Analysis of Olive Tree Flowering Behavior Based on Thermal Requirements: A Case Study from the Northern Mediterranean Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Olive Variety
2.3. Meteorological Data
2.4. Phenological Data
2.5. Calculations of Chill Units and Growing Degree Hours
3. Results and Discussion
3.1. Late Spring Frost
| Year | Timeframe of Frost | Lowest Tmax [°C] | Number of Days with the Tmax Below 0 °C | Lowest Tmin [°C] | 14-Day Average Tmax [°C] Before the Occurrence of Tmax Below 0 | Damage | Reference |
|---|---|---|---|---|---|---|---|
| 1929 | 11 February–13 February 1929 Winter frost | / | / | −14.3 | / | 90% of trees affected by frost | [60] |
| 1956 | 2 February–20 February 1956 Winter frost | / | / | −12.8 | / | 30% of trees affected by frost | [60] |
| 1985 | 1 January–18 January 1985 Winter frost | −5.6 | 6 | −9.3 | 7.64 | 60% of trees affected by frost | [60] |
| 1996 | 27 December–29 December 1996 Winter frost | −4.6 | 3 | −8.5 | 10.52 | Young trees completely damaged | [61] |
| 2012 | 1 February–12 February 2012 Winter frost | −2.4 | 4 | −5.9 | 6.79 | 10% of trees affected by frost | [62] |
| 2018 | 25 February–1 March 2018 Winter frost | −3.2 | 2 | −5.7 | 9.2 | 30% of trees affected by frost | [62] |
| 2021 | 4 April–9 April 2021 Spring frost | 10.8 | 0 | −2.3 | / | More than 50% reduced yield | [30] |
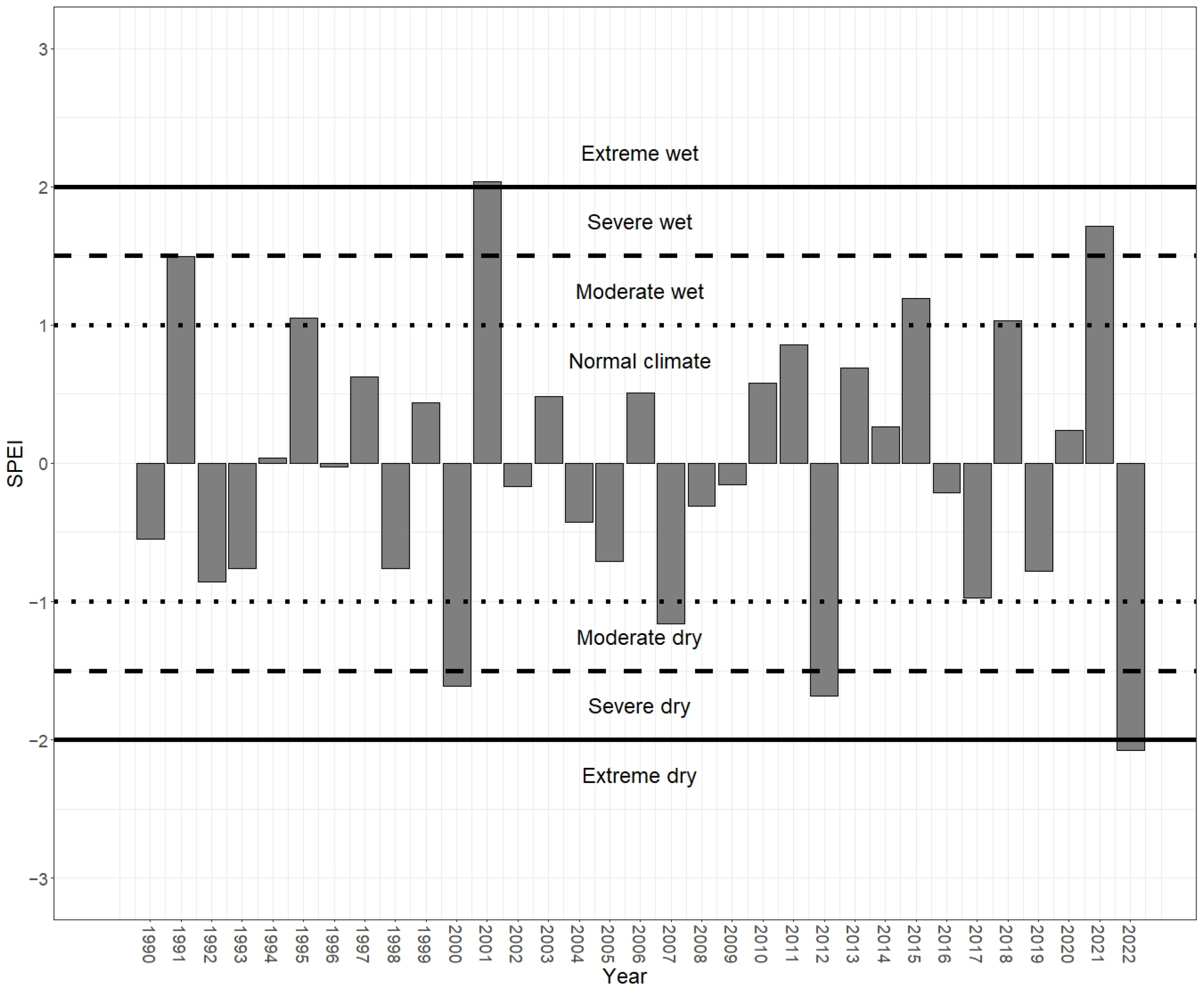
3.2. Spring Drought
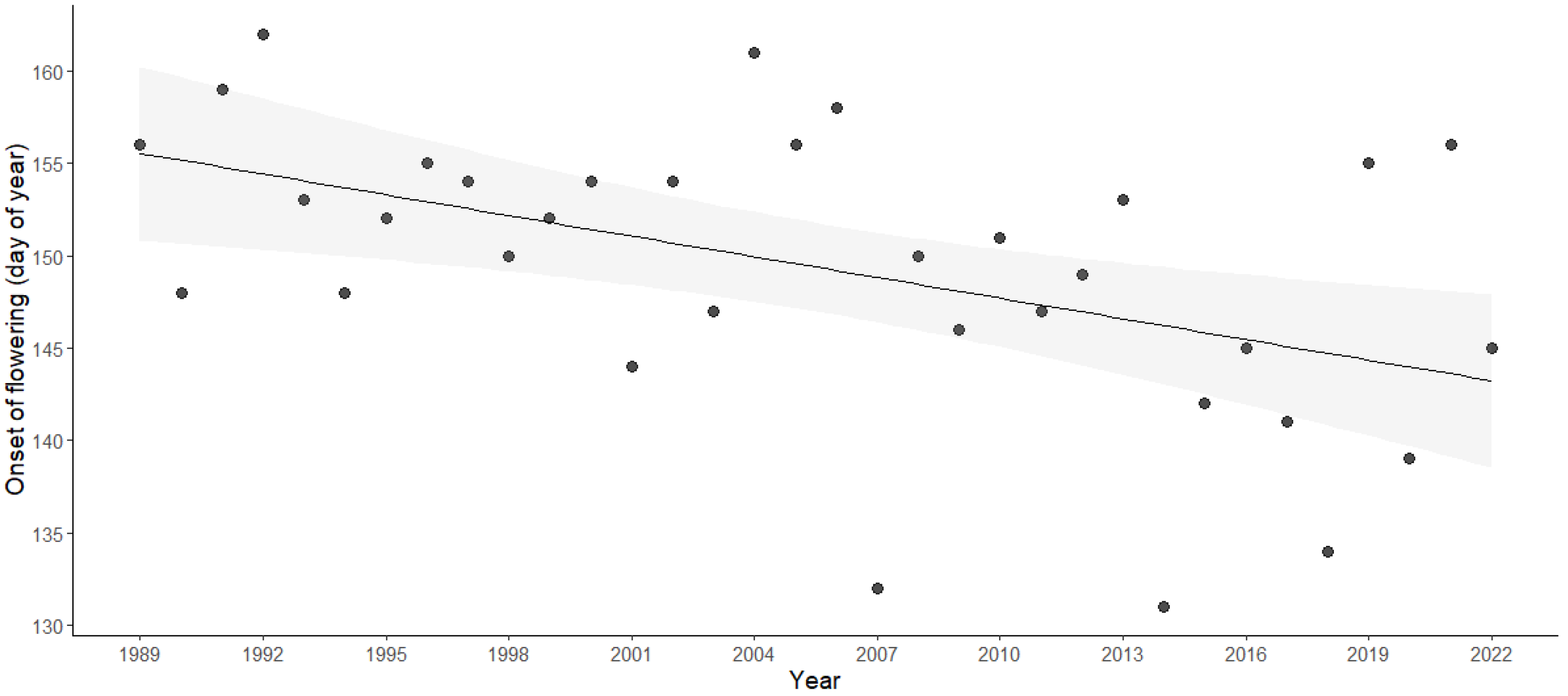
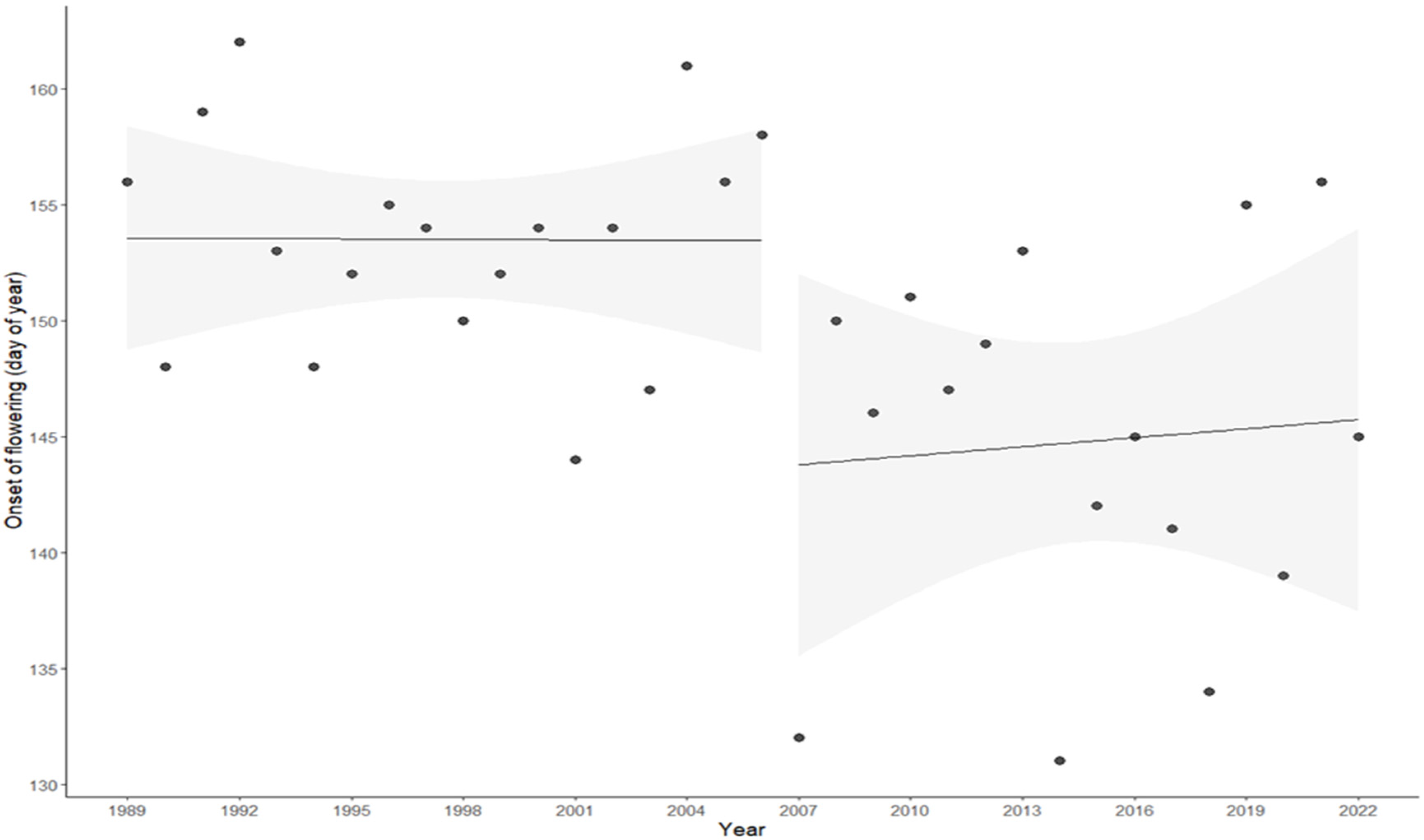
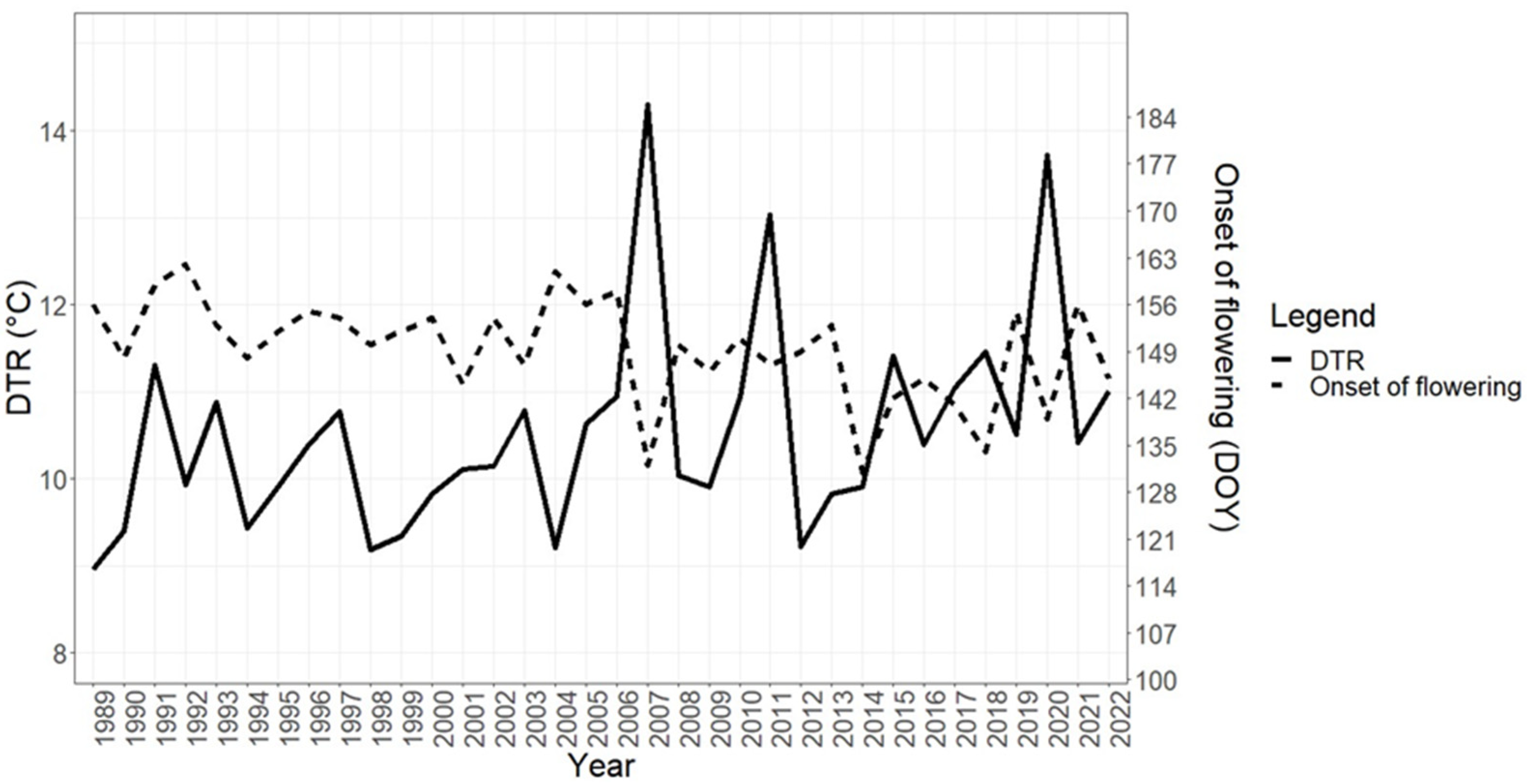
3.3. Changes in the Flowering of Olive Trees and Diurnal Temperature Range
3.4. Cold (Chilling) and Heat (Forcing) Requirements
3.4.1. Estimation of the Thermal Accumulation Period
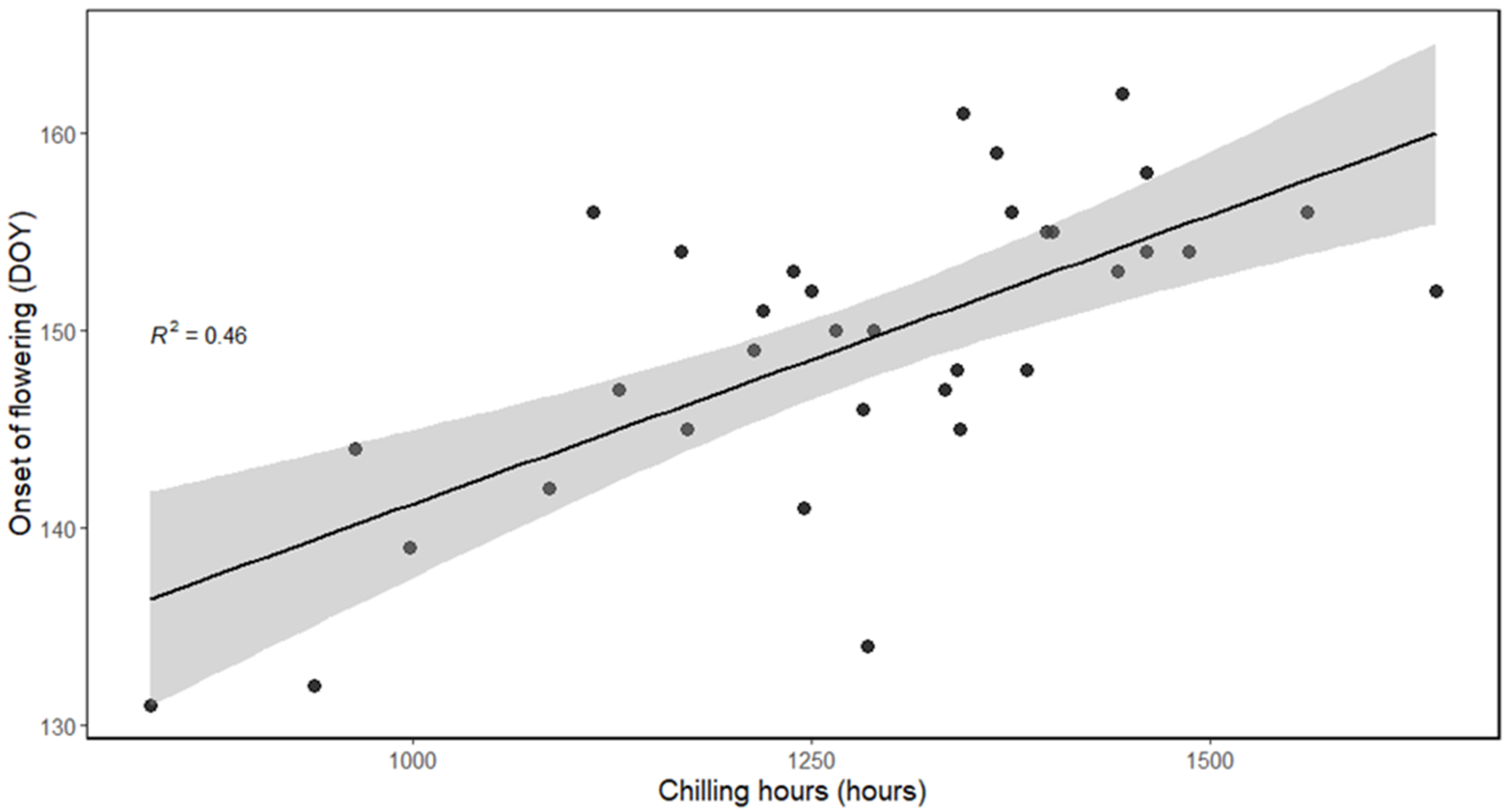
