Drought Coincided with, but Does Not Explain, Late Holocene Megafauna Extinctions in SW Madagascar
Abstract
1. Introduction
1.1. Climate Change in SW Madagascar
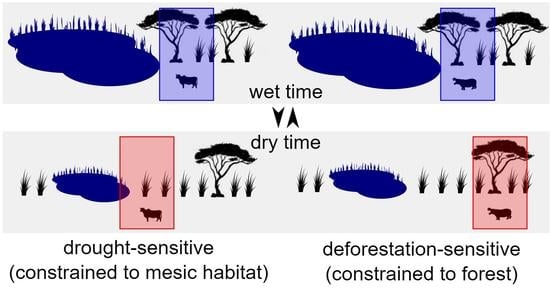
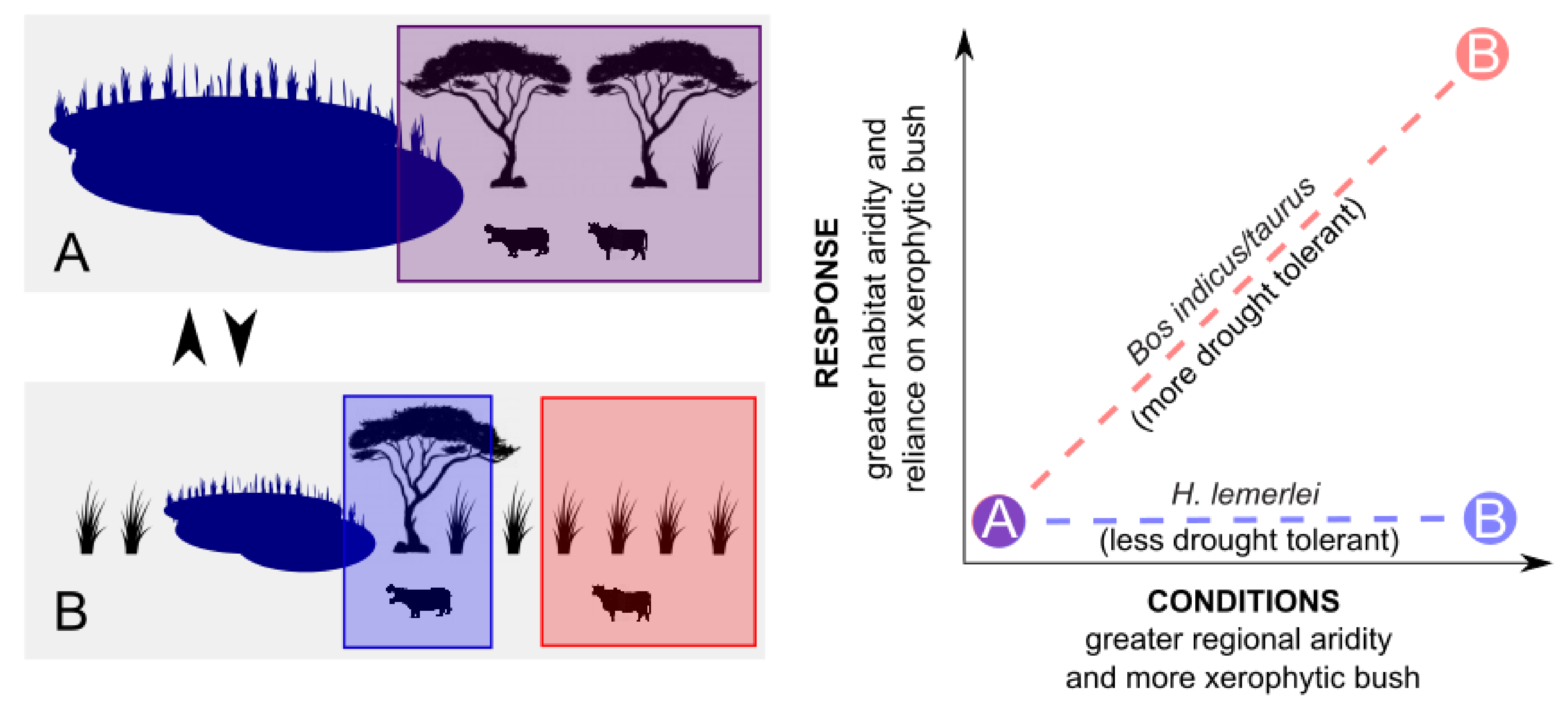
1.2. Animal Responses to Climate Change
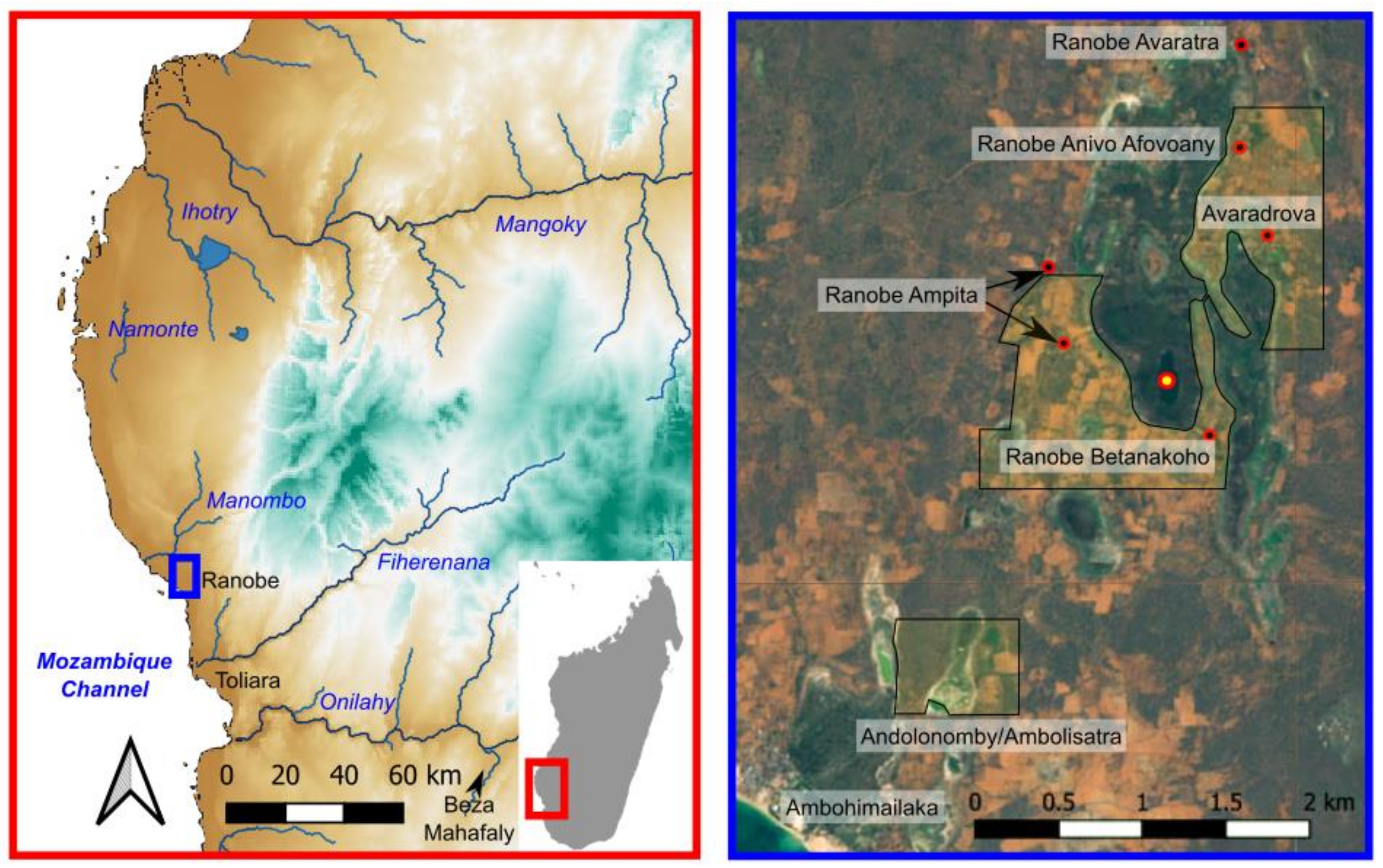
1.3. Regional Setting
2. Materials and Methods
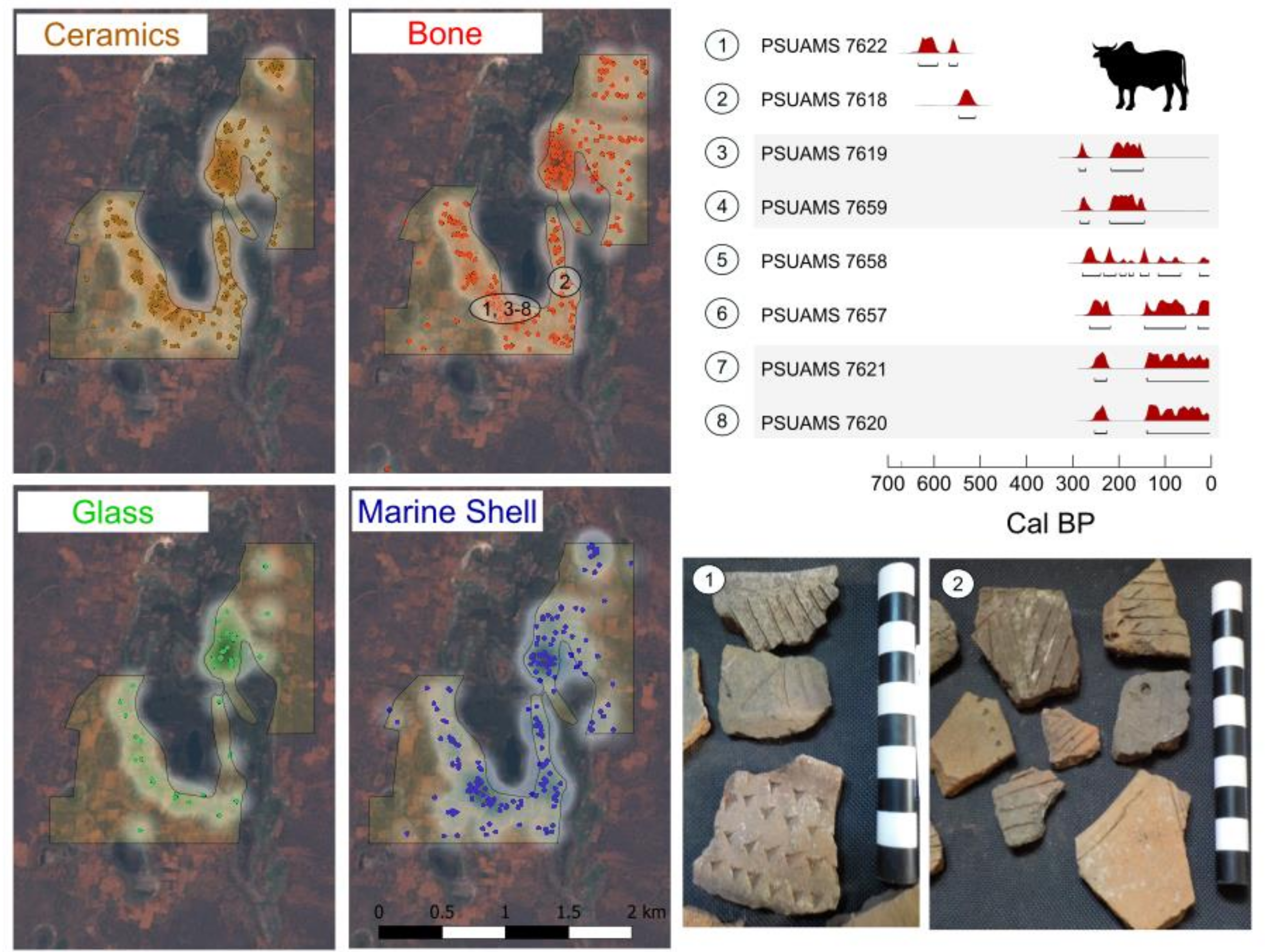
2.1. Sediment Collection and Archaeological Survey
2.2. Chronology
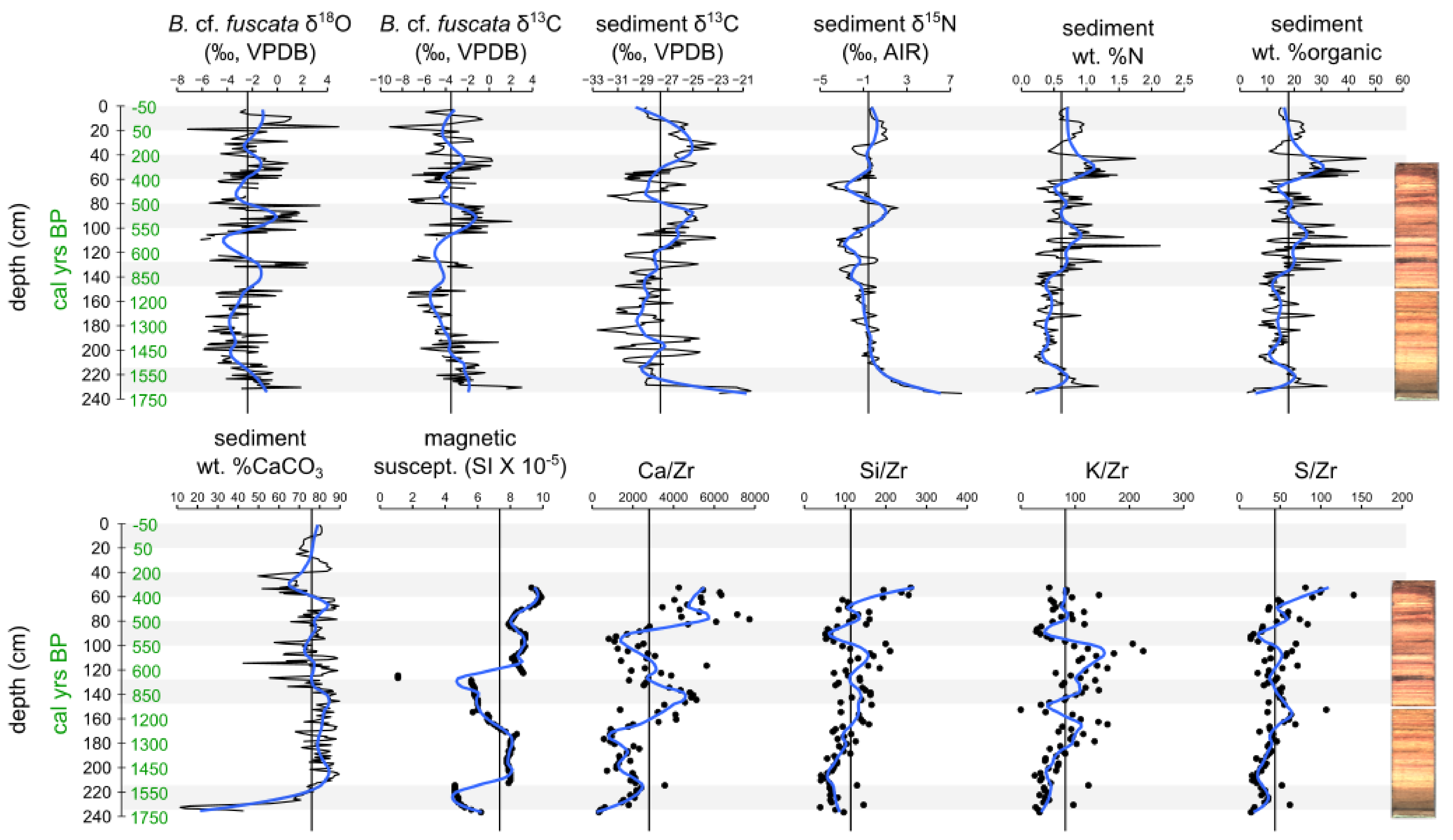
2.3. Geochemical Data
3. Results
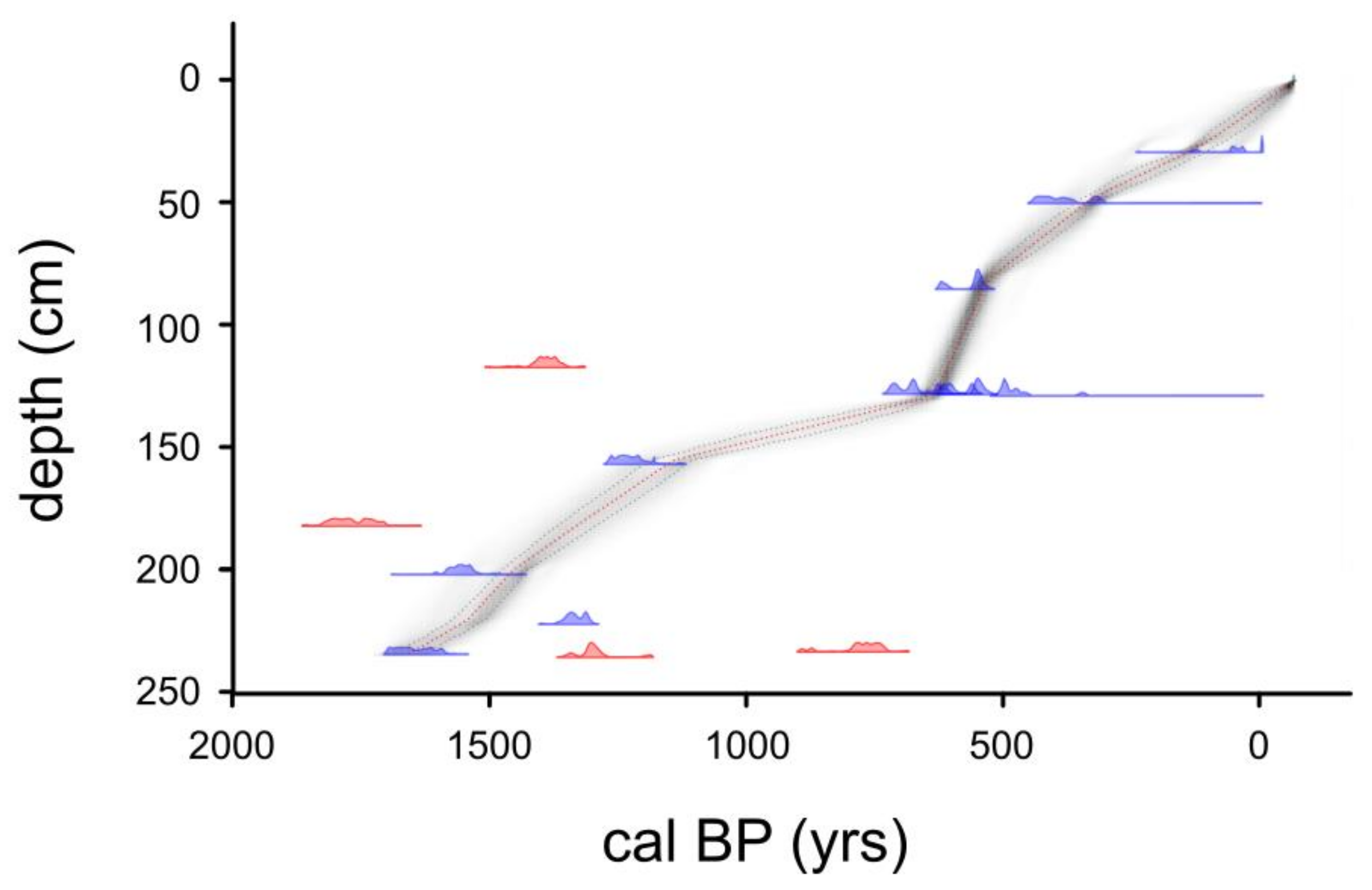
3.1. Sediment Core Chronology
3.2. Archaeological Survey
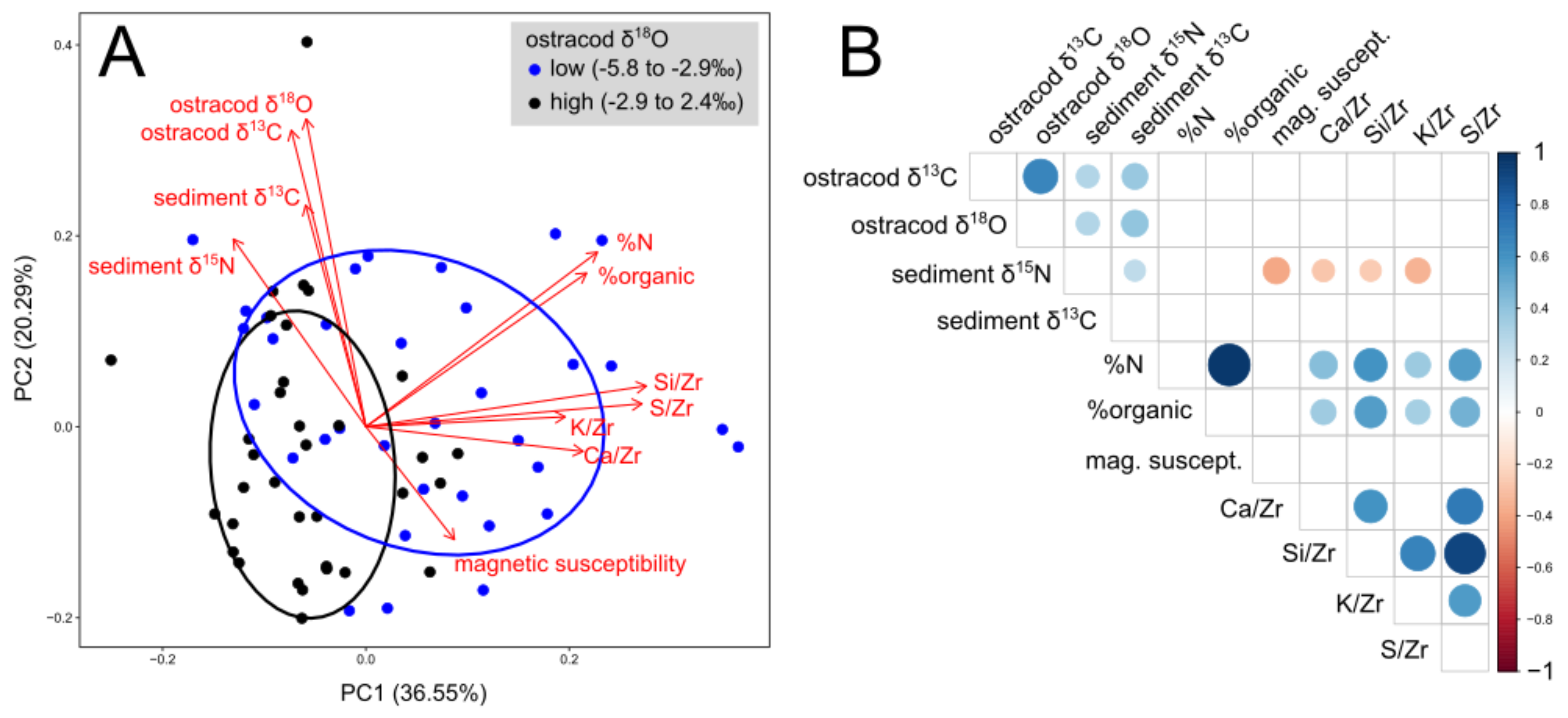
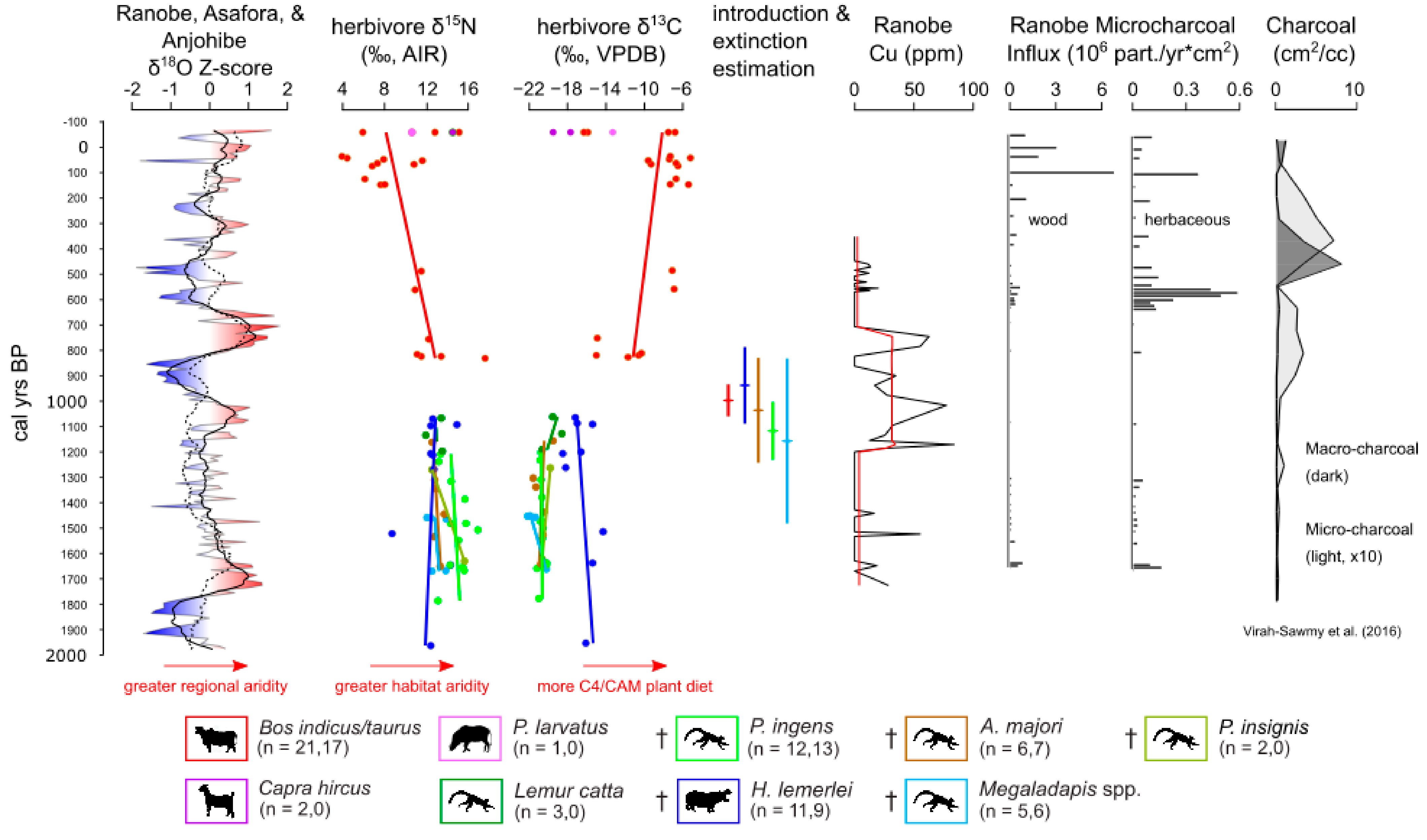
3.3. Geochemical Data
3.4. Charcoal and Herbivore Data
4. Discussion
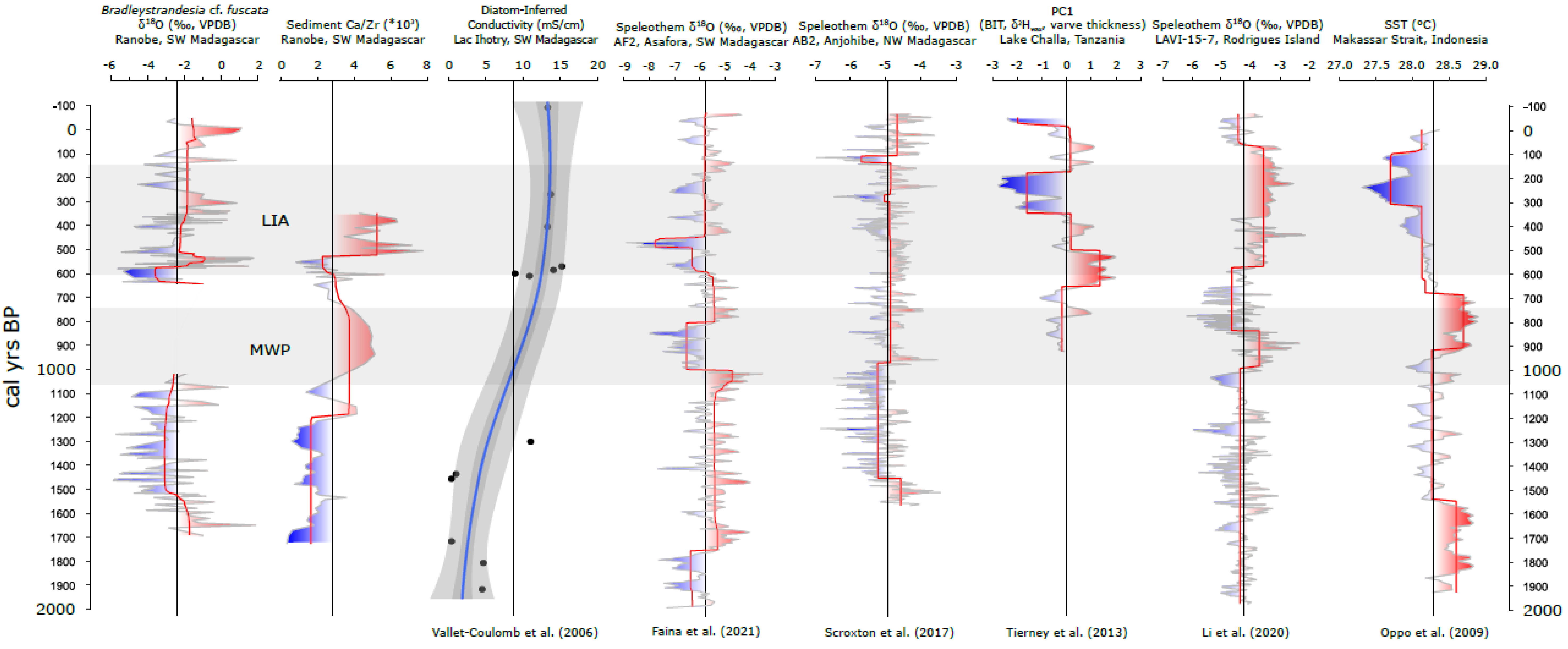
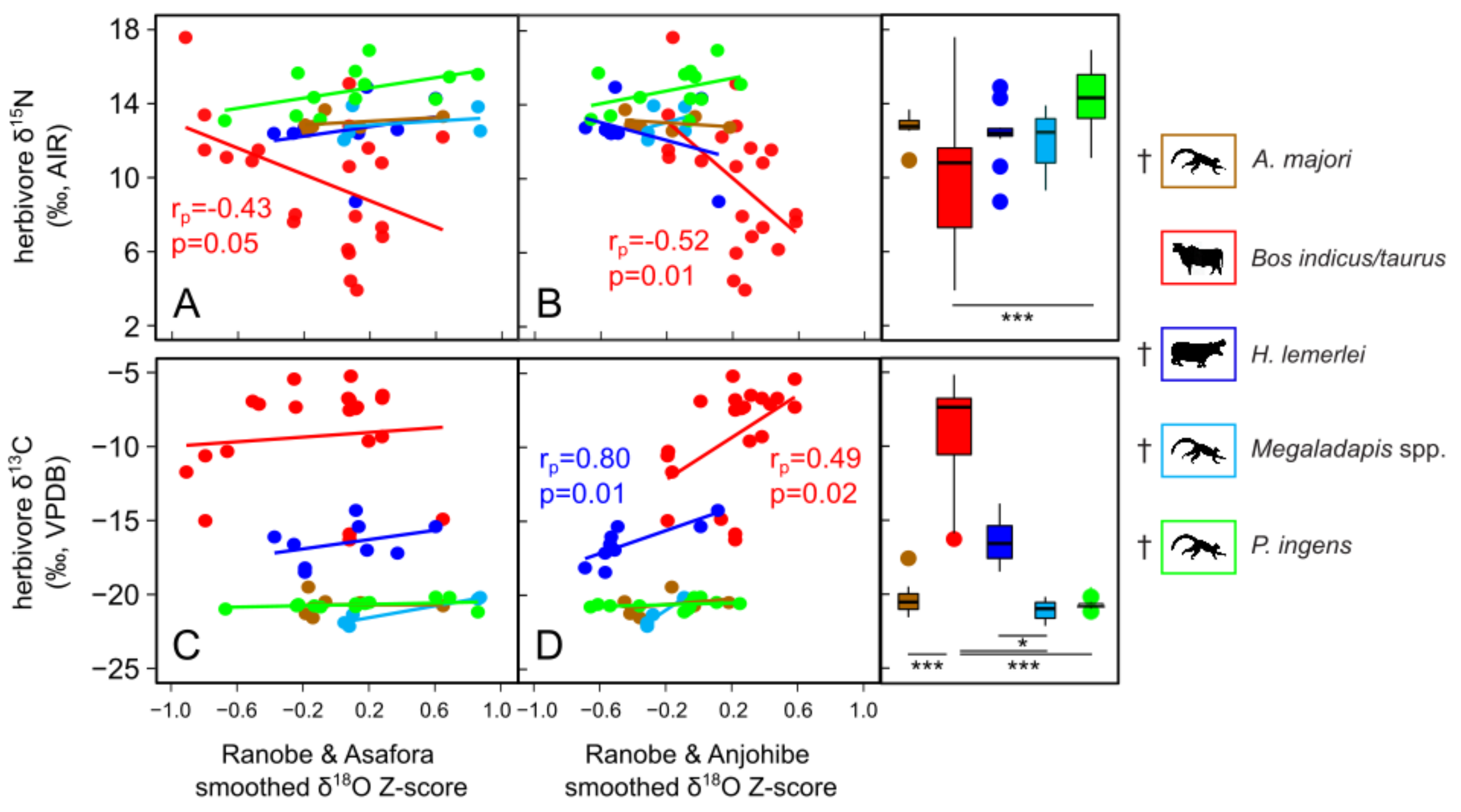
4.1. Drought and Relationships among Paleoclimate Records
4.2. Chronology and Regional Comparison
4.3. Human Activity and Vegetation Change
4.4. Herbivore Activity
4.5. Implications for Land Management and Conservation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Douglass, K.; Cooper, J. Archaeology, environmental justice, and climate change on islands of the Caribbean and southwestern Indian Ocean. Proc. Natl. Acad. Sci. USA 2020, 117, 8254–8262. [Google Scholar] [CrossRef]
- Bhalla, N. Drought Leaves 366,000 ‘One Step Away from Famine’ in Madagascar: U.N. In Reuters. 2019. Available online: https://www.reuters.com/places/africa/article/us-madagascar-aid-drought/drought-leaves-366000-one-step-away-from-famine-in-madagascar-u-n-idUSKCN1T72HD (accessed on 30 June 2021).
- Tadross, M.; Randriamarolaza, L.; Rabefitia, Z.; Yip, Z.K. Climate Change in Madagascar, Recent Past and Future; World Bank: Washington, DC, USA, 2008; p. 18. [Google Scholar]
- Douglass, K.; Walz, J.; Morales, E.Q.; Marcus, R.; Myers, G.; Pollini, J. Historical perspectives on contemporary human–environment dynamics in southeast Africa. Conserv. Biol. 2019, 33, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Dietl, G.P.; Kidwell, S.M.; Brenner, M.; Burney, D.A.; Flessa, K.W.; Jackson, S.T.; Koch, P.L. Conservation paleobiology: Leveraging knowledge of the past to inform conservation and restoration. Annu. Rev. Earth Planet. Sci. 2015, 43, 79–103. [Google Scholar] [CrossRef]
- Wilcox, B.A. Supersaturated island faunas: A species-age relationship for lizards on post-Pleistocene land-bridge islands. Science 1978, 199, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, F.M. Archaeology, ecological history, and conservation. Annu. Rev. Anthropol. 2005, 34, 43–65. [Google Scholar] [CrossRef]
- Virah-Sawmy, M.; Gillson, L.; Gardner, C.J.; Anderson, A.; Clark, G.; Haberle, S. A landscape vulnerability framework for identifying integrated conservation and adaptation pathways to climate change: The case of Madagascar’s spiny forest. Landsc. Ecol. 2016, 31, 637–654. [Google Scholar] [CrossRef]
