Changes in Climate and Their Implications for Cattle Nutrition and Management
Abstract
1. Introduction
2. Discussion
2.1. Impact of Climate Change on Soil
Soil Microbes and Nutrient Cycling
2.2. Impact of Climate Change on Forage Plants
2.2.1. Plant Growth, Establishment, and Phenology
2.2.2. Plant Species Composition, Forage Biomass, and Nutritional Quality
2.2.3. Plant Secondary Compounds
2.3. Impact of Climate Change on Cattle
2.3.1. Heat Stress
2.3.2. Cold Stress
2.3.3. Health
2.4. Impact of Climate Change on Water for Cattle
Water Availability and Quality
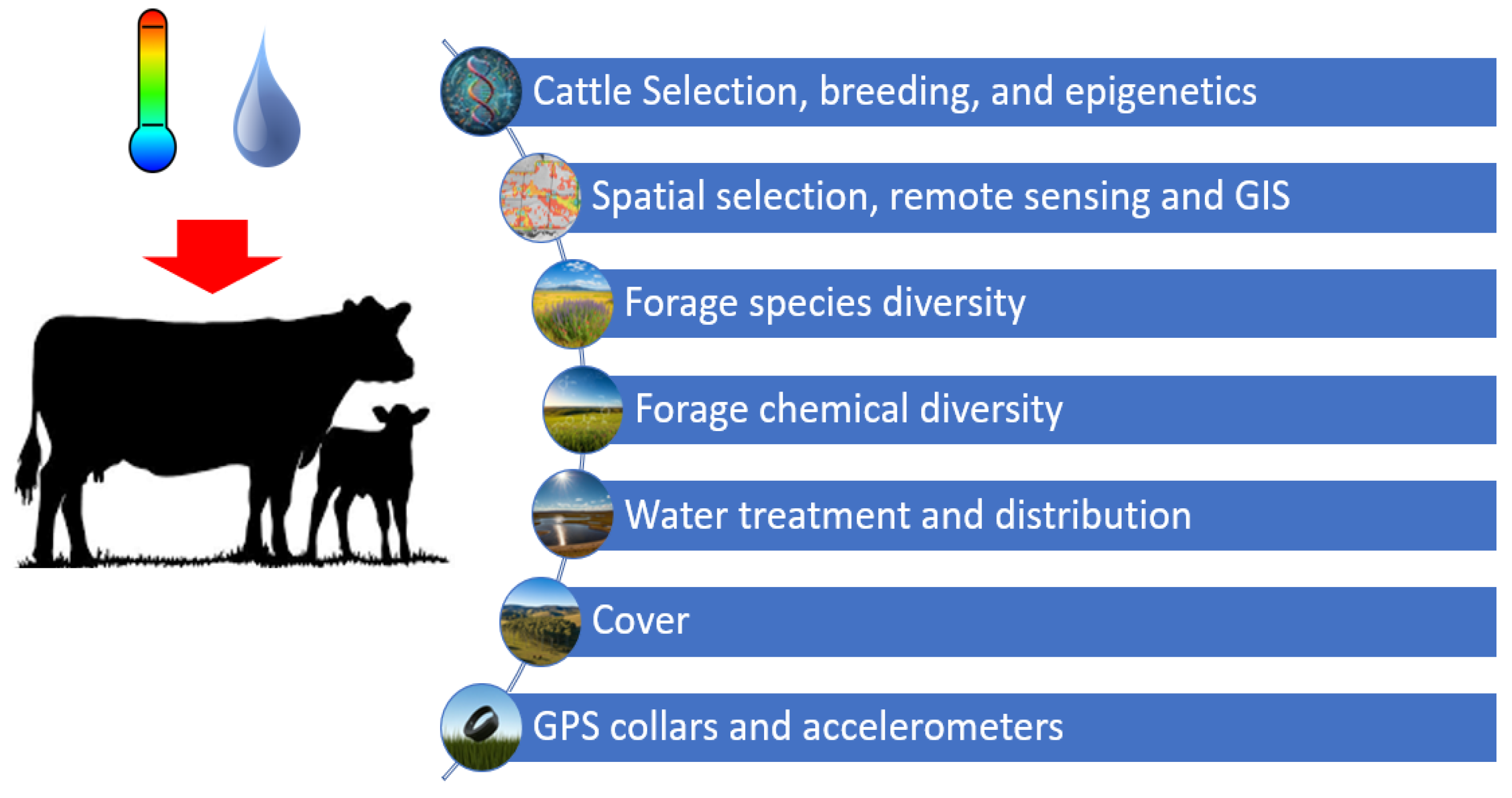
2.5. Mitigation Strategies
2.5.1. Cattle Selection, Breeding, and Epigenetics
2.5.2. Spatial Selection, Remote Sensing, and GIS
2.5.3. Forage Species and Chemical Diversity to Attenuate the Negative Impacts of Climate Fluctuations on Livestock Performance
2.5.4. Water Treatment, Water Points, and Strategic Shade Allocation
2.5.5. Real-Time GPS Coupled with Accelerometer Collars
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, A.; Anand, A.; Arora, D.; Bhattacharya, A.M.; Chahal, P.; Chauhan, R.; Dhall, A.; Dikshit, V.; Gulati, R.; Haque, A.J.; et al. Conservation, Sustainability, and Environmental Justice in India; Lexington Books: Lanham, MD, USA, 2020. [Google Scholar]
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Le Quéré, C.; Peters, G.P.; Peters, W.; Pongratz, J.; et al. Global Carbon Budget 2021. Earth Syst. Sci. Data 2022, 14, 1917–2005. [Google Scholar] [CrossRef]
- Soon, W.; Connolly, R.; Connolly, M.; Akasofu, S.I.; Baliunas, S.; Berglund, J.; Bianchini, A.; Briggs, W.M.; Butler, C.J.; Cionco, R.G.; et al. The Detection and Attribution of Northern Hemisphere Land Surface Warming (1850–2018) in Terms of Human and Natural Factors: Challenges of Inadequate Data. Climate 2023, 11, 179. [Google Scholar] [CrossRef]
- Forster, P.M.; Smith, C.J.; Walsh, T.; Lamb, W.F.; Lamboll, R.; Hauser, M.; Ribes, A.; Rosen, D.; Gillett, N.; Palmer, M.D.; et al. Indicators of Global Climate Change 2022: Annual Update of Large-Scale Indicators of the State of the Climate System and Human Influence. Earth Syst. Sci. Data 2023, 15, 2295–2327. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, M.; Tao, M.; Zhou, W.; Lu, X.; Xiong, Y.; Li, F.; Wang, Q. The Role of Satellite Remote Sensing in Mitigating and Adapting to Global Climate Change. Sci. Total Environ. 2023, 904, 166820. [Google Scholar] [CrossRef] [PubMed]
- Henchion, M.; Moloney, A.P.; Hyland, J.; Zimmermann, J.; McCarthy, S. Review: Trends for Meat, Milk and Egg Consumption for the next Decades and the Role Played by Livestock Systems in the Global Production of Proteins. Animal 2021, 15, 100287. [Google Scholar] [CrossRef]