3.4.2. Models for Calculating Chilling Requirements Using Different Chilling Models
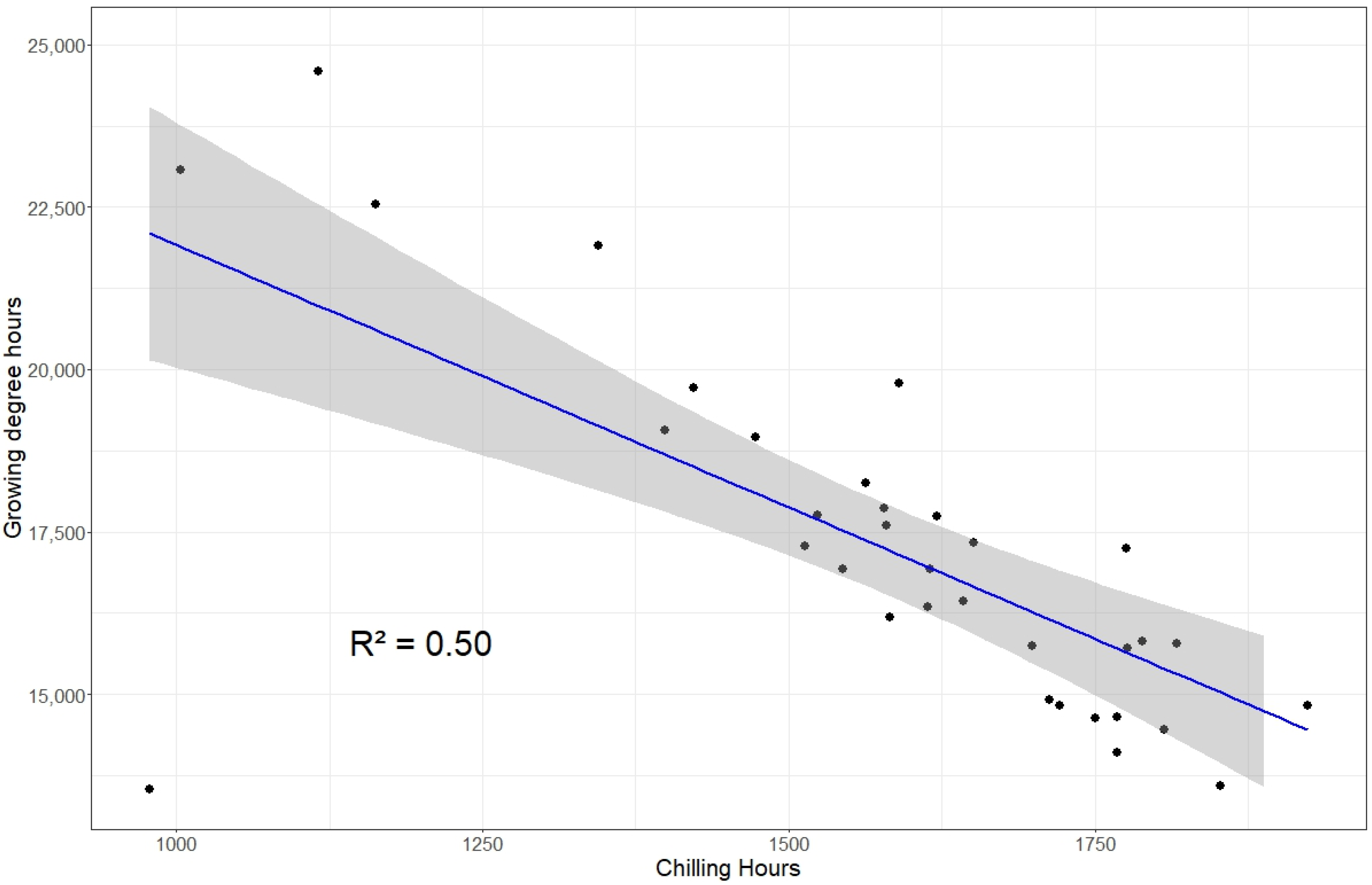
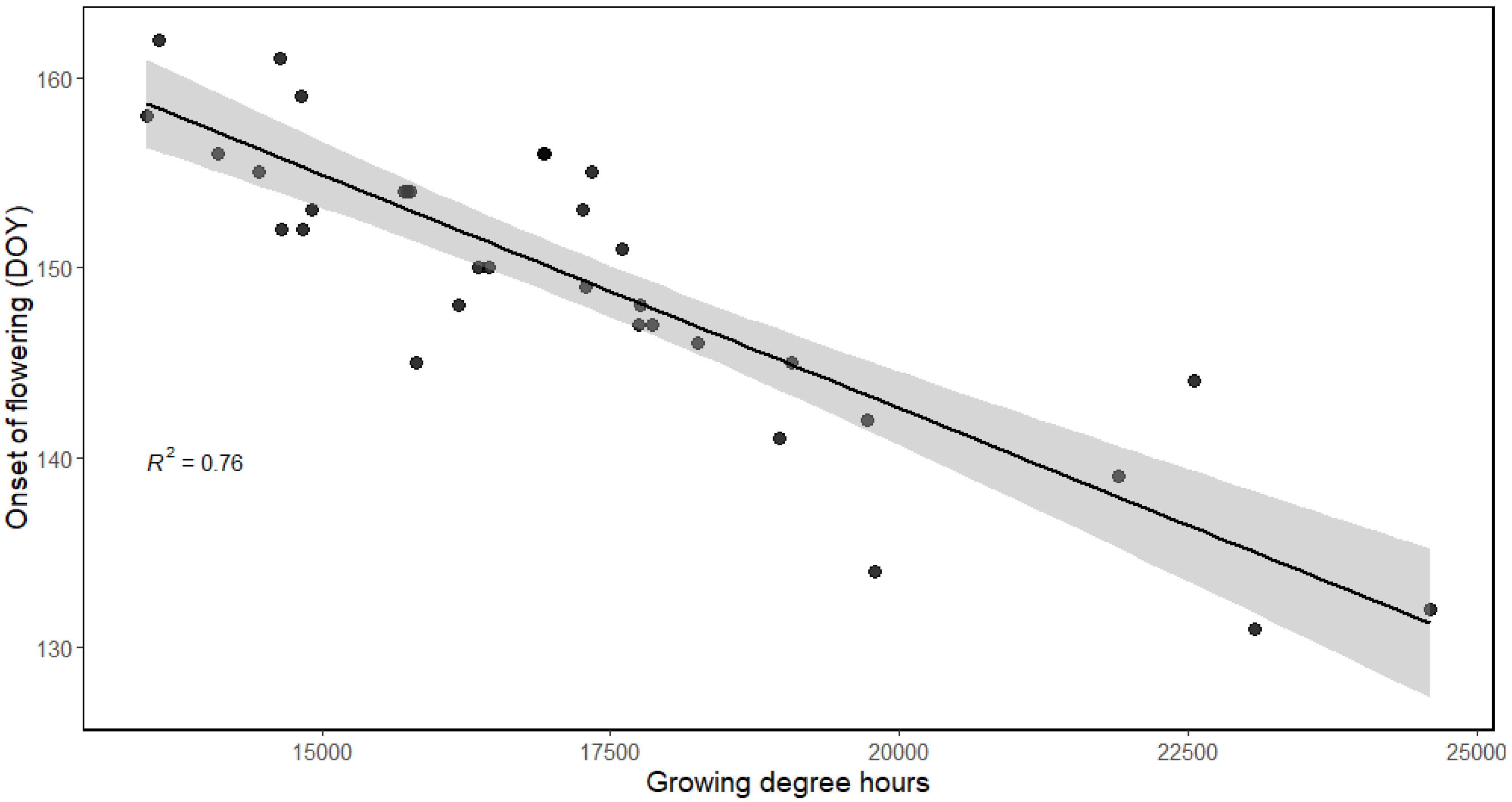
3.4.3. Model for Calculating Growing Degree Hours (GDHs)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B
Appendix C

Appendix D

References
- Vitasse, Y.; Rebetez, M. Unprecedented risk of spring frost damage in Switzerland and Germany in 2017. Clim. Change 2018, 149, 233–246. [Google Scholar] [CrossRef]
- Campoy, J.A.; Ruiz, D.; Egea, J. Dormancy in temperate fruit trees in a global warming context: A review. Sci. Hortic. 2011, 130, 357–372. [Google Scholar] [CrossRef]
- Rubio-Valdés, G.; Cabello, D.; Rallo, L.; Rapoport, H.F. Bud dormancy release dynamics in the olive tree and validation of the use of explants to determine chilling requirement fulfillment. SSRN Electron. J. 2022, 11, 3461. [Google Scholar] [CrossRef]
- Haberman, A.; Bakhshian, O.; Cerezo-Medina, S.; Paltiel, J.; Adler, C.; Ben-Ari, G.; Mercado, J.A.; Pliego-Alfaro, F.; Lavee, S.; Samach, A. A possible role for FT-encoding genes in interpreting environmental and internal cues affecting olive (Olea europaea L.) flower induction. Plant Cell Environ. 2017, 40, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Rapoport, H.F.; Cabello, D.; Rallo, L. Chilling accumulation, dormancy release temperature, and the role of leaves in olive reproductive budburst: Evaluation using shoot explants. Sci. Hortic. 2018, 231, 241–252. [Google Scholar] [CrossRef]
- Lang, G.A.; Early, J.D.; Martin, G.C.; Darnell, R.L. Endo-, Para-, and Ecodormancy: Physiological Terminology and Classification for Dormancy Research. HortScience 1987, 22, 371–377. [Google Scholar] [CrossRef]