- Faina, P.; Burns, S.J.; Godfrey, L.R.; Crowley, B.E.; Scroxton, N.; McGee, D.; Sutherland, M.R.; Ranivoharimanana, L. Comparing the paleoclimates of northwestern and southwestern Madagascar during the late Holocene: Implications for the role of climate in megafaunal extinction. Malagasy Nat. 2021, 15. [Google Scholar]
- Vallet-Coulomb, C.; Gasse, F.; Robison, L.; Ferry, L.; Van Campo, E.; Chalie, F. Hydrological modeling of tropical closed Lake Ihotry (SW Madagascar): Sensitivity analysis and implications for paleohydrological reconstructions over the past 4000 years. J. Hydrol. 2006, 331, 257–271. [Google Scholar] [CrossRef]
- Razanatsoa, E. Impact of Human Land-Use and Rainfall Variability in Tropical Dry Forests of Southwest Madagascar during the Late Holocene; University of Cape Town: Cape Town, South Africa, 2019. [Google Scholar]
- Wells, N. Some hypotheses on the Mesozoic and Cenozoic paleoenvironmental history of Madagascar. In The Natural History of Madagascar; Goodman, S., Benstead, J., Eds.; University of Chicago Press: Chicago, IL, USA, 2003; pp. 16–34. [Google Scholar]
- Saji, N.; Yamagata, T. Possible impacts of Indian Ocean dipole mode events on global climate. Clim. Res. 2003, 25, 151–169. [Google Scholar] [CrossRef]
- Battistini, R. Conditions de gisements des sites littoraux de subfossiles et causes de la disparition de la faune des grands animaux dans le sud-ouest et l’extrême sud de Madagascar. Taloha 1971, 4, 7–18. [Google Scholar]
- Camoin, G.; Montaggioni, L.; Braithwaite, C. Late glacial to post glacial sea levels in the Western Indian Ocean. Mar. Geol. 2004, 206, 119–146. [Google Scholar] [CrossRef]
- Li, H.; Sinha, A.; Andre, A.A.; Spoetl, C.; Vonhof, H.; Meunier, A.; Kathayat, G.; Duan, P.; Voarintsoa, N.R.G.; Ning, Y.; et al. A multimillennial climatic context for the megafaunal extinctions in Madagascar and Mascarene Islands. Sci. Adv. 2020, 6, eabb2459. [Google Scholar] [CrossRef]
- Curtis, J.H.; Hodell, D.A.; Brenner, M. Climate variability on the Yucatan Peninsula (Mexico) during the past 3500 years, and implications for Maya cultural evolution. Quat. Res. 1996, 46, 37–47. [Google Scholar] [CrossRef]
- Kylander, M.E.; Ampel, L.; Wohlfarth, B.; Veres, D. High-resolution X-ray fluorescence core scanning analysis of Les Echets (France) sedimentary sequence: New insights from chemical proxies. J. Quat. Sci. 2011, 26, 109–117. [Google Scholar] [CrossRef]
- Haberzettl, T.; Corbella, H.; Fey, M.; Janssen, S.; Luecke, A.; Mayr, C.; Ohlendorf, C.; Schaebitz, F.; Schleser, G.H.; Wille, M.; et al. Lateglacial and Holocene wet—Dry cycles in southern Patagonia: Chronology, sedimentology and geochemistry of a lacustrine record from Laguna Potrok Aike, Argentina. Holocene 2007, 17, 297–310. [Google Scholar] [CrossRef]
- Piva, A.; Asioli, A.; Schneider, R.R.; Trincardi, F.; Andersen, N.; Colomenero-Hidalgo, E.; Dennielou, B.; Flores, J.A.; Vigliotti, L. Climatic cycles as expressed in sediments of the PROMESS1 borehole PRAD1-2, central Adriatic, for the last 370 ka: 1. Integrated stratigraphy. Geochem. Geophys. Geosyst. 2008, 9. [Google Scholar] [CrossRef]