- Cheng, M.; McCarl, B.; Fei, C. Climate Change and Livestock Production: A Literature Review. Atmosphere 2022, 13, 140. [Google Scholar] [CrossRef]
- Kelly, A.E.; Goulden, M.L. Rapid Shifts in Plant Distribution with Recent Climate Change. Proc. Natl. Acad. Sci. USA 2008, 105, 11823–11826. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Spasojevic, M.J.; Li, D. Climate and Plant Community Diversity in Space and Time. Proc. Natl. Acad. Sci. USA 2020, 117, 4464–4470. [Google Scholar] [CrossRef] [PubMed]
- Sprunger, C.D.; Lindsey, A.; Lightcap, A. Above- and Belowground Linkages during Extreme Moisture Excess: Leveraging Knowledge from Natural Ecosystems to Better Understand Implications for Row-Crop Agroecosystems. J. Exp. Bot. 2023, 74, 2845–2859. [Google Scholar] [CrossRef] [PubMed]
- Izaurralde, R.C.; Thomson, A.M.; Morgan, J.A.; Fay, P.A.; Polley, H.W.; Hatfield, J.L. Climate Impacts on Agriculture: Implications for Forage and Rangeland Production. Agron. J. 2011, 103, 371–381. [Google Scholar] [CrossRef]
- Apgaua, D.M.G.; Tng, D.Y.P.; Forbes, S.J.; Ishida, Y.F.; Vogado, N.O.; Cernusak, L.A.; Laurance, S.G.W. Elevated Temperature and CO2 Cause Differential Growth Stimulation and Drought Survival Responses in Eucalypt Species from Contrasting Habitats. Tree Physiol. 2019, 39, 1806–1820. [Google Scholar] [CrossRef] [PubMed]
- Blond, B.; Majkić, M.; Spasojević, J.; Hristov, S.; Radinović, M.; Nikolić, S.; Anđušić, L.; Čukić, A.; Došenović Marinković, M.; Vujanović, B.D.; et al. Influence of Heat Stress on Body Surface Temperature and Blood Metabolic, Endocrine, and Inflammatory Parameters and Their Correlation in Cows. Metabolites 2024, 14, 104. [Google Scholar] [CrossRef]
- Crous, K.Y. Plant Responses to Climate Warming: Physiological Adjustments and Implications for Plant Functioning in a Future, Warmer World. Am. J. Bot. 2019, 106, 1049. [Google Scholar] [CrossRef]
- Kennedy, P.M.; Milligan, L.P. Effects of Cold Exposure on Digestion, Microbial Synthesis and Nitrogen Transformations in Sheep. Br. J. Nutr. 1978, 39, 105–117. [Google Scholar] [CrossRef]
- Bell, M.J.; Eckard, R.J.; Harrison, M.T.; Neal, J.S.; Cullen, B.R. Effect of Warming on the Productivity of Perennial Ryegrass and Kikuyu Pastures in South-Eastern Australia. Crop Pasture Sci. 2013, 64, 61–70. [Google Scholar] [CrossRef]
- Havrilla, C.A.; Bradford, J.B.; Yackulic, C.B.; Munson, S.M.; Caroline Havrilla, C.A.; Jarvis, S. Divergent Climate Impacts on C 3 versus C 4 Grasses Imply Widespread 21st Century Shifts in Grassland Functional Composition. Divers. Distrib. 2023, 29, 379–394. [Google Scholar] [CrossRef]