- Farga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean Olive Orchards under Climate Change: A Review of Future Impacts and Adaptation Strategies. Agronomy 2020, 11, 56. [Google Scholar] [CrossRef]
- Osborne, C.P.; Chuine, I.; Viner, D.; Woodward, F.I. Olive phenology as a sensitive indicator of future climatic warming in the Mediterranean. Plant Cell Environ. 2000, 23, 701–710. [Google Scholar] [CrossRef]
- Galán, C.; García-Mozo, H.; Vázquez, L.; Ruiz, L.; de la Guardia, C.D.; Trigo, M.M. Heat requirement for the onset of the Olea europaea L. pollen season in several sites in Andalusia and the effect of the expected future climate change. Int. J. Biometeorol. 2005, 49, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Gómez-del-Campo, M.; Rapoport, H. Descripción de la iniciación floral, floración, cuajado, caída de frutos y endurecimiento del hueso. Agric. Rev. Agropecuaria 2008, 907, 400–406. [Google Scholar]
- Tombesi, A. Biologia fiorale e di fruttificazione. In Olea. Trattato di Olivicoltura; Fiorino, P., Ed.; Edagricole: Bologna, Italy, 2003. [Google Scholar]
- Rallo, L.; Cuevas, J. Fructificación y producción. In El Cultivo del Olivo; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; Mundi-Prensa y Junta de Andalucía: Madrid, Spain, 2004. [Google Scholar]
- Lavee, S. Biología y fisiología del olivo. In Enciclopedia Mundial del Olivo; Consejo Oleícola Internacional: Madrid, Spain, 1996. [Google Scholar]
- Ayerza, R.; Sibbett, G.S. Thermal adaptability of olive (Olea europaea L.) to the Arid Chaco of Argentina. Agric. Ecosyst. Environ. 2001, 84, 277–285. [Google Scholar] [CrossRef]