- Aufgebauer, A.; Panagiotopoulos, K.; Wagner, B.; Schaebitz, F.; Viehberg, F.A.; Vogel, H.; Zanchetta, G.; Sulpizio, R.; Leng, M.J.; Damaschke, M. Climate and environmental change in the Balkans over the last 17 ka recorded in sediments from Lake Prespa (Albania/FYR of Macedonia/Greece). Quat. Int. 2012, 274, 122–135. [Google Scholar] [CrossRef]
- Hixon, S.W.; Douglass, K.G.; Crowley, B.E.; Rakotozafy, L.M.A.; Clark, G.; Anderson, A.; Haberle, S.; Ranaivoarisoa, J.F.; Buckley, M.; Fidiarisoa, S.; et al. Late Holocene spread of pastoralism coincides with endemic megafaunal extinction on Madagascar. Proc. R. Soc. Lond. B 2021, 288, 20211204. [Google Scholar]
- Feldt, T.; Schlecht, E. Analysis of GPS trajectories to assess spatio-temporal differences in grazing patterns and land use preferences of domestic livestock in southwestern Madagascar. Pastoralism 2016, 6, 5. [Google Scholar] [CrossRef]
- Burney, D.A. Climate Change and Fire Ecology as Factors in the Quaternary Biogeography of Madagascar. In Biogeography of Madagascar; Lourenco, W.R., Ed.; OSTROM: Paris, France, 1996; pp. 49–58. [Google Scholar]
- Godfrey, L.R.; Scroxton, N.; Crowley, B.E.; Burns, S.J.; Sutherland, M.R.; Perez, V.R.; Faina, P.; McGee, D.; Ranivoharimanana, L. A new interpretation of Madagascar’s megafaunal decline: The “Subsistence Shift Hypothesis”. J. Hum. Evol. 2019, 130, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Burney, D.A.; Robinson, G.S.; Burney, L.P. Sporormiella and the late Holocene extinctions in Madagascar. Proc. Natl. Acad. Sci. USA 2003, 100, 10800–10805. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Crowley, B.E.; Samonds, K.E. Stable carbon isotope values confirm a recent increase in grasslands in northwestern Madagascar. Holocene 2013, 23, 1066–1073. [Google Scholar] [CrossRef]
- Crowley, B.E.; Godfrey, L.R.; Bankoff, R.J.; Perry, G.H.; Culleton, B.J.; Kennett, D.J.; Sutherland, M.R.; Samonds, K.E.; Burney, D.A. Island-wide aridity did not trigger recent megafaunal extinctions in Madagascar. Ecography 2017, 40, 901–912. [Google Scholar] [CrossRef]
- Crowley, B.E.; Thoren, S.; Rasoazanabary, E.; Vogel, E.R.; Barrett, M.A.; Zohdy, S.; Blanco, M.B.; McGoogan, K.C.; Arrigo-Nelson, S.J.; Irwin, M.T.; et al. Explaining geographical variation in the isotope composition of mouse lemurs (Microcebus). J. Biogeogr. 2011, 38, 2106–2121. [Google Scholar] [CrossRef]
- Dewar, R.E.; Richard, A.F. Evolution in the hypervariable environment of Madagascar. Proc. Natl. Acad. Sci. USA 2007, 104, 13723–13727. [Google Scholar] [CrossRef]
- Grandidier, G. Les animaux disparus de Madagascar. Gisements, époques et causes de leur disparition. Revue de Madagascar 1905, 7, 111–128. [Google Scholar]
- Chanudet, C. Conditions géographiques et archéologiques de la disparition des subfossiles à Madagascar. In Mémoire de Maîtrise; Université de Bretagne Occidentale: Brest, France, 1975. [Google Scholar]
- Burney, D.A. Late Holocene environmental changes in arid southwestern Madagascar. Quat. Res. 1993, 40, 98–106. [Google Scholar] [CrossRef]
- MacPhee, R.D.; Burney, D.A. Dating of modified femora of extinct dwarf Hippopotamus from southern Madagascar: Implications for constraining human colonization and vertebrate extinction events. J. Archaeol. Sci. 1991, 18, 695–706. [Google Scholar] [CrossRef]
