Climate Change and Arbovirus: A Review and Bibliometric Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Database
2.2. Search Strategy
2.3. Validation
2.4. Data Analysis
3. Results
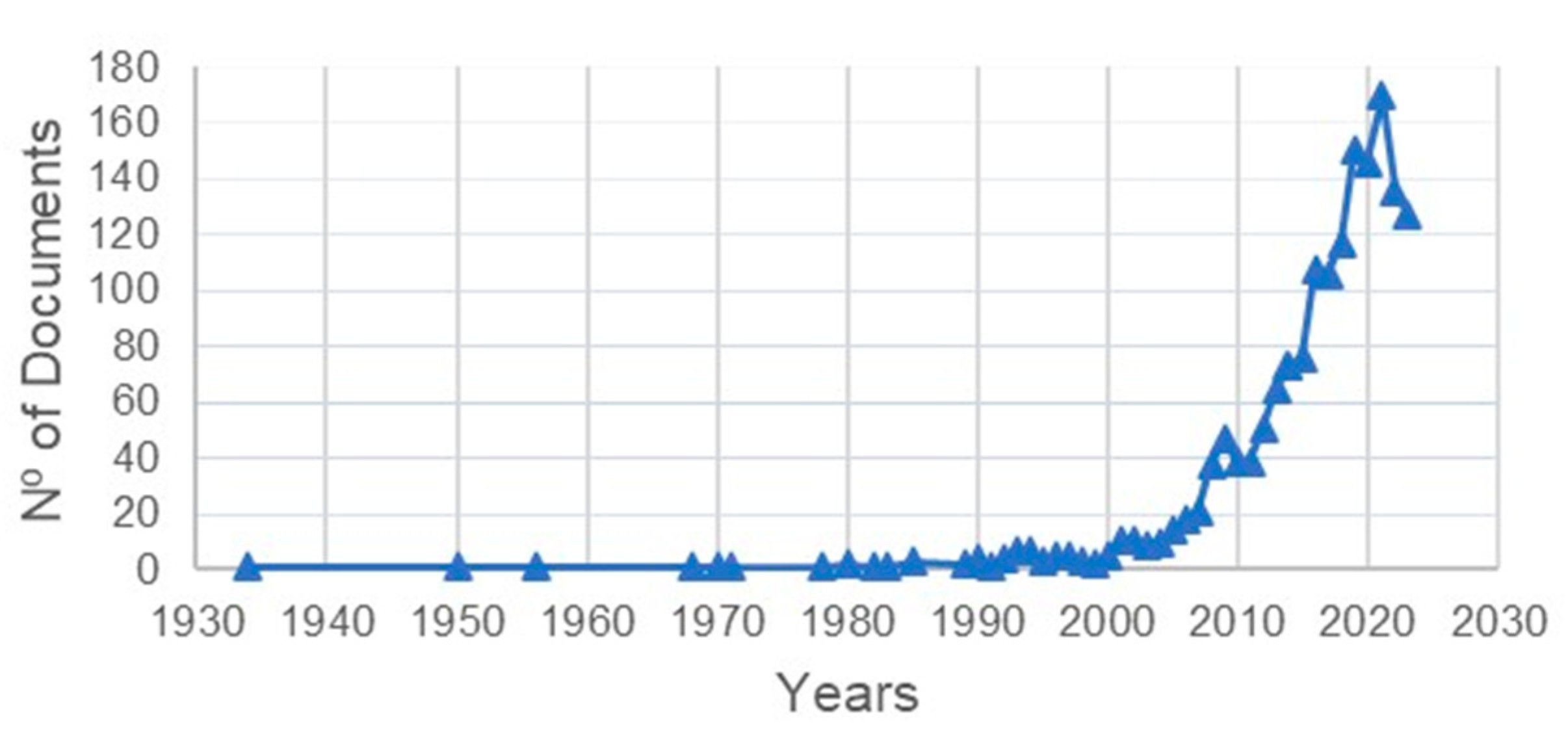
3.1. Volume, Growth of Publications and Types
3.2. Number of Citations
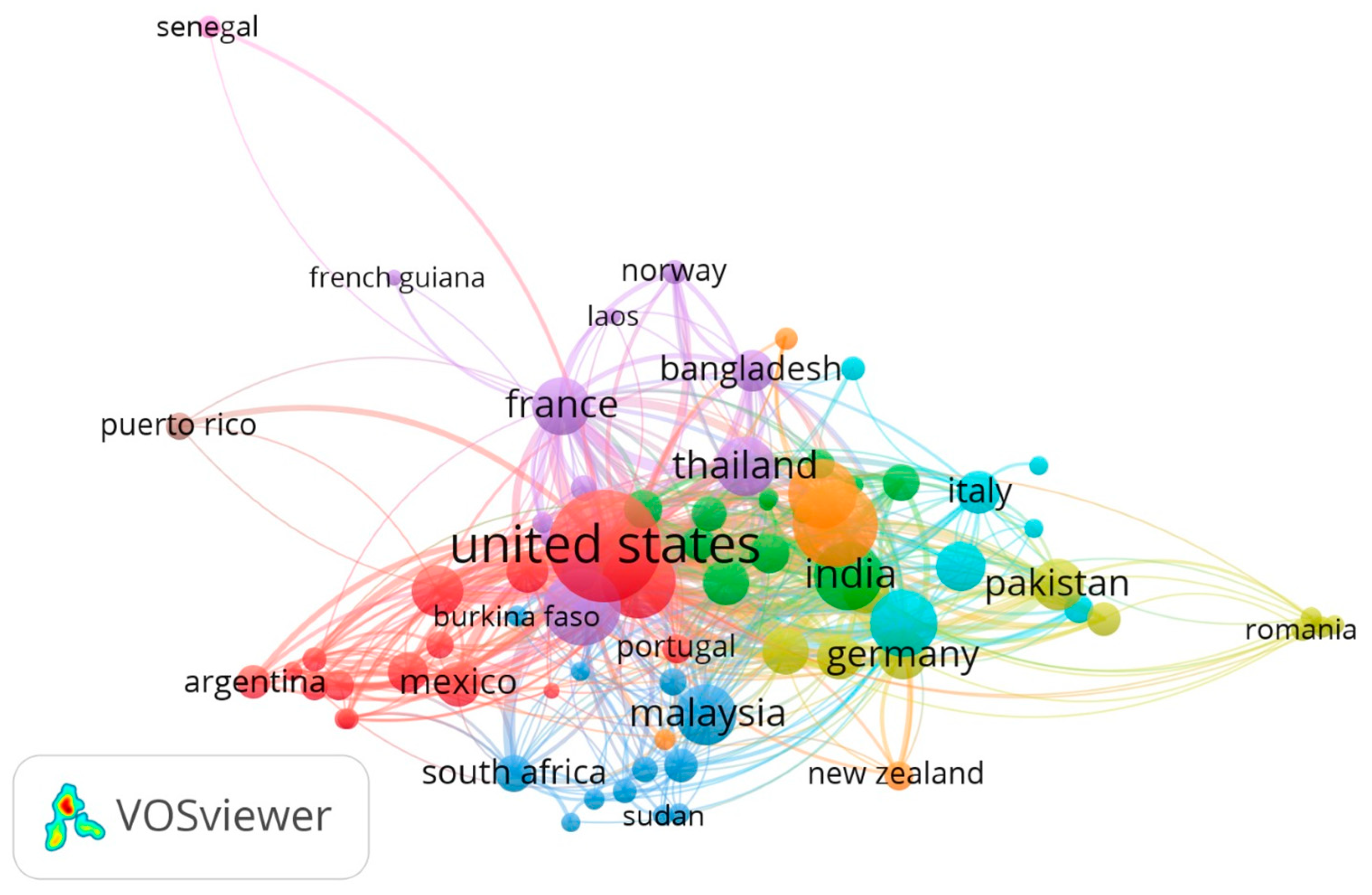
3.3. Country, Author and Publication Journal
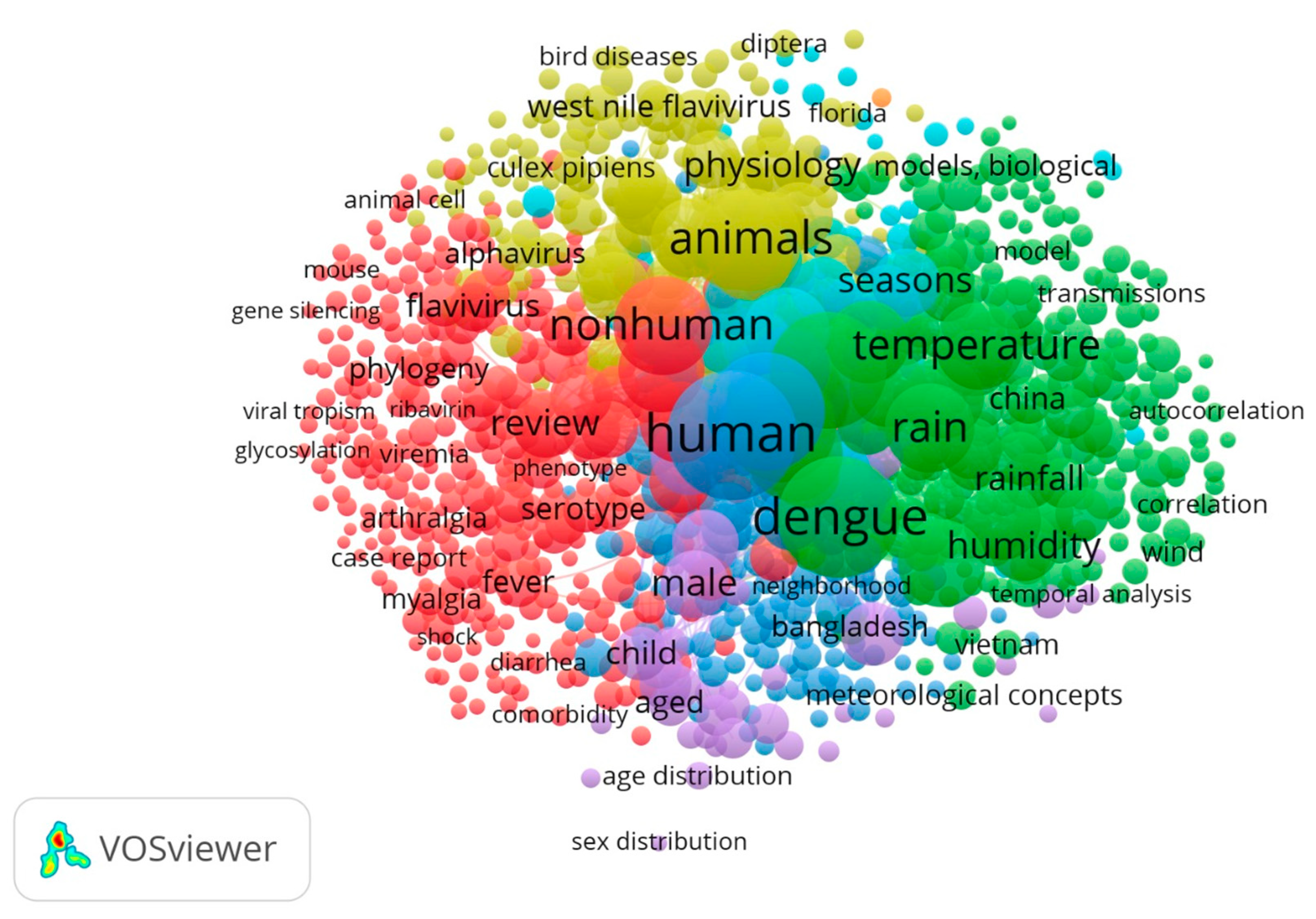
3.4. Keywords
4. Discussion
4.1. Environmental Movements and Arbovirus Publications
4.2. Chronology of Research into Arboviruses and Climate
4.3. Expansion of Arboviruses to New Ecological Niches
4.4. Global Academic Production
4.5. Dengue, Zika and Chikungunya
4.6. Other Arboviruses: West Nile Fever, Japanese Encephalitis, Yellow Fever and Ross River Fever
4.7. Prevention, Control and Surveillance
5. Limitations
6. Considerations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitehorn, J.; Yacoub, S. Global warming and arboviral infections. Clin. Med. 2019, 19, 149–152. [Google Scholar] [CrossRef]
- Celik, S. The Effects of Climate Change on Human Behaviors. In Environment, Climate, Plant and Vegetation Growth; Fahad, S., Hasanuzzaman, M., Alam, M., Ullah, H., Saeed, M., Ali Khan, I., Adnan, M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 577–589. [Google Scholar]
