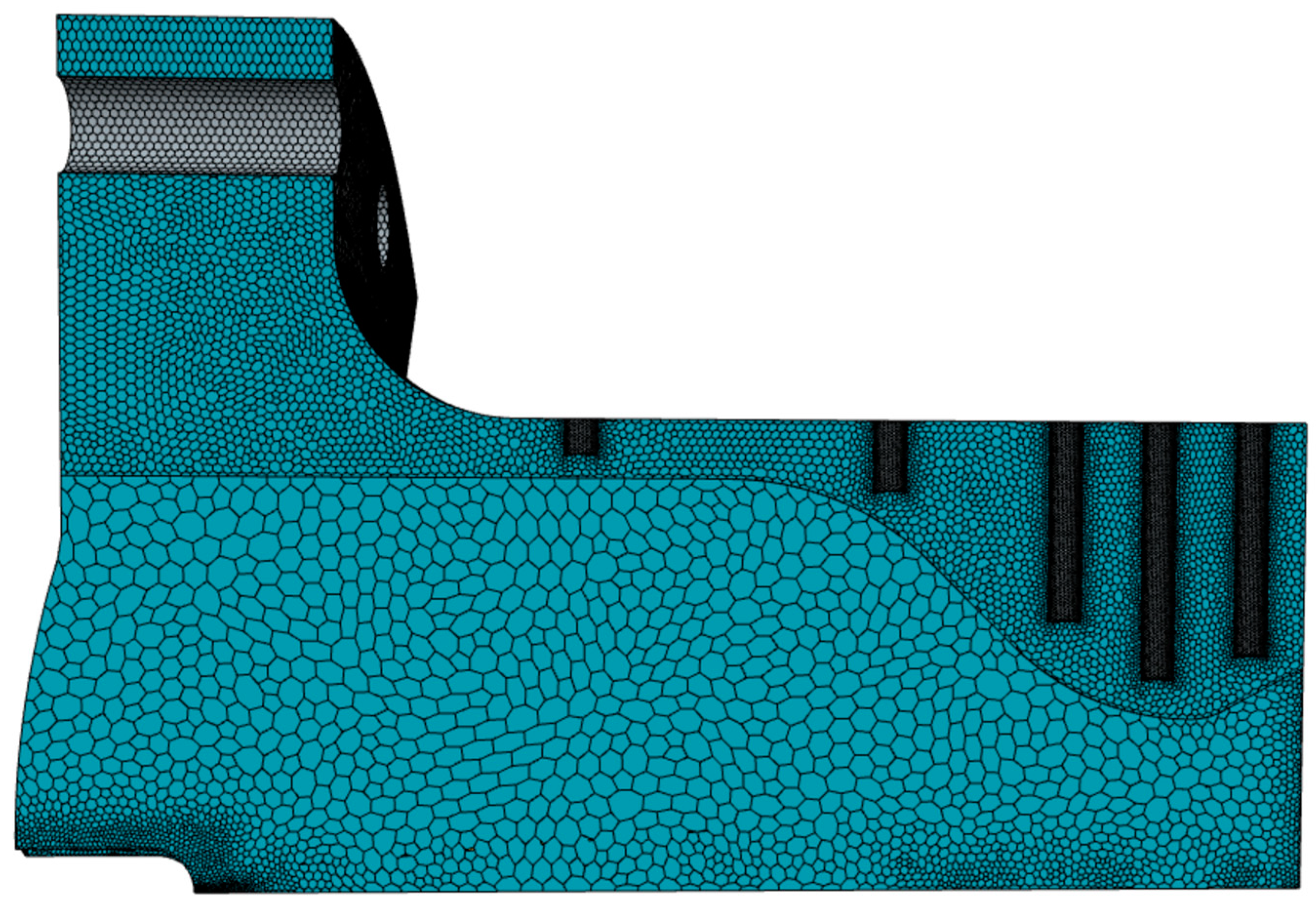
Table 6 and
Figure 11 present the boundary conditions applied to the solid regions, and the resulting grid for the entire model, respectively.
The boundary conditions set for the outer surfaces were estimated for the convective heat transfer due to the environmental conditions where the system was tested. These settings will be employed in simulating the combustion of the engine at different thrust outputs, with their respective inputs. The simulation assumes the required conditions are achieved at the engine fuel and oxidizer inlets. In this simulation, fuel film injection for cooling was excluded due to limitations in STAR-CCM+, particularly in model compatibility. When trying to simulate fuel–oxygen combustion with multiphase injections, STAR-CCM+ could not accurately model the interaction between the fuel film used for wall cooling and the combustion process. As a result, the effects of fuel film evaporation, and its influence on wall temperature and combustion gases, were not captured. To address this, the authors developed a workaround using 1D heat transfer calculations and a separate 3D fuel film cooling (FFC) model to ensure adequate cooling during hot-fire tests. They used conduction and convection formulas to analyze wall temperatures across various FFC levels. In the 3D FFC model, the combustion chamber was assumed to be at a high temperature, allowing the team to study cooling effects without initializing the combustion model. They found that with proper FFC levels, the surface temperature could remain below the metallurgical limit of Inconel 718 (melting point temperature = 1260–1336 °C). The authors plan to publish detailed findings on FFC in a future paper. To know more about the fuel film cooling injection orifices and injection orientation, please refer to [
27].