- Battistini, R.; Vérin, P. Témoignages archéologiques sur la côte vezo de l’embouchure de l’Onilahy à la Baie des Assassins. Taloha 1971, 4, 51–63. [Google Scholar]
- Fisher, M.M.; Brenner, M.; Reddy, K.R. A simple, inexpensive piston corer for collecting undisturbed sediment/water interface profiles. J. Paleolimnol. 1992, 7, 157–161. [Google Scholar] [CrossRef]
- Hogg, A.G.; Heaton, T.J.; Hua, Q.; Palmer, J.G.; Turney, C.S.M.; Southon, J.; Bayliss, A.; Blackwell, P.G.; Boswijk, G.; Ramsey, C.B.; et al. SHCal20 Southern Hemisphere calibration, 0–55,000 years cal BP. Radiocarbon 2020, 62, 1–20. [Google Scholar] [CrossRef]
- Blaauw, M.; Christen, J.A. Flexible paleoclimate age-depth models using an autoregressive gamma process. Bayesian Anal. 2011, 6, 457–474. [Google Scholar] [CrossRef]
- Philippsen, B. The freshwater reservoir effect in radiocarbon dating. Herit. Sci. 2013, 1, 24. [Google Scholar] [CrossRef]
- Guiry, E.J.; Szpak, P.; Richards, M.P. Effects of lipid extraction and ultrafiltration on stable carbon and nitrogen isotopic compositions of fish bone collagen. Rapid Commun. Mass Spectrom. 2016, 30, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.A.; Nelson, D.E.; Vogel, J.S.; Southon, J.R. Improved collagen extraction by modified Longin method. Radiocarbon 1988, 30, 171–177. [Google Scholar] [CrossRef]
- McClure, S.B.; Puchol, O.G.; Culleton, B.J. AMS dating of human bone from Cova de la Pastora: New evidence of ritual continuity in the prehistory of eastern Spain. Radiocarbon 2010, 52, 25–32. [Google Scholar] [CrossRef]
- Kennett, D.J.; Plog, S.; George, R.J.; Culleton, B.J.; Watson, A.S.; Skoglund, P.; Rohland, N.; Mallick, S.; Stewardson, K.; Kistler, L.; et al. Archaeogenomic evidence reveals prehistoric matrilineal dynasty. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Stafford, T.W.; Brendel, K.; Duhamel, R.C. Radiocarbon, 13 C and 15 N analysis of fossil bone: Removal of humates with XAD-2 resin. Geochim. Et Cosmochim. Acta 1988, 52, 2257–2267. [Google Scholar] [CrossRef]
- Stafford, T.W.; Hare, P.E.; Currie, L.; Jull, A.J.T.; Donahue, D.J. Accelerator radiocarbon dating at the molecular level. J. Archaeol. Sci. 1991, 18, 35–72. [Google Scholar] [CrossRef]
- Lohse, J.C.; Culleton, B.J.; Black, S.L.; Kennett, D.J. A Precise Chronology of Middle to Late Holocene Bison Exploitation in the Far Southern Great Plains. J. Tex. Archaeol. Hist. 2014, 1, 94–126. [Google Scholar] [CrossRef]
- Beaumont, W.; Beverly, R.; Southon, J.; Taylor, R.E. Bone preparation at the KCCAMS laboratory. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2010, 268, 906–909. [Google Scholar] [CrossRef]
- DeNiro, M.J. Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature 1985, 317, 806–809. [Google Scholar] [CrossRef]
- Van Klinken, G.J. Bone collagen quality indicators for palaeodietary and radiocarbon measurements. J. Archaeol. Sci. 1999, 26, 687–695. [Google Scholar] [CrossRef]
- Crowley, B.E.; Godfrey, L.R. Why all those spines?: Anachronistic defences in the Didiereoideae against now extinct lemurs. S. Afr. J. Sci. 2013, 109, 1–7. [Google Scholar] [CrossRef]