- Samset, B.H.; Fuglestvedt, J.S.; Lund, M.T. Delayed emergence of a global temperature response after emission mitigation. Nat. Commun. 2020, 11, 3261. [Google Scholar] [CrossRef]
- Climate Change 2022: Impacts, Adaptation and Vulnerability. 2022. Available online: www.ipcc.ch/report/ar6/wg2/ (accessed on 10 October 2023).
- van Daalen, K.R.; Romanello, M.; Rocklöv, J.; Semenza, J.C.; Tonne, C.; Markandya, A.; Dasandi, N.; Jankin, S.; Achebak, H.; Ballester, J.; et al. The 2022 Europe report of the Lancet Countdown on health and climate change: Towards a climate resilient future. Lancet Public Health 2022, 7, e942–e965. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Climatic changes and their role in emergence and re-emergence of diseases. Environ. Sci. Pollut. Res. 2020, 27, 22336–22352. [Google Scholar] [CrossRef]
- Tidman, R.; Abela-Ridder, B.; de Castañeda, R.R. The impact of climate change on neglected tropical diseases: A systematic review. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Githeko, A.K.; Lindsay, S.W.; Confalonieri, U.E.; Patz, J.A. Climate change and vector-borne diseases: A regional analysis. Bull. World Health Organ. 2000, 78, 1136–1147. Available online: https://www.scielosp.org/pdf/bwho/v78n9/v78n9a09.pdf (accessed on 10 October 2023).