- Torres, M.; Pierantozzi, P.; Searles, P.; Rousseaux, M.C.; García-Inza, G.; Miserere, A.; Bodoira, R.; Contreras, C.; Maestri, D. Olive Cultivation in the Southern Hemisphere: Flowering, Water Requirements and Oil Quality Responses to New Crop Environments. Front. Plant Sci. 2017, 8, 1830. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Llanque, F.J.; Rapoport, H.F.; Baumann Samanez, H. Irrigation withholding effects on olive reproductive bud development for conditions with insufficient winter chilling. Acta Hortic. 2014, 1057, 113–119. [Google Scholar] [CrossRef]
- Medina-Alonso, M.G.; Cabezas Luque, J.M.; Ríos Mesa, D.; Lorite, I.J. Flowering Phenology of Olive Cultivars in Two Climate Zones with Contrasting Temperatures (Subtropical and Mediterranean). Agriculture 2023, 13, 1312. [Google Scholar] [CrossRef]
- Rapoport, H.F.; Hammami, S.; Martins, P.; Pérez-Priego, O.; Orgaz, F. Influence of water deficits at different times during olive tree inflorescence and flower development. Environ. Exp. Bot. 2011, 77, 227–233. [Google Scholar] [CrossRef]
- Gucci, R.; Fereras, E.; Goldhamer, D.A. Olive. In Crop Yield Response to Water, FAO Irrigation and Drainage Paper 66; Steduto, P., Hsiao, T.C., Fereres, E., Raes, D., Eds.; FAO: Rome, Italy, 2012; pp. 300–313. [Google Scholar]
- Grattan, S.R.; Berenguer, M.J.; Connell, J.H.; Polito, V.S.; Vossen, P.M. Olive oil production as influenced by different quantities of applied water. Agric. Water Manag. 2006, 85, 133–140. [Google Scholar] [CrossRef]
- Rapoport, H.F.; Moreno-Alías, I. Botánica y morfología. In El Cultivo del Olivo, 7th ed.; Barranco, D.R., Fernández-Escobar, R., Rallo, L., Eds.; Editorial Mundi-Prensa: Madrid, Spain, 2017; pp. 35–65. [Google Scholar]
- Stephenson, A.G. Flower and fruit abortion: Proximate causes and ultimate functions. Annu. Rev. Ecol. Syst. 1981, 12, 253–279. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Rossetto, G.; Maddau, L.; Vatrano, T.; Bregant, C. Diversity and pathogenicity of Botryosphaeriaceae and Phytophthora species associated with emerging olive diseases in Italy. Agriculture 2023, 13, 1575. [Google Scholar] [CrossRef]