- Buck, C.E.; Bard, E. A calendar chronology for Pleistocene mammoth and horse extinction in North America based on Bayesian radiocarbon calibration. Quat. Sci. Rev. 2007, 26, 2031–2035. [Google Scholar] [CrossRef]
- Battarbee, R.W. The Eutrophication of Lough Erne Inferred from Changes in the Diatom Assemblages of ²¹⁰Pb-and ¹³⁷Cs-Dated Sediment Cores. Proc. R. Ir. Acad. Sect. B Biol. Geol. Chem. Sci. 1986, 141–168. [Google Scholar]
- Walsh, M.K.; Prufer, K.M.; Culleton, B.J.; Kennett, D.J. A late Holocene paleoenvironmental reconstruction from Agua Caliente, southern Belize, linked to regional climate variability and cultural change at the Maya polity of Uxbenká. Quat. Res. 2014, 82, 38–50. [Google Scholar] [CrossRef]
- Erdman, C.; Emerson, J.W. bcp: An R package for performing a Bayesian analysis of change point problems. J. Stat. Softw. 2007, 23, 1–13. [Google Scholar] [CrossRef]
- Barry, D.; Hartigan, J.A. A Bayesian analysis for change point problems. J. Am. Stat. Assoc. 1993, 88, 309–319. [Google Scholar]
- Scroxton, N.; Burns, S.J.; McGee, D.; Hardt, B.; Godfrey, L.R.; Ranivoharimanana, L.; Faina, P. Hemispherically in-phase precipitation variability over the last 1700 years in a Madagascar speleothem record. Quat. Sci. Rev. 2017, 164, 25–36. [Google Scholar] [CrossRef]
- Douglass, K. The Diversity of Late Holocene Shellfish Exploitation in Velondriake, Southwest Madagascar. J. Isl. Coast. Archaeol. 2016, 12, 1–27. [Google Scholar] [CrossRef]
- Oppo, D.W.; Rosenthal, Y.; Linsley, B.K. 2,000-year-long temperature and hydrology reconstructions from the Indo-Pacific warm pool. Nature 2009, 460, 1113. [Google Scholar] [CrossRef]
- Tierney, J.E.; Smerdon, J.E.; Anchukaitis, K.J.; Seager, R. Multidecadal variability in East African hydroclimate controlled by the Indian Ocean. Nature 2013, 493, 389. [Google Scholar] [CrossRef]
- Talbot, M. A review of the palaeohydrological interpretation of carbon and oxygen isotopic ratios in primary lacustrine carbonates. Chem. Geol. Isot. Geosci. Sect. 1990, 80, 261–279. [Google Scholar] [CrossRef]
- Li, H.-C.; Ku, T.-L. δ13C–δ18O covariance as a paleohydrological indicator for closed-basin lakes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997, 133, 69–80. [Google Scholar] [CrossRef]
- Amundson, R.; Austin, A.T.; Schuur, E.A.G.; Yoo, K.; Matzek, V.; Kendall, C.; Uebersax, A.; Brenner, D.; Baisden, W.T. Global patterns of the isotopic composition of soil and plant nitrogen. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Austin, A.T.; Vitousek, P. Nutrient dynamics on a precipitation gradient in Hawai’i. Oecologia 1998, 113, 519–529. [Google Scholar] [CrossRef]
- Hecky, R.E.; Bootsma, H.A.; Mugidde, R.M.; Bugenyi, F.W.B. Phosphorus Pumps, Nitrogen Sinks, and Silicon Drains: Plumbing Nutrients in the African Great Lakes. In The Limnology, Climatology, and Paleoclimatology of the East African Lakes; Johnson, T.C., Odada, E.O., Whittaker, K.T., Eds.; Routledge: Oxfordshire, UK, 1996. [Google Scholar]
- Talbot, M.R. Nitrogen isotopes in palaeolimnology. In Tracking Environmental Change Using Lake Sediments; Last, W.M., Smol, J.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; Volume 2, pp. 401–439. [Google Scholar]