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Young, P.R. Arboviruses: A Family on the Move. In Dengue and Zika: Control and Antiviral Treatment Strategies. Advances in Experimental Medicine and Biology; Hilgenfeld, R., Vasudevan, S., Eds.; Springer: Singapore, 2018; Volume 1062. [Google Scholar] [CrossRef]
- Carreto, C.; Gutiérrez-Romero, R.; Rodríguez, T. Climate-driven mosquito-borne viral suitability index: Measuring risk transmission of dengue, chikungunya and Zika in Mexico. Int. J. Health Geogr. 2022, 21, 15. [Google Scholar] [CrossRef]
- Mordecai, E.A.; Caldwell, J.M.; Grossman, M.K.; Lippi, C.A.; Johnson, L.R.; Neira, M.; Rohr, J.R.; Ryan, S.J.; Savage, V.; Shocket, M.S.; et al. Thermal biology of mosquito-borne disease. Ecol. Lett. 2019, 22, 1690–1708. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Laboratory Testing for Zika Virus and Dengue Virus Infections: Interim Guidance, 14 July 2022; WHO/ZIKV_DENV/LAB/2022.1; World Health Organization: Geneva, Switzerland, 2022; Available online: https://apps.who.int/iris/handle/10665/359857 (accessed on 10 October 2023).