- Sanna, F.; Mori, N.; Santoiemma, G.; Pozzebon, A.; Scaccini, D.; Marangoni, F.; Sella, L. Halyomorpha halys (Hemiptera: Pentatomidae) as the major contributor to early olive drop in northern Italy. J. Econ. Entomol. 2024, 117, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Kolainis, S.; Koletti, A.; Lykogianni, M.; Karamanou, D.; Gkizi, D.; Tjamos, S.E.; Paraskeuopoulos, A.; Aliferis, K.A. An integrated approach to improve plant protection against olive anthracnose caused by the Colletotrichum acutatum species complex. PLoS ONE 2020, 15, e0233916. [Google Scholar] [CrossRef] [PubMed]
- Staut, M.; Kovačič, G.; Ogrin, D. The spatial cognition of Mediterranean in Slovenia: (in)consistency between perception and physical definitions. Acta Geogr. Slov. 2007, 47, 105–131. [Google Scholar] [CrossRef]
- Urbanc, M. Perception of Land among Slovenians in the Context of Landscape Changes in Slovenian Istria [Istra]. Mitteilungen Osterr. Geogr. Ges. 2011, 153, 199–220. [Google Scholar] [CrossRef]
- Segnalini, M.; Nardone, A.; Bernabucci, U.; Vitali, A.; Ronchi, B.; Lacctera, N. Dynamics of the temperature-humidity index in the Mediterranean basin. Int. J. Biometeorol. 2011, 55, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, C.; Tubiello, F.N. Impacts of global climate change on Mediterranean agriculture: Current methodologies and future directions. An introductory essay. Mitig. Adapt. Strateg. Glob. Change 1997, 1, 219–232. [Google Scholar] [CrossRef]