- Moreno, A.; Giralt, S.; Valero-Garces, B.; Saez, A.; Bao, R.; Prego, R.; Pueyo, J.J.; Gonzalez-Samperiz, P.; Taberner, C. A 14 kyr record of the tropical Andes: The Lago Chungará sequence (18 S, northern Chilean Altiplano). Quat. Int. 2007, 161, 4–21. [Google Scholar] [CrossRef]
- Li, T.; Wang, B.; Chang, C.P.; Zhang, Y. A theory for the Indian Ocean dipole–zonal mode. J. Atmos. Sci. 2003, 60, 2119–2135. [Google Scholar] [CrossRef]
- Virah-Sawmy, M.; Willis, K.J.; Gillson, L. Evidence for drought and forest declines during the recent megafaunal extinctions in Madagascar. J. Biogeogr. 2010, 37, 506–519. [Google Scholar] [CrossRef]
- Domic, A.I. Influence of late Holocene climate change, megafaunal extinction, and human occupation on terrestrial and aquatic ecosystems in SW Madagascar. Front. Ecol. Evol. 2021. submitted. [Google Scholar]
- Guyard, H.; Chapron, E.; St-Onge, G.; Anselmetti, F.S.; Arnaud, F.; Magand, O.; Francus, P.; Melieres, M.A. High-altitude varve records of abrupt environmental changes and mining activity over the last 4000 years in the Western French Alps (Lake Bramant, Grandes Rousses Massif). Quat. Sci. Rev. 2007, 26, 2644–2660. [Google Scholar] [CrossRef]
- Davies, S.J.; Metcalfe, S.E.; MacKenzie, A.B.; Newton, A.J.; Endfield, G.H.; Farmer, J.G. Environmental changes in the Zirahuén Basin, Michoacán, Mexico, during the last 1000 years. J. Paleolimnol. 2004, 31, 77–98. [Google Scholar] [CrossRef]
- Anderson, A.; Clark, G.; Haberle, S.; Higham, T.; Nowak-Kemp, M.; Prendergast, A.; Radimilahy, C.; Rakotozafy, L.M.; Ramilisonina; Schwenninger, J.L.; et al. New evidence of megafaunal bone damage indicates late colonization of Madagascar. PLoS ONE 2018, 13, e0204368. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.R.; Godfrey, L.R.; Nowak-Kemp, M.; Burney, D.A.; Ratsimbazafy, J.; Vasey, N. Evidence of early butchery of giant lemurs in Madagascar. J. Hum. Evol. 2005, 49, 722–742. [Google Scholar] [CrossRef]
- Verin, P.; Battistini, R. Les anciens habitats de Rezoky et d’Asambalahy. Taloha 1971, 4, 29–49. [Google Scholar]
- Douglass, K.M.G. An Archaeological Investigation of Settlement and Resource Exploitation Patterns in the Velondriake Marine Protected Area, Southwest Madagascar, ca. 900 BC to AD 1900; Yale University: New Haven, CT, USA, 2016. [Google Scholar]
- Burney, D.A. Late Holocene vegetational change in central Madagascar. Quat. Res. 1987, 28, 130–143. [Google Scholar] [CrossRef]
- Burns, S.J.; Godfrey, L.R.; Faina, P.; McGee, D.; Hardt, B.; Ranivoharimanana, L.; Randrianasy, J. Rapid human-induced landscape transformation in Madagascar at the end of the first millennium of the Common Era. Quat. Sci. Rev. 2016, 134, 92–99. [Google Scholar] [CrossRef]
- Crowley, B.E.; Godfrey, L.R.; Guilderson, T.P.; Zermeno, P.; Koch, P.L.; Dominy, N.J. Extinction and ecological retreat in a community of primates. Proc. R. Soc. Lond. B Biol. Sci. 2012, 279, 3597–3605. [Google Scholar] [CrossRef]