- Webb, C.E.; Doggett, S.L. Exotic mosquito threats require strategic surveillance and response planning. Public Health Res. Pract. 2016, 26, 2651656. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.L.B.; de Albuquerque, M.; de Brito, C.A.A.; Franca, R.F.D.; Moreira, A.J.P.; Machado, M.I.D.; Melo, R.P.; Medialdea-Carrera, R.; Mesquita, S.D.; Santos, M.L.; et al. Neurological disease in adults with Zika and chikungunya virus infection in Northeast Brazil: A prospective observational study. Lancet Neurol. 2020, 19, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Weetman, D.; Kamgang, B.; Badolo, A.; Moyes, C.L.; Shearer, F.M.; Coulibaly, M.; Pinto, J.; Lambrechts, L.; McCall, P.J. Aedes Mosquitoes and Aedes-Borne Arboviruses in Africa: Current and Future Threats. Int. J. Environ. Res. Public Health 2018, 15, 220. [Google Scholar] [CrossRef] [PubMed]
- Bartlow, A.W.; Manore, C.; Xu, C.; Kaufeld, K.A.; Valle, S.D.; Ziemann, A.; Fairchild, G.; Fair, J.M. Forecasting zoonotic infectious disease response to climate change: Mosquito vectors and a changing environment. Vet. Sci. 2019, 6, 40. [Google Scholar] [CrossRef]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of recent and future climate change on vector-borne diseases. Ann. N. Y. Acad. Sci. 2019, 1436, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Sweileh, W.M. Bibliometric analysis of peer-reviewed literature on climate change and human health with an emphasis on infectious diseases. Glob. Health 2020, 16, 44. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Leal Filho, W.; Ternova, L.; Parasnis, S.A.; Kovaleva, M.; Nagy, G.J. Climate change and zoonoses: A review of concepts, definitionsand bibliometrics. Int. J. Environ. Res. Public Health 2022, 19, 893. [Google Scholar] [CrossRef]
- Scopus. ScopusContentCoverageGuideWEB.pdf. 2023. Available online: https://assets.ctfassets.net/o78em1y1w4i4/EX1iy8VxBeQKf8aN2XzOp/c36f79db25484cb38a5972ad9a5472ec/Scopus_ContentCoverage_Guide_WEB.pdf (accessed on 10 October 2023).
- CDC. Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID); Division of Vector-Borne Diseases (DVBD). 2022. Available online: https://search.cdc.gov/search/?query=vector%20borne%20diseases&dpage=1 (accessed on 10 October 2023).
- Zyoud, S.H. Dengue research: A bibliometric analysis of worldwide and Arab publications during 1872–2015. Virol. J. 2016, 13, 78. [Google Scholar] [CrossRef]
- JCR. Journal Citation Reports Help Center. 2022. Available online: https://journalcitationreports.zendesk.com/hc/en-gb/articles/28350221001745-2022 (accessed on 10 October 2023).
- Quantum GIS. Internet. 2023. Available online: http://qgis.osgeo.org/ (accessed on 10 October 2023).
- Zupic, I.; Cater, T. Bibliometric Methods in Management and Organization. Organ. Res. Methods 2015, 18, 429–472. [Google Scholar] [CrossRef]
- Raboaca, M.S.; Bizon, N.; Grosu, O.V. Energy management strategies for hybrid electric vehicles-vosviwer bibliometric analysis. In Proceedings of the 2020 12th International Conference on Electronics, Computers and Artificial Intelligence (ECAI), Bucharest, Romania, 25–27 June 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–8. [Google Scholar]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Murray, N.E.A.; Quam, M.B.; Wilder-Smith, A. Epidemiology of dengue: Past, present and future prospects. Clin. Epidemiol. 2013, 5, 299–309. [Google Scholar] [CrossRef]
- Hales, S.; De Wet, N.; Maindonald, J.; Woodward, A. Potential effect of population and climate changes on global distribution of dengue fever: An empirical model. Lancet 2002, 360, 830–834. [Google Scholar] [CrossRef]
- Kyle, J.L.; Harris, E. Global spread and persistence of dengue. Annu. Rev. Microbiol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, M. Designing the United Nations Environment Programme: A story of compromise and confrontation. Int. Environ. Agreem. Politics Law. Econ. 2007, 7, 337–361. [Google Scholar] [CrossRef]
- Bodansky, D. The history of the global climate change regime. In International Relations and Global Climate Change, 1st ed.; Press Cambridge: London, UK, 2001; pp. 34–40. [Google Scholar]
- Gupta, J. A history of international climate change policy. Wiley Interdiscip. Rev. Clim. Change 2010, 1, 636–653. [Google Scholar] [CrossRef]
- McMichael, A.J.; Neira, M.; Heymann, D.L. World Health Assembly 2008: Climate change and health. Lancet 2008, 371, 1895–1896. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barret, K. IPCC, 2023: Climate Change 2023: Synthesis Report, Summary for Policymakers. In Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 8–40. [Google Scholar]