- Sanz-Sáez, Á.; Erice, G.; Aguirreolea, J.; Irigoyen, J.J.; Sánchez-Díaz, M. Alfalfa Yield under Elevated CO2 and Temperature Depends on the Sinorhizobium Strain and Growth Season. Environ. Exp. Bot. 2012, 77, 267–273. [Google Scholar] [CrossRef]
- Sisco, M.R.; Bosetti, V.; Weber, E.U. When Do Extreme Weather Events Generate Attention to Climate Change? Clim. Chang. 2017, 143, 227–241. [Google Scholar] [CrossRef]
- Giridhar, K.; Samireddypalle, A.; Giridhar, K.; Samireddypalle, A. Impact of Climate Change on Forage Availability for Livestock. In Climate Change Impact on Livestock: Adaptation and Mitigation; Springer: New Delhi, India, 2015; pp. 97–112. [Google Scholar] [CrossRef]
- Chaplin-Kramer, R.; George, M.R. Effects of Climate Change on Range Forage Production in the San Francisco Bay Area. PLoS ONE 2013, 8, e57723. [Google Scholar] [CrossRef]
- Visconti-Moreno, E.F.; Valenzuela-Balcázar, I.G. Pores Distribution Influences the Soil Microorganism’s Response to Changes in Temperature and Moisture. Eurasian J. Soil Sci. 2023, 12, 28–36. [Google Scholar] [CrossRef]
- Agom, O.; Gbadebo, A. Effects of Climate Change and Global Warming on Enzymes. Rev. Gestão Soc. Ambient. 2024, 18, e06079. [Google Scholar] [CrossRef]
- Choudhary, P.; Bhatt, S.; Chatterjee, S. From Freezing to Functioning: Cellular Strategies of Cold-Adapted Bacteria for Surviving in Extreme Environments. Arch. Microbiol. 2024, 206, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Najar, I.N.; Sharma, P.; Tamang, S.; Mondal, K.; Das, S.; Sherpa, M.T.; Thakur, N. Temperature—A Critical Abiotic Paradigm That Governs Bacterial Heterogeneity in Natural Ecological System. Environ. Res. 2023, 234, 116547. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Ma, Z.; Zhang, J.; Sun, B.; Zhou, J.; Liang, Y. Coupling Temperature-Dependent Spatial Turnover of Microbes and Plants Using the Metabolic Theory of Ecology. New Phytol. 2023, 238, 383–392. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Yu, M.; Chen, X. Response of Extreme Rainfall to Atmospheric Warming and Wetting: Implications for Hydrologic Designs Under a Changing Climate. J. Geophys. Res. Atmos. 2023, 128, e2022JD038430. [Google Scholar] [CrossRef]
- Li, W.; Su, T.; Shen, Y.; Ma, H.; Zhou, Y.; Lu, Q.; Wang, G.; Liu, Z.; Li, J. Effects of Warming Seasonal Rotational Grazing on Plant Communities’ Structure and Diversity in Desert Steppe. Ecol. Evol. 2023, 13, e9748. [Google Scholar] [CrossRef] [PubMed]
- Rojas, Y.P.; Ghezzehei, T. Determining Soil Microbial Activity Based on Soil Moisture and Average Functions. In Proceedings of the EGU General Assembly 2023, Vienna, Austria, 24–28 April 2023; p. EGU23-899. [Google Scholar] [CrossRef]