- Podgornik, M.; Valenčič, V.; Bešter, E.; Butinar, B.; Fantinič, J.; Hladnik, T.; Kozlovič, G.; Volk, S.; Fičur, K.; Bučar-Miklavčič, M. Climate change is already taking its toll on olive growing. Olive Olive Grow. Assoc. Newsl. 2021, 22, 16–17. [Google Scholar]
- Repolusk, P. The Koper Hills. In Slovenia: Landscapes and People; Perko, D., Oražen Adamič, M., Eds.; Mladinska Knjiga: Ljubljana, Slovenia, 2001; pp. 268–280. [Google Scholar]
- Ogrin, D. Modern climate change in Slovenia. In Slovenia: A Geographical Overview; Orožen Adamič, M., Ed.; Association of the Geographical Societies of Slovenia: Ljubljana, Slovenia, 2004; p. 159. [Google Scholar]
- Podgornik, M.; Pintar, M.; Bučar Miklavčič, M.; Bandelj, D. Different quantities of applied water on Olea europaea L. cultivated under humid conditions. J. Irrig. Drain. Eng. 2017, 143, 9. [Google Scholar] [CrossRef]
- ARSO, 2023a. Climate Data. Available online: https://meteo.arso.gov.si/met/sl/climate/tables/statistike_1950_2020/letalisce_portoroz// (accessed on 20 May 2023).
- Bandelj, D.; Jakše, J.; Javornik, B. DNA fingerprinting of olive varieties by microsatellite markers. Food Technol. Biotechnol. 2002, 40, 185–190. [Google Scholar]
- Parmegiani, P.; Scarbolo, E. Olive trees and oil in the Friuli Venezia Giulia Region: Historical notes, production, characterization, specificity. In Proceedings of the Perspectives for Horticultural and Viticulture in the Alpine Region in the Third Millennium, Udine, Italy, 8–10 November 2000; pp. 604–606. [Google Scholar]
- ARSO, 2023b. Archive Data. Available online: https://meteo.arso.gov.si/met/sl/archive/ (accessed on 20 May 2023).
- Beguería, S.; Vicente-Serrano, S.M. SPEI: Calculation of the Standardized Precipitation-Evapotranspiration Index. R package version 1.8.1, 2023. Available online: https://CRAN.R-project.org/package=SPEI (accessed on 20 April 2023).