- Ravenel, M.P. Our correspondent speaks of the American Public Health Association Meeting at Pasadena—The Poliomyelitis Situation—The Drought and Typhoid—Dengue Fever in Florida—And Medical Insurance. Public Health 1934, 48, 77–78. [Google Scholar] [CrossRef]
- Kumm, H.W. Seasonal variations in rainfall: Prevalence of Haemagogus and incidence of jungle yellow fever in Brazil and Colombia. Trans. R. Soc. Trop. Med. Hyg. 1950, 43, 673–682. [Google Scholar] [CrossRef]
- Doherty, R.L.; Standfast, H.A.; Wetters, E.J.; Whitehead, R.H.; Barrow, G.J.; Gorman, B.M. Virus isolation and serological studies of arthropodborne virus infections in a high rainfall area of North Queensland. Trans. R. Soc. Trop. Med. Hyg. 1968, 62, 862–867. [Google Scholar] [CrossRef]
- Standfast, H.A.; Barrow, G.J. Studies of the epidemiology of arthropod-borne virus infections at Mitchell River Mission, Cape York Peninsula, North Queensland. Trans. R. Soc. Trop. Med. Hyg. 1968, 62, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Grady, G.F.; Maxfield, H.K.; Hildreth, S.W.; Timperi, R.J.; Gilfillan, R.F.; Rosenau, B.J.; Francy, D.B.; Calisher, C.H.; Marcus, L.C.; Madoff, M.A. Eastern equine encephalitis in massachusetts, 1957-1976: A prospective study centered upon analyses of mosquitoes. Am. J. Epidemiol. 1978, 107, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Li, C.F.; Lim, T.W.; Han, L.L.; Fang, R. Rainfall, abundance of Aedes aegypti and dengue infection in Selangor, Malaysia. S. Asian J. Trop. Med. Public Health 1985, 16, 560–568. [Google Scholar] [PubMed]
- Doggett, S.L.; Russell, R.C.; Clancy, J.; Haniotis, J.; Cloonan, M.J. Barmah Forest virus epidemic on the south coast of New South Wales, Australia, 1994-1995: Viruses, vectors, human casesand environmental factors. J. Med. Entomol. 1999, 36, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Patz, J.A.; Balbus, J.M. Methods for assessing public health vulnerability to global climate change. Clim. Res. 1996, 6, 113–125. [Google Scholar] [CrossRef]
- Patz, J.A.; Martens, W.J.M.; Focks, D.A.; Jetten, T.H. Dengue fever epidemic potential as projected by general circulation models of global climate change. Environ. Health Perspect. 1998, 106, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.C. Mosquito-borne arboviruses in Australia: The current scene and implications of climate change for human health. Int. J. Parasitol. 1998, 28, 9673874. [Google Scholar] [CrossRef] [PubMed]
- Hubálek, Z.; Halouzka, J. West Nile fever-A reemerging mosquito-borne viral disease in Europe. Emerg. Infect. Dis. 1999, 5, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Cazelles, B.; Chavez, M.; McMichael, A.J.; Hales, S. Nonstationary influence of El Nino on the synchronous dengue epidemics in Thailand. PLoS Med. 2005, 2, e106. [Google Scholar] [CrossRef]
- Kelly-Hope, L.A.; Purdie, D.M.; Kay, B.H. El Nino southern oscillation and Ross River virus outbreaks in Australia. Vector-Borne Zoonotic Dis. 2004, 4, 210–213. [Google Scholar] [CrossRef]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef]
- Bonnin, L.; Tran, A.; Herbreteau, V.; Marcombe, S.; Boyer, S.; Mangeas, M.; Menkes, C. Predicting the Effects of Climate Change on Dengue Vector Densities in Southeast Asia through Process-Based Modeling. Environ. Health Perspect. 2022, 130, 127002. [Google Scholar] [CrossRef]
- Kamal, M.; Kenawy, M.A.; Rady, M.H.; Khaled, A.S.; Samy, A.M. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS ONE 2018, 13, e0210122. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Gasparrini, A.; Van Meerbeeck, C.J.; Lippi, C.A.; Mahon, R.; Trotman, A.R.; Rollock, L.; Hinds, A.Q.J.; Ryan, S.J.; Stewart-Ibarra, A.M. Nonlinear and delayed impacts of climate on dengue risk in Barbados: A modelling study. PLoS Med. 2018, 15, e1002613. [Google Scholar] [CrossRef]
- Outammassine, A.; Zouhair, S.; Loqman, S. Global potential distribution of three underappreciated arboviruses vectors ( Aedes japonicus, Aedes vexans and Aedes vittatus) under current and future climate conditions. Transbound. Emerg. Dis. 2022, 69, E1160–E1171. [Google Scholar] [CrossRef] [PubMed]
- Findlater, A.; Bogoch, I.I. Human mobility and the global spread of infectious diseases: A focus on air travel. Trends Parasitol. 2018, 34, 772–783. [Google Scholar] [CrossRef]
- Medlock, J.M.; Hansford, K.M.; Schaffner, F.; Versteirt, V.; Hendrickx, G.; Zeller, H.; Bortel, W.V. A review of the invasive mosquitoes in Europe: Ecology, public health risksand control options. Vector-Borne Zoonotic Dis. 2012, 12, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Plowright, R.K.; Reaser, J.K.; Locke, H.; Woodley, S.J.; Patz, J.A.; Becker, D.J.; Oppler, G.; Hudson, P.J.; Tabor, G.M. Land use-induced spillover: A call to action to safeguard environmental, animaland human health. Lancet Planet. Health 2021, 5, e237–e245. [Google Scholar] [CrossRef]