- Chatterjee, D.; Saha, S. Response of Soil Properties and Soil Microbial Communities to the Projected Climate Change. In Advances in Crop Environment Interaction; Springer: Singapore, 2018; pp. 87–136. [Google Scholar]
- Li, D.; Wu, C.; Wu, J. Soil Fungal Community Has Higher Network Stability than Bacterial Community in Response to Warming and Nitrogen Addition in a Subtropical Primary Forest. Appl. Environ. Microbiol. 2024, 90, e00001-24. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, K.; Zhao, Y.; Zhao, Y.; Wang, B.; Wang, Y.; He, T.; Xu, X.; Bai, T.; Zhang, Y.; et al. Climate Warming Suppresses Abundant Soil Fungal Taxa and Reduces Soil Carbon Efflux in a Semi-Arid Grassland. mLife 2023, 2, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Q.; Zhang, Z.; Li, W.; Liu, W.; Xiao, N.; Liu, H.; Wang, L.; Li, Z.; Ma, J.; et al. Decreased Soil Multifunctionality Is Associated with Altered Microbial Network Properties under Precipitation Reduction in a Semiarid Grassland. iMeta 2023, 2, e106. [Google Scholar] [CrossRef]
- Huntley, B.J. Soil, Water and Nutrients. In Ecology Angola; Springer International Publishing: Cham, Switzerland, 2023; pp. 127–147. [Google Scholar] [CrossRef]
- Naylor, D.; McClure, R.; Jansson, J. Trends in Microbial Community Composition and Function by Soil Depth. Microorganisms 2022, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Bainard, L.D.; Evans, B.; Malis, E.; Yang, T.; Bainard, J.D. Influence of Annual Plant Diversity on Forage Productivity and Nutrition, Soil Chemistry, and Soil Microbial Communities. Front. Sustain. Food Syst. 2020, 4, 560479. [Google Scholar] [CrossRef]
- Danar Dewa, D.; Buchori, I. Impacts of Rapid Urbanization on Spatial Dynamics of Land Use-Based Carbon Emission and Surface Temperature Changes in the Semarang Metropolitan Region, Indonesia Dimas Danar Dewa · Imam Buchori. Environ. Monit. Assess. 2023, 195, 259. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Günther, M.; Pischinger, S.; Kramer, U.; Nederlof, C.; van Almsick, T. Influence of Desulfurization Strategies for Methane Gaseous Direct Injection Engine on Carbon Dioxide Emissions. J. Nat. Gas. Sci. Eng. 2022, 108, 104822. [Google Scholar] [CrossRef]
- Koutsoyiannis, D.; Kundzewicz, Z.W. Atmospheric Temperature and CO2: Hen-Or-Egg Causality? Sci 2020, 2, 83. [Google Scholar] [CrossRef]
- Lacis, A.A.; Schmidt, G.A.; Rind, D.; Ruedy, R.A. Atmospheric CO2: Principal Control Knob Governing Earth’s Temperature. Science 2010, 330, 356–359. [Google Scholar] [CrossRef]
- Sage, R.F.; Kubien, D.S. The Temperature Response of C3 and C4 Photosynthesis. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef]