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J. Climate 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Sanz-Cortés, F.; Martinez-Calvo, J.; Badenes, M.L.; Bleiholder, H.; Hack, H.; Llácer, G.; Meier, U. Phenological growth stages of olive trees (Olea europaea). Ann. Appl. Biol. 2005, 146, 73–80. [Google Scholar] [CrossRef]
- Rojo, J.; Orlandi, F.; Ben Dhiab, A.; Lara, B.; Picornell, A.; Oteros, J.; Msallem, M.; Fornaciari, M.; Pérez-Badia, R. Estimation of Chilling and Heat Accumulation Periods Based on the Timing of Olive Pollination. Forests 2020, 11, 835. [Google Scholar] [CrossRef]
- Luedeling, E.; Kunz, A.; Blanke, M.M. Identification of Chilling and Heat Requirements of Cherry Trees—A Statistical Approach. Int. J. Biometeorol. 2013, 57, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Richardson, E.A.; Seeley, S.D.; Walker, D.R. A model for estimating the completion of rest for Redhaven and Elberta peach trees. HortScience 1974, 9, 331–332. [Google Scholar] [CrossRef]
- Weinberger, J.H. Chilling Requirements of Peach Varieties. In Proceedings of the American Society for Horticultural Science; American Society for Horticultural Science: Alexandria, VA, USA, 1950; Volume 56, pp. 122–128. [Google Scholar]
- Gabaldón-Leal, C.; Ruiz-Ramos, M.; de la Rosa, R.; León, L.; Belaj, A.; Rodríguez, A.; Santos, C.; Lorite, I.J. Impact of Changes in Mean and Extreme Temperatures Caused by Climate Change on Olive Flowering in Southern Spain. Int. J. Climatol. 2017, 37, 940–957. [Google Scholar] [CrossRef]
- Erez, A.; Fishman, S.; Linsley-Noakes, G.C.; Allan, P. The dynamic model for rest completion in peach buds. Acta Hortic. 1990, 276, 165–174. [Google Scholar] [CrossRef]
- Fishman, S.; Erez, A.; Couvillon, G.A. The Temperature Dependence of Dormancy Breaking in Plants: Mathematical Analysis of a Two-Step Model Involving a Cooperative Transition. J. Theor. Biol. 1987, 124, 473–483. [Google Scholar] [CrossRef]
- Anderson, J.L.; Richardson, E.A.; Kesner, C.D. Validation of Chill Unit and Flower Bud Phenology Models for “Montmorency” Sour Cherry. Acta Hortic. 1986, 84, 71–78. [Google Scholar] [CrossRef]
- Darbyshire, R.; Webb, L.; Goodwin, I.; Barlow, S. Winter Chilling Trends for Deciduous Fruit Trees. Agric. For. Meteorol. 2011, 151, 1074–1085. [Google Scholar] [CrossRef]
- Ahani, A.; Mousavi, S. FAO56 1.0; November 2023. Available online: https://cran.r-project.org/package=FAO56 (accessed on 20 November 2023).
- Žnidaršič, Z.; Gregorič, G.; Sušnik, A.; Pogačar, T. Frost risk assessment in Slovenia in the period of 1981–2020. Atmosphere 2023, 14, 683. [Google Scholar] [CrossRef]
- Petruccelli, R.; Bartolini, G.; Ganino, T.; Zelasco, S.; Lombardo, L.; Perri, E.; Durante, M.; Bernardi, R. Cold stress, freezing adaptation, varietal susceptibility of Olea europaea L.: A review. Plants 2022, 11, 1367. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Rowland, L.J.; Tanino, K. Induction and release of bud dormancy in woody perennials: A science comes of age. HortScience 2003, 38, 911. [Google Scholar] [CrossRef]
- van der Schoot, C.; Rinne, P.L.H. Dormancy cycling at the shoot apical meristem: Transitioning between self-organization and self-arrest. Plant Sci. 2011, 180, 120–131. [Google Scholar] [CrossRef] [PubMed]
- López-Bernal, A.; García-Tejera, O.; Testi, L.; Orgaz, F.; Villalobos, F.J. Studying and modelling winter dormancy in olive trees. Agric. For. Meteorol. 2020, 280, 107776. [Google Scholar] [CrossRef]
- Lavee, S.; Rallo, L.; Rapoport, H.F.; Troncoso, A. The floral biology of the olive: Effect of flower number, type and distribution on fruit set. Sci. Hortic. 1996, 66, 149–158. [Google Scholar] [CrossRef]
- Lenz, A.; Hoch, G.; Körner, C.; Vitasse, Y. Convergence of leaf-out timing towards minimum risk of freezing damage in temperate trees. Funct. Ecol. 2016, 30, 1480–1490. [Google Scholar] [CrossRef]
- Coville, F.V. The influence of cold in stimulating the growth of plants. Proc. Natl. Acad. Sci. USA 1920, 6, 434–435. [Google Scholar] [CrossRef] [PubMed]
- Delpierre, N.; Vitasse, Y.; Chuine, I.; Guillemot, J.; Bazot, S.; Rutishauser, T.; Rathgeber, C.B.K. Temperate and boreal forest tree phenology: From organ-scale processes to terrestrial ecosystem models. Ann. For. Sci. 2016, 73, 5–25. [Google Scholar] [CrossRef]
- Ogrin, D. Olive growing in Slovenian Istria and climatic limitations to its development. Morav. Geogr. Rep. 2007, 15, 34–40. [Google Scholar]
- Ogrin, D. On the Olive Tree Frost in Slovenian Istria in December 1996. Disaster 1997, 11, 34–38. [Google Scholar]
- Vrhovnik, I.; Vesel, V.; Podgornik, M. Characteristics of the 2018 Olive Tree Frost Compared to Previous Frost Events. Olive Newsl. Olive Grow. Assoc. 2018, 21, 23–25. [Google Scholar]
- Sanzani, S.M.; Schena, L.; Nigro, F.; Sergeeva, V.; Ippolito, A.; Salerno, M.G. Abiotic diseases of olive. J. Plant Pathol. 2012, 294, 469–491. [Google Scholar]
- Sibbett, G.S.; Osgood, J. Site selection and preparation, tree spacing and design, planting, and initial training. In Olive Production Manual; Sibbett, G.S., Ferguson, L., Coviello, J.L., Lindstrand, M., Eds.; University of California, Agriculture and Natural Resources: Oakland, CA, USA, 2005; pp. 49–54. [Google Scholar]
- Sedmak, D. Slovenian Littoral Municipalities—Koper, Izola, Piran: Investment Plan for Olive Orchards 1986–1990; SOZD Timav, DO Droga Portorož; TOK Agraria: Koper, Slovenia, 1985; p. 37. [Google Scholar]
- Toreti, A.; Masante, D.; Acosta Navarro, J.; Bavera, D.; Cammalleri, C.; De Jager, A.; Di Ciollo, C.; Hrast Essenfelder, A.; Maetens, W.; Magni, D.; et al. Drought in Europe July 2022; EUR 31147 EN; Publications Office of the European Union: Luxembourg, 2022; ISBN 978-92-76-54953-6. [Google Scholar] [CrossRef]
- Gambella, F.; Bianchini, L.; Cecchini, M.; Egidi, G.; Ferrara, A.; Salvati, L.; Colantoni, A.; Morea, D. Moving toward the north? The spatial shift of olive groves in Italy. Agric. Econ. 2021, 67, 129–135. [Google Scholar] [CrossRef]
- Zupanc, V.; Pintar, M.; Podgornik, M. Olive production on cultivated terraces in northern Istria. Ann. Ser. Hist. Sociol. 2018, 28, 795–810. [Google Scholar] [CrossRef]
- Bertalanič, R.; Dolinar, M.; Honzak, L.; Lokošek, N.; Medved, A.; Vertačnik, G.; Vlahović, Ž. Climate Change Projections for Slovenia over the 21st Century; Ministry of the Environment and Spatial Planning, Slovenian Environment Agency: Ljubljana, Slovenia, 2019.