- Patz, J.A.; Olson, S.H.; Uejio, C.K.; Gibbs, H.K. Disease Emergence from Global Climate and Land Use Change. Med. Clin. N. Am. 2008, 92, 1473–1491. [Google Scholar] [CrossRef]
- Bruinsma, J. World Agriculture: Towards 2015/2030: An FAO Study; Routledge: London, UK, 2017. [Google Scholar] [CrossRef]
- Powell, J.R.; Tabachnick, W.J. History of domestication and spread of Aedes aegypti-a review. Mem. Inst. Oswaldo Cruz 2013, 108, 11–17. [Google Scholar] [CrossRef]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.M.; Pfeiffer, M.; Steffens, O.; Schneider, F.; Gerger, V.; Phuyal, P.; Braun, M.; Magdeburg, A.; Ahrens, B.; Groneberg, D.A.; et al. The ecophysiological plasticity of Aedes aegypti and Aedes albopictus concerning overwintering in cooler ecoregions is driven by local climate and acclimation capacity. Sci. Total Environ. 2021, 778, 146128. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Hansford, K.M.; Vaux, A.G.C.; Cull, B.; Gillingham, E.; Leach, S. Assessment of the public health threats posed by vector-borne disease in the United Kingdom (UK). Int. J. Environ. Res. Public Health 2018, 15, 2145. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.; Scheday, S.; Boenecke, J.; Gogoi, A.; Maharaj, A.; Korovou, S. Climate Change, Health and Mosquito-Borne Diseases: Trends and Implications to the Pacific Region. Int. J. Environ. Res. Public Health 2019, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, C.; Bi, P.; Vardoulakis, S.; Huang, C. Local actions to health risks of heatwaves and dengue fever under climate change: Strategies and barriers among primary healthcare professionals in southern China. Environ. Res. 2020, 187, 109688. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Siu, E.; Chuang, K.-Y. A bibliometric analysis of dengue-related publications in the Science Citation Index Expanded. Future Virol. 2016, 11, 631–648. [Google Scholar] [CrossRef]
- Li, C.; Ojeda-Thies, C.; Renz, N.; Margaryan, D.; Perka, C.; Trampuz, A. The global state of clinical research and trends in periprosthetic joint infection: A bibliometric analysis. Int. J. Infect. Dis. 2020, 96, 696–709. [Google Scholar] [CrossRef]
- Mota, F.B.; Galina, A.C.; da Silva, R.M. Mapping the dengue scientific landscape worldwide: A bibliometric and network analysis. Mem. Inst. Oswaldo Cruz 2017, 112, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Fang, K.; Zheng, Y.; Wang, H.-l.; Wu, J. Global burden and trends of neglected tropical diseases from 1990 to 2019. J. Travel Med. 2022, 29, taac031. [Google Scholar] [CrossRef]
- Soni, S.; Gill, V.J.S.; Singh, J.; Chhabra, J.; Gill, G.J.S.; Bakshi, R. Dengue, Chikungunyaand Zika: The Causes and Threats of Emerging and Re-emerging Arboviral Diseases. Cureus 2023, 15, e41717. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Wang, G.; Xu, J.; Zhang, T. An ecological assessment of the potential pandemic threat of Dengue Virus in Zhejiang province of China. BMC Infect. Dis. 2023, 23, 473. [Google Scholar] [CrossRef] [PubMed]
- Sahu, M.C.; Samantaray, R.K.; Pal, A.; Pati, S. Recent advances on pathogenesis, diagnosis, prevention, immunological aspectsand vectors of dengue: A review. Asian Pac. J. Trop. Biomed. 2023, 13, 325–338. [Google Scholar] [CrossRef]
- Ogunlade, S.T.; Adekunle, A.I.; Meehan, M.T.; McBryde, E.S. Quantifying the impact of Wolbachia releases on dengue infection in Townsville, Australia. Sci. Rep. 2023, 13, 14932. [Google Scholar] [CrossRef] [PubMed]
- Bartholomeeusen, K.; Daniel, M.; LaBeaud, D.A.; Gasque, P.; Peeling, R.W.; Stephenson, K.E.; Ng, L.F.P.; Ariën, K.K. Chikungunya fever. Nat. Rev. Dis. Primers 2023, 9, 17. [Google Scholar] [CrossRef]
- Mayer, S.V.; Tesh, R.B.; Vasilakis, N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2017, 166, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Obadia, T.; Gutierrez-Bugallo, G.; Duong, V.; Nunez, A.I.; Fernandes, R.S.; Kamgang, B.; Hery, L.; Gomard, Y.; Abbo, S.R.; Jiolle, D.; et al. Zika vector competence data reveals risks of outbreaks: The contribution of the European ZIKAlliance project. Nat. Commun. 2022, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S.; Morens, D.M. Zika virus in the Americas—Yet another arbovirus threat. N. Engl. J. Med. 2016, 374, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Lessler, J.; Chaisson, L.H.; Kucirka, L.M.; Bi, Q.; Grantz, K.; Salje, H.; Carcelen, A.C.; Ott, C.T.; Sheffield, J.S.; Ferguson, N.M. Assessing the global threat from Zika virus. Science 2016, 353, aaf8160. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.-M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Puntasecca, C.J.; King, C.H.; LaBeaud, A.D. Measuring the global burden of chikungunya and Zika viruses: A systematic review. PLoS Neglected Trop. Dis. 2021, 15, e0009055. [Google Scholar] [CrossRef] [PubMed]