- Jobe, T.O.; Rahimzadeh Karvansara, P.; Zenzen, I.; Kopriva, S. Ensuring Nutritious Food Under Elevated CO2 Conditions: A Case for Improved C4 Crops. Front. Plant Sci. 2020, 11, 563726. [Google Scholar] [CrossRef] [PubMed]
- Wijewardene, I.; Shen, G.; Zhang, H. Enhancing Crop Yield by Using Rubisco Activase to Improve Photosynthesis under Elevated Temperatures. Stress Biol. 2021, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G. Mechanisms Linking Drought, Hydraulics, Carbon Metabolism, and Vegetation Mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef]
- Buanafina, M.M.d.O.; Morris, P. The Impact of Cell Wall Feruloylation on Plant Growth, Responses to Environmental Stress, Plant Pathogens and Cell Wall Degradability. Agronomy 2022, 12, 1847. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Kharel, T.; Sauer, T.; Adams, T.C.; Philipp, D.; Thomas, A.L.; Owens, P.R. Spatial Monitoring Technologies for Coupling the Soil Plant Water Animal Nexus. Sci. Rep. 2022, 12, 3508. [Google Scholar] [CrossRef] [PubMed]
- More, S.J.; Ravi, V.; Raju, S. Tropical Tuber Crops. In Vegetables for Nutrition and Entrepreneurship; Springer: Singapore, 2019; Volume 1, pp. 719–758. [Google Scholar] [CrossRef]
- Sershen; Perumal, A.; Varghese, B.; Govender, P.; Ramdhani, S.; Berjak, P. Effects of Elevated Temperatures on Germination and Subsequent Seedling Vigour in Recalcitrant Trichilia Emetica Seeds. S. Afr. J. Bot. 2014, 90, 153–162. [Google Scholar] [CrossRef]
- Bhattacharya, A. Physiological Processes in Plants Under Low Temperature Stress; Springer: Singapore, 2022. [Google Scholar]
- Abobatta, W.F. The Influence of Climate Change on Interactions between Environmental Stresses and Plants. In Plant Stress Mitigators: Types, Techniques and Functions; Academic Press: Cambridge, MA, USA, 2023; pp. 425–434. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Naeem, M.; Ali, I.; Tabassum, T.; Nazir, U. Žemės Ūkio Augalų Reakcija į Sausros Sukurtą Stresą: Apžvalga. Zemdirbyste 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Yadav, S.K. Cold Stress Tolerance Mechanisms in Plants. A Review. Agron. Sustain. Dev. 2010, 30, 515–527. [Google Scholar] [CrossRef]
- Bhandari, K.; Nayyar, H. Low Temperature Stress in Plants: An Overview of Roles of Cryoprotectants in Defense. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment; Springer: New York, NY, USA, 2014; Volume 1, pp. 193–265. ISBN 9781461485919. [Google Scholar]
- Ojo, T.A.; Kirkman, K.; Tedder, M. Effects of Warming and Rainfall Variation on Grass Phenology and Regenerative Responses in Mesic Grassland. S. Afr. J. Bot. 2024, 174, 107–115. [Google Scholar] [CrossRef]