- Zeileis, A.; Leisch, F.; Hornik, K.; Kleiber, C. strucchange: An R Package for Testing for Structural Change in Linear Regression Models. J. Stat. Softw. 2002, 7, 1–38. [Google Scholar] [CrossRef]
- Zeileis, A.; Kleiber, C.; Krämer, W.; Hornik, K. Testing and Dating of Structural Changes in Practice. Comput. Stat. Data Anal. 2003, 44, 109–123. [Google Scholar] [CrossRef]
- Pohlert, T. trend: Non-Parametric Trend Tests and Change-Point Detection. R Package Version 1.1.6, 2023. Available online: https://CRAN.R-project.org/package=trend (accessed on 25 May 2025).
- Pareja-Bonilla, D.; Arista, M.; Morellato, L.P.C.; Ortiz, P.L. Better Soon than Never: Climate Change Induces Strong Phenological Reassembly in the Flowering of a Mediterranean Shrub Community. Ann. Bot. 2025, 135, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Fitter, A.H.; Fitter, R.S. Rapid Changes in Flowering Time in British Plants. Science 2002, 296, 1689–1691. [Google Scholar] [CrossRef] [PubMed]
- Büntgen, U.; Piermattei, A.; Krusic, P.J.; Esper, J.; Sparks, T.; Crivellaro, A. Plants in the UK flower a month earlier under recent warming. Proc. R. Soc. B 2022, 289, 20212456. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.A.; Ahmed, M.; Hassan, F.; Afzal, O.; Mehmood, M.Z.; Qadir, G.; Asif, M.; Komal, S.; Hussain, T. Impact of temperature fluctuations on plant morphological and physiological traits. In Building Climate Resilience in Agriculture: Theory, Practice, and Future Perspective; Jatoi, W.N., Mubeen, M., Ahmad, A., Cheema, M.A., Lin, Z., Hashmi, M.Z., Eds.; Springer: Cham, Switzerland, 2022; pp. 25–52. [Google Scholar] [CrossRef]
- Bartolini, S.; Viti, R. Olive Floral Biology and Climatic Elements: Twenty-Eight Years of Observations. Acta Hortic. 2018, 1229, 45. [Google Scholar] [CrossRef]
- Fornaciari, M.; Orlandi, F.; Tedeschini, E. Long term analysis on Olive flowering and climatic relationships in central Italy. Eur. J. Agron. 2025, 162, 127435. [Google Scholar] [CrossRef]
- Oteros, J.; García-Mozo, H.; Vázquez, L.; Mestre, A.; Domínguez-Vilches, E.; Galán, C. Modelling olive phenological response to weather and topography. Agric. Ecosyst. Environ. 2013, 179, 62–68. [Google Scholar] [CrossRef]
- Maniriho, F. Flower differentiation and fruiting dynamics in olive trees (Olea europaea): Eco-physiological analysis in the Mediterranean basin. Adv. Hortic. Sci. 2022, 36, 53–62. [Google Scholar] [CrossRef]
- Horton, D.E.; Johnson, N.C.; Singh, D.; Swain, D.L.; Rajaratnam, B.; Diffenbaugh, N.S. Contribution of Changes in Atmospheric Circulation Patterns to Extreme Temperature Trends. Nature 2015, 522, 465–469. [Google Scholar] [CrossRef] [PubMed]
- El Yaacoubi, A.; Oukabli, A.; Legave, J.-M.; Ainane, T.; Mouhajir, A.; Zouhair, R.; Hafidi, M. Response of almond flowering and dormancy to Mediterranean temperature conditions in the context of adaptation to climate variations. Sci. Hortic. 2019, 257, 108687. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Ge, Q.; Dai, J. The interactive effects of chilling, photoperiod, and forcing temperature on flowering phenology of temperate woody plants. Front. Plant Sci. 2020, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Omran, M.A.A.; Mofeed, A.S.; Mohamed, H.M. Effect of chilling requirements on flowering and productivity of some native and foreign olive cultivars under the Egyptian conditions. Middle East J. Appl. Sci. 2019, 9, 421–433. [Google Scholar]
- De Melo-Abreu, J.P.; Barranco, D.; Cordeiro, A.M.; Tous, J.; Rogadoe, B.M.; Villalobos, F.J. Modelling olive flowering date using chilling for dormancy release and thermal time. Agric. For. Meteorol. 2004, 125, 117–127. [Google Scholar] [CrossRef]
- Belaj, A.; de la Rosa, R.; León, L.; Gabaldón-Leal, C.; Santos, C.; Porras, R.; de la Cruz-Blanco, M.; Lorite, I.J. Phenological diversity in a world olive germplasm bank: Potential use for breeding programs and climate change studies. Span. J. Agric. Res. 2020, 18, e0701. [Google Scholar] [CrossRef]
- Aybar, V.E.; De Melo-Abreu, J.P.; Searles, P.S.; Matias, A.C.; Del Rio, C.; Caballero, J.M.; Rousseaux, M.C. Evaluation of olive flowering at low latitude sites in Argentina using a chilling requirement model. Span. J. Agric. Res. 2015, 13, e0901. [Google Scholar] [CrossRef]
- Yu, H.; Luedeling, E.; Xu, J. Winter and spring warming result in delayed spring phenology on the Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2010, 107, 22151–22156. [Google Scholar] [CrossRef] [PubMed]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podgornik, M.; Fantinič, J.; Pogačar, T.; Zupanc, V. Analysis of Olive Tree Flowering Behavior Based on Thermal Requirements: A Case Study from the Northern Mediterranean Region. Climate 2025, 13, 156. https://doi.org/10.3390/cli13080156
Podgornik M, Fantinič J, Pogačar T, Zupanc V. Analysis of Olive Tree Flowering Behavior Based on Thermal Requirements: A Case Study from the Northern Mediterranean Region. Climate. 2025; 13(8):156. https://doi.org/10.3390/cli13080156
Chicago/Turabian StylePodgornik, Maja, Jakob Fantinič, Tjaša Pogačar, and Vesna Zupanc. 2025. "Analysis of Olive Tree Flowering Behavior Based on Thermal Requirements: A Case Study from the Northern Mediterranean Region" Climate 13, no. 8: 156. https://doi.org/10.3390/cli13080156
APA StylePodgornik, M., Fantinič, J., Pogačar, T., & Zupanc, V. (2025). Analysis of Olive Tree Flowering Behavior Based on Thermal Requirements: A Case Study from the Northern Mediterranean Region. Climate, 13(8), 156. https://doi.org/10.3390/cli13080156