- Kucharz, E.J.; Cebula-Byrska, I. Chikungunya fever. Eur. J. Intern. Med. 2012, 23, 325–329. [Google Scholar] [CrossRef]
- Burt, F.J.; Rolph, M.S.; Rulli, N.E.; Mahalingam, S.; Heise, M.T. Chikungunya: A re-emerging virus. Lancet 2012, 379, 662–671. [Google Scholar] [CrossRef]
- Ronca, S.E.; Ruff, J.C.; Murray, K.O. A 20-year historical review of West Nile virus since its initial emergence in North America: Has West Nile virus become a neglected tropical disease? PLoS Neglected Trop. Dis. 2021, 15, e0009190. [Google Scholar] [CrossRef]
- Luethy, D. Eastern, Western, and Venezuelan Equine Encephalitis and West Nile Viruses: Clinical and Public Health Considerations. Vet. Clin. Equine Pract. 2023, 39, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Filgueira, L.; Lannes, N. Review of emerging Japanese encephalitis virus: New aspects and concepts about entry into the brain and inter-cellular spreading. Pathogens 2019, 8, 111. [Google Scholar] [CrossRef]
- Hsieh, J.T.; St. John, A.L. Japanese encephalitis virus and its mechanisms of neuroinvasion. PLoS Pathog. 2020, 16, e1008260. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, P.; Duong, V.; Boyer, S.; Burgess, G.; Williams, D.T.; Dussart, P.; Horwood, P.F. The ecology and evolution of Japanese encephalitis virus. Pathogens 2021, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.B.; Vrati, S.; Kalia, M. Pathobiology of Japanese encephalitis virus infection. Mol. Asp. Med. 2021, 81, 100994. [Google Scholar] [CrossRef]
- Auerswald, H.; Maquart, P.-O.; Chevalier, V.; Boyer, S. Mosquito vector competence for Japanese encephalitis virus. Viruses 2021, 13, 1154. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Wilson, M.E. Yellow fever control: Current epidemiology and vaccination strategies. Trop. Dis. Travel. Med. Vaccines 2020, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Giovanetti, M.; de Mendonça, M.C.L.; Fonseca, V.; Mares-Guia, M.A.; Fabri, A.; Xavier, J.; de Jesus, J.G.; Gräf, T.; dos Santos Rodrigues, C.D.; Dos Santos, C.C. Yellow fever virus reemergence and spread in Southeast Brazil, 2016–2019. J. Virol. 2019, 94. [Google Scholar] [CrossRef]
- Monath, T.P.; Vasconcelos, P.F. Yellow fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.I.O.; Sacchetto, L.; De Rezende, I.M.; Trindade, G.d.S.; LaBeaud, A.D.; De Thoisy, B.; Drumond, B.P. Recent sylvatic yellow fever virus transmission in Brazil: The news from an old disease. Virol. J. 2020, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Damtew, Y.T.; Tong, M.; Varghese, B.M.; Hansen, A.; Liu, J.; Dear, K.; Zhang, Y.; Morgan, G.; Driscoll, T.; Capon, T.; et al. Associations between temperature and Ross river virus infection: A systematic review and meta-analysis of epidemiological evidence. Acta Trop. 2022, 231, 106454. [Google Scholar] [CrossRef]
- Harley, D.; Sleigh, A.; Ritchie, S. Ross River virus transmission, infectionand disease: A cross-disciplinary review. Clin. Microbiol. Rev. 2001, 14, 909–932. [Google Scholar] [CrossRef]
- Koolhof, I.S.; Gibney, K.B.; Bettiol, S.; Charleston, M.; Wiethoelter, A.; Arnold, A.-L.; Campbell, P.T.; Neville, P.J.; Aung, P.; Shiga, T. The forecasting of dynamical Ross River virus outbreaks: Victoria, Australia. Epidemics 2020, 30, 100377. [Google Scholar] [CrossRef]
- Mostafavi, H.; Tharmarajah, K.; Vider, J.; West, N.P.; Freitas, J.R.; Cameron, B.; Foster, P.S.; Hueston, L.P.; Lloyd, A.R.; Mahalingam, S. Interleukin-17 contributes to Ross River virus-induced arthritis and myositis. PLoS Pathog. 2022, 18, e1010185. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Hurst, C.; Glass, K.; Harley, D.; Viennet, E. Spatial and temporal patterns of Ross River virus in Queensland, 2001–2020. Trop. Med. Infect. Dis. 2021, 6, 145. [Google Scholar] [CrossRef]
- Schaffner, F.; Bellini, R.; Petrić, D.; Scholte, E.-J.; Zeller, H.; Marrama Rakotoarivony, L. Development of guidelines for the surveillance of invasive mosquitoes in Europe. Parasites Vectors 2013, 6, 209. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Gubler, D.J.; Weaver, S.C.; Monath, T.P.; Heymann, D.L.; Scott, T.W. Epidemic arboviral diseases: Priorities for research and public health. Lancet Infect. Dis. 2017, 17, e101–e106. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, M.; Brainard, J.; Hooper, L.; Hunter, P.R. Public health interventions for Aedes control in the time of Zikavirus–A meta-review on effectiveness of vector control strategies. PLoS Neglected Trop. Dis. 2016, 10, e0005176. [Google Scholar] [CrossRef] [PubMed]
- Tsheten, T.; Gray, D.J.; Clements, A.C.; Wangdi, K. Epidemiology and challenges of dengue surveillance in the WHO South-East Asia Region. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 583–599. [Google Scholar] [CrossRef]