- Plos, C.; Hensen, I.; Korell, L.; Auge, H.; Römermann, C. Plant Species Phenology Differs between Climate and Land-Use Scenarios and Relates to Plant Functional Traits. Ecol. Evol. 2024, 14, e11441. [Google Scholar] [CrossRef] [PubMed]
- Kangombe, F.N.; Throop, H.; Sala, O.; Vivoni, E.; Pigg, K.; Hultine, K.; Kwembeya, E. Environmental Drivers of Vegetative and Flowering Phenology in Drylands. Doctoral Dissertation, Arizona State University, Tempe, AZ, USA, 2023. [Google Scholar]
- Fang, H.; Sha, M.; Xie, Y.; Lin, W.; Qiu, D.; Tu, J.; Tan, X.; Li, X.; Sha, Z. Shifted Global Vegetation Phenology in Response to Climate Changes and Its Feedback on Vegetation Carbon Uptake. Remote Sens. 2023, 15, 2288. [Google Scholar] [CrossRef]
- Schüle, M.; Heinken, T.; Fartmann, T. Long-Term Effects of Environmental Alterations in Protected Grasslands—Land-Use History Determines Changes in Plant Species Composition. Ecol. Eng. 2023, 188, 106878. [Google Scholar] [CrossRef]
- Liu, H.; Mi, Z.; Lin, L.; Wang, Y.; Zhang, Z.; Zhang, F.; Wang, H.; Liu, L.; Zhu, B.; Cao, G.; et al. Shifting Plant Species Composition in Response to Climate Change Stabilizes Grassland Primary Production. Proc. Natl. Acad. Sci. USA 2018, 115, 4051–4056. [Google Scholar] [CrossRef]
- French, K.E. Species Composition Determines Forage Quality and Medicinal Value of High Diversity Grasslands in Lowland England. Agric. Ecosyst. Environ. 2017, 241, 193–204. [Google Scholar] [CrossRef]
- Zhang, G.; Dai, E.; Dawaqiongda; Luobu; Fu, G. Effects of Climate Change and Fencing on Forage Nutrition Quality of Alpine Grasslands in the Northern Tibet. Plants 2023, 12, 3182. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, Y.; Aoki, R.; Kinugasa, T.; Sasaki, T. Predicted Effects of Simulated Ambient Warming and Moisture on Forage Nutrient Quality and Community Composition in Mongolian an Arid Grassland. Rangel. J. 2022, 44, 159–164. [Google Scholar] [CrossRef]
- Petit Bon, M.; Bråthen, K.A.; Ravolainen, V.T.; Ottaviani, G.; Böhner, H.; Jónsdóttir, I.S. Herbivory and Warming Have Opposing Short-Term Effects on Plant-Community Nutrient Levels across High-Arctic Tundra Habitats. J. Ecol. 2023, 111, 1514–1530. [Google Scholar] [CrossRef]
- Sebastià, M.T.; Banagar, F.; Palero, N.; Ibáñez, M.; Plaixats, J. Quality Production of Sainfoin Swards Challenged by Global Change in Mountain Areas in the Western Mediterranean. Agronomy 2023, 14, 6. [Google Scholar] [CrossRef]
- Habermann, E.; Dias de Oliveira, E.A.; Contin, D.R.; San Martin, J.A.B.; Curtarelli, L.; Gonzalez-Meler, M.A.; Martinez, C.A. Stomatal Development and Conductance of a Tropical Forage Legume Are Regulated by Elevated [CO2] under Moderate Warming. Front. Plant Sci. 2019, 10, 609. [Google Scholar] [CrossRef] [PubMed]