- Hixon, S.W.; Smith, E.A.E.; Crowley, B.E.; Perry, G.H.; Randrianasy, J.; Ranaivoarisoa, J.F.; Kennett, D.J.; Newsome, S.D. Nitrogen isotope (δ15N) patterns for amino acids in lemur bones are inconsistent with aridity driving megafaunal extinction in south-western Madagascar. J. Quat. Sci. 2018, 33, 958–968. [Google Scholar] [CrossRef]
- Rasolondrainy, T.V.R. Decision-Making in the Face of Unpredictable Climate and Intergroup Conflicts in Southwest Madagascar, Sixteenth to Nineteenth Centuries CE; Yale University: New Haven, CT, USA, 2019. [Google Scholar]
- Feldt, T.; Antsonantenainarivony, O.; Schlecht, E. Feed selection on dry rangelands in southwestern Madagascar: Implications for ruminant nutrition in view of ecological and social challenge. J. Arid. Environ. 2017, 144, 81–90. [Google Scholar] [CrossRef]
- Kaufmann, J.C.; Tsirahamba, S. Forests and thorns: Conditions of change affecting Mahafale pastoralists in southwestern Madagascar. Conserv. Soc. 2006, 4, 231–261. [Google Scholar]
- Nogué, S.; de Nascimento, L.; Froyd, C.A.; Wilmshurst, J.M.; de Boer, E.J.; Coffey, E.E.D.; Whittaker, R.J.; Fernandez-Palacios, J.M.; Willis, K.J. Island biodiversity conservation needs palaeoecology. Nat. Ecol. Evol. 2017, 1, 1–9. [Google Scholar] [CrossRef]
- Castilla, J.C. Coastal marine communities: Trends and perspectives from human-exclusion experiments. Trends Ecol. Evol. 1999, 14, 280–283. [Google Scholar] [CrossRef]
- Bird, R.B.; Bird, D.W.; Codding, B.F.; Parker, C.H.; Jones, J.H. The “fire stick farming” hypothesis: Australian Aboriginal foraging strategies, biodiversity, and anthropogenic fire mosaics. Proc. Natl. Acad. Sci. USA 2008, 105, 14796–14801. [Google Scholar] [CrossRef] [PubMed]
- Crowley, B.E.; Godfrey, L.R.; Irwin, M.T. A glance to the past: Subfossils, stable isotopes, seed dispersal, and lemur species loss in southern Madagascar. Am. J. Primatol. 2011, 73, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, L.R.; Jungers, W.L.; Schwartz, G.T.; Irwin, M.T. Ghosts and Orphans. In Elwyn Simons: A Search for Origins; Springer: Berlin/Heidelberg, Germany, 2008; pp. 361–395. [Google Scholar]
- Federman, S.; Dornburg, A.; Daly, D.C.; Downie, A.; Perry, G.H.; Yoder, A.D.; Sargis, E.J.; Richard, A.F.; Donoghue, M.J.; Baden, A.L. Implications of lemuriform extinctions for the Malagasy flora. Proc. Natl. Acad. Sci. USA 2016, 113, 5041–5046. [Google Scholar] [CrossRef]
- Andriantsaralaza, S.; Pedrono, M.; Tassin, J.; Roger, E.; Rakouth, B.; Danthu, P. The role of extinct giant tortoises in the germination of extant baobab Adansonia rubrostipa seeds in Madagascar. Afr. J. Ecol. 2014, 52, 246–249. [Google Scholar] [CrossRef]
- Pedrono, M.; Rambeloson, E.; Clausen, A. Giant Tortoises Make a Comeback in Madagascar. Nature 2020, 587, 548. [Google Scholar] [CrossRef]
- Pedrono, M.; Griffiths, O.L.; Clausen, A.; Smith, L.L.; Griffiths, C.J.; Wilme, L.; Burney, D.A. Using a surviving lineage of Madagascar’s vanished megafauna for ecological restoration. Biol. Conserv. 2013, 159, 501–506. [Google Scholar] [CrossRef]
- Ganzhorn, J.U.; Lowry, P.P.; Schatz, G.E.; Sommer, S. The biodiversity of Madagascar: One of the world’s hottest hotspots on its way out. Oryx 2001, 35, 346–348. [Google Scholar] [CrossRef]