- Banu, A.N.; Balasubramanian, C. Myco-synthesis of silver nanoparticles using Beauveria bassiana against dengue vector, Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2014, 113, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.B.; Kasai, S.; Scott, J.G. Pyrethroid resistance in Aedes aegypti and Aedes albopictus: Important mosquito vectors of human diseases. Pestic. Biochem. Physiol. 2016, 133, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Morton Jr, J.C.; Ramirez, J.L.; Souza-Neto, J.A.; Dimopoulos, G. The entomopathogenic fungus Beauveria bassiana activate toll and JAK-STAT pathway-controlled effector genes and anti-dengue activity in Aedes aegypti. Insect Biochem. Mol. Biol. 2012, 42, 126–132. [Google Scholar] [CrossRef]
- Ogunlade, S.T.; Adekunle, A.I.; McBryde, E.S.; Meehan, M.T. Modelling the ecological dynamics of mosquito populations with multiple co-circulating Wolbachia strains. Sci. Rep. 2022, 12, 14. [Google Scholar] [CrossRef]
- Utarini, A.; Indriani, C.; Ahmad, R.A.; Tantowijoyo, W.; Arguni, E.; Ansari, M.R.; Supriyati, E.; Wardana, D.S.; Meitika, Y.; Ernesia, I. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N. Engl. J. Med. 2021, 384, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Keesing, F.; Belden, L.K.; Daszak, P.; Dobson, A.; Harvell, C.D.; Holt, R.D.; Hudson, P.; Jolles, A.; Jones, K.E.; Mitchell, C.E. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 2010, 468, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, L.S.; Ferreira, M.S.; Fernandes, D.; Padda, H.; Travassos-da-Rosa, E.S.; Deem, S.L.; Vasconcelos, P.F.; Martins, L.C. Individual, household and environmental factors associated with arboviruses in rural human populations, Brazil. Zoonoses Public Health 2021, 68, 203–212. [Google Scholar] [CrossRef] [PubMed]

| Rank | Author Name | Frequency (n = 1644) | h-Index * |
|---|---|---|---|
| 1st | Hu, W. | 34 | 44 |
| 2nd | Tong, S. | 24 | 79 |
| 3rd | Liu, Q. | 23 | 54 |
| 4th | Rocklöv, J. | 22 | 54 |
| 5th | Bi, P. | 16 | 49 |
| 6th | Lowe, R. | 16 | 35 |
| 7th | Harley, D. | 14 | 24 |
| 8th | Wilder-Smith, A. | 13 | 62 |
| 9th | Bambrick, H. | 12 | 24 |
| 10th | Viennet, E. | 12 | 14 |
| Rank | Research Center | Frequency (n = 1644) | Country |
|---|---|---|---|
| 1st | The University of Queensland | 50 | Australia |
| 2nd | Queensland University of Technology | 47 | Australia |
| 3rd | Fundação Oswaldo Cruz | 43 | Brazil |
| 4th | London School of Hygiene & Tropical Medicine | 41 | U.K. |
| 5th | Chinese Center for Disease Control and Prevention | 34 | China |
| 6th | The Australian National University | 32 | Australia |
| 7th | University of Florida | 30 | United States |
| 8th | Universidade de São Paulo | 28 | Brazil |
| 9th | QIMR Berghofer Medical Research Institute | 27 | Australia |
| 10th | Institut Pasteur Paris | 26 | France |
| Rank | Journal | Frequency (n = 1644) | JIF * |
|---|---|---|---|
| 1st | PLOS Neglected Tropical Diseases | 100 (6.1%) | 3.8 |
| 2nd | PLOS ONE | 48 (2.9%) | 3.7 |
| 3rd | American Journal of Tropical Medicine and Hygiene | 42 (2.5%) | 3.3 |
| 4th | Journal of Medical Entomology | 33 (2.0%) | 2.1 |
| 5th | Inter. Journal of Environmental Research and Public Health | 31 (1.9%) | 4614 |
| 6th | Acta Tropica | 24 (1.45%) | 2.7 |
| 7th | Viruses | 23 (1.4%) | 4.7 |
| 8th | Parasites & Vectors | 21 (1.3%) | 3.2 |
| 9th | Science of The Total Environment | 21 (1.3%) | 9.8 |
| 10th | BMC Infectious Diseases | 20 (1.2%) | 3.7 |
| Disease/Pathogen | Frequency (Keyword by Filter) |
|---|---|
| Dengue/virus/fever/hemorrhagic fever | 1468 |
| West Nile virus/fever/flavivirus | 371 |
| Zika virus/fever/virus infection | 354 |
| Chikungunya/virus/fever | 281 |
| Japanese encephalitis/virus/encephalitis, Japanese | 164 |
| Yellow fever | 53 |
| Ross River vírus | 48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sant’Anna, M.W.; Ferreira, M.L.; da Silva, L.F.; Côrtes, P.L. Climate Change and Arbovirus: A Review and Bibliometric Analysis. Climate 2025, 13, 35. https://doi.org/10.3390/cli13020035
Sant’Anna MW, Ferreira ML, da Silva LF, Côrtes PL. Climate Change and Arbovirus: A Review and Bibliometric Analysis. Climate. 2025; 13(2):35. https://doi.org/10.3390/cli13020035
Chicago/Turabian StyleSant’Anna, Maryly Weyll, Maurício Lamano Ferreira, Leonardo Ferreira da Silva, and Pedro Luiz Côrtes. 2025. "Climate Change and Arbovirus: A Review and Bibliometric Analysis" Climate 13, no. 2: 35. https://doi.org/10.3390/cli13020035
APA StyleSant’Anna, M. W., Ferreira, M. L., da Silva, L. F., & Côrtes, P. L. (2025). Climate Change and Arbovirus: A Review and Bibliometric Analysis. Climate, 13(2), 35. https://doi.org/10.3390/cli13020035