- Jones, J.P.; Ratsimbazafy, J.; Ratsifandrihamanana, A.N.; Watson, J.E.M.; Andrianandrasana, H.T.; Cabeza, M.; Cinner, J.E.; Goodman, S.M.; Hawkins, F.; Mittermeier, R.A.; et al. Last chance for Madagascar’s biodiversity. Nat. Sustain. 2019, 2, 350–352. [Google Scholar] [CrossRef]
- Randriamiharisoa, L.O.; Rakotondravony, D.; Raherilalao, M.J.; Ranirison, A.; Wilme, L.; Ganzhorn, J.U. Effects of transhumance route on the richness and composition of bird communities in Tsimanampesotse National Park. Madag. Conserv. Dev. 2015, 10, 110–115. [Google Scholar] [CrossRef][Green Version]
- Ratovonamana, Y.; Rajeriarison, C.; Roger, E.; Keifer, I.; Ganzhorn, J.U. Impact of livestock grazing on forest structure, plant species composition and biomass in southwestern Madagascar. Scr. Bot. Belg. 2013, 50, 82–92. [Google Scholar]
- Randriamalala, J.R.; Radosy, H.O.; Razanaka, S.; Randriambanona, H.; Herve, D. Effects of goat grazing and woody charcoal production on xerophytic thickets of southwestern Madagascar. J. Arid. Environ. 2016, 128, 65–72. [Google Scholar] [CrossRef]
- Razanatsoa, E.; Virah-Sawmy, M.; Woodborne, S.; Callanan, C.; Gillson, L. Adaptation of subsistence strategies of the southwestern Malagasy in the face of climate change. Malagasy Nat. 2021, 15, 1–31. [Google Scholar]
- Gardner, C.J.; Davies, Z.G. Rural bushmeat consumption within multiple-use protected areas: Qualitative evidence from southwest Madagascar. Hum. Ecol. 2014, 42, 21–34. [Google Scholar] [CrossRef]
- Benstead, J.P.; De Rham, P.H.; Gattolliat, J.L.; Gibon, F.M.; Loiselle, P.V.; Sartori, M.; Sparks, J.S.; Stiassny, M.L.J. Conserving Madagascar’s freshwater biodiversity. BioScience 2003, 53, 1101–1111. [Google Scholar] [CrossRef]
- Bamford, A.J.; Razafindrajao, F.; Young, R.P.; Hilton, G.M. Profound and pervasive degradation of Madagascar’s freshwater wetlands and links with biodiversity. PLoS ONE 2017, 12, e0182673. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hixon, S.W.; Curtis, J.H.; Brenner, M.; Douglass, K.G.; Domic, A.I.; Culleton, B.J.; Ivory, S.J.; Kennett, D.J. Drought Coincided with, but Does Not Explain, Late Holocene Megafauna Extinctions in SW Madagascar. Climate 2021, 9, 138. https://doi.org/10.3390/cli9090138
Hixon SW, Curtis JH, Brenner M, Douglass KG, Domic AI, Culleton BJ, Ivory SJ, Kennett DJ. Drought Coincided with, but Does Not Explain, Late Holocene Megafauna Extinctions in SW Madagascar. Climate. 2021; 9(9):138. https://doi.org/10.3390/cli9090138
Chicago/Turabian StyleHixon, Sean W., Jason H. Curtis, Mark Brenner, Kristina G. Douglass, Alejandra I. Domic, Brendan J. Culleton, Sarah J. Ivory, and Douglas J. Kennett. 2021. "Drought Coincided with, but Does Not Explain, Late Holocene Megafauna Extinctions in SW Madagascar" Climate 9, no. 9: 138. https://doi.org/10.3390/cli9090138
APA StyleHixon, S. W., Curtis, J. H., Brenner, M., Douglass, K. G., Domic, A. I., Culleton, B. J., Ivory, S. J., & Kennett, D. J. (2021). Drought Coincided with, but Does Not Explain, Late Holocene Megafauna Extinctions in SW Madagascar. Climate, 9(9), 138. https://doi.org/10.3390/cli9090138