- Bykova, O.; Chuine, I.; Morin, X. Highlighting the Importance of Water Availability in Reproductive Processes to Understand Climate Change Impacts on Plant Biodiversity. Perspect. Plant Ecol. Evol. Syst. 2019, 37, 20–25. [Google Scholar] [CrossRef]
- Neal, J.S.; Fulkerson, W.J.; Hacker, R.B. Differences in Water Use Efficiency among Annual Forages Used by the Dairy Industry under Optimum and Deficit Irrigation. Agric. Water Manag. 2011, 98, 759–774. [Google Scholar] [CrossRef]
- Carter, P.R.; Sheaffer, C.C. Alfalfa Response to Soil Water Deficits. I. Growth, Forage Quality, Yield, Water Use, and Water-Use Efficiency1. Crop Sci. 1983, 23, 669–675. [Google Scholar] [CrossRef]
- Peterson, P.R.; Sheaffer, C.C.; Hall, M.H. Drought Effects on Perennial Forage Legume Yield and Quality. Agron. J. 1992, 84, 774–779. [Google Scholar] [CrossRef]
- Dumont, B.; Andueza, D.; Niderkorn, V.; Lüscher, A.; Porqueddu, C.; Picon-Cochard, C. A Meta-Analysis of Climate Change Effects on Forage Quality in Grasslands: Specificities of Mountain and Mediterranean Areas. Grass Forage Sci. 2015, 70, 239–254. [Google Scholar] [CrossRef]
- Küchenmeister, K.; Küchenmeister, F.; Kayser, M.; Wrage-Mönnig, N.; Isselstein, J. Influence of Drought Stress on Nutritive Value of Perennial Forage Legumes. Int. J. Plant Prod. 2013, 7, 693–710. [Google Scholar]
- Wilson, J.R. Effects of Water Stress on in Vitro Dry Matter Digestibility and Chemical Composition of Herbage of Tropical Pasture Species. Aust. J. Agric. Res. 1983, 34, 377–390. [Google Scholar] [CrossRef]
- Buxton, D.R. Quality-Related Characteristics of Forages as Influenced by Plant Environment and Agronomic Factors. Anim. Feed. Sci. Technol. 1996, 59, 37–49. [Google Scholar] [CrossRef]
- Reddy, G.S.N.; Matsumoto, G.I.; Shivaji, S. Sporosarcina Macmurdoensis Sp. Nov., from a Cyanobacterial Mat Sample from a Pond in the McMurdo Dry Valleys, Antarctica. Int. J. Syst. Evol. Microbiol. 2003, 53, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.-L.; Gonzalez-Dugo, V.; Gastal, F. How Much Do Water Deficits Alter the Nitrogen Nutrition Status of Forage Crops? Nutr. Cycl. Agroecosystems 2021, 88, 231–243. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Ge, G.; Han, G.; Jia, Y. Influence of Drought Stress on Afalfa Yields and Nutritional Composition. BMC Plant Biol. 2018, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Halim, R.A.; Buxton, D.R.; Hattendorf, M.J.; Carlson, R.E. Water-Stress Effects on Alfalfa Forage Quality After Adjustment for Maturity Differences. Agron. J. 1989, 81, 189–194. [Google Scholar] [CrossRef]
- Kalu, B.A.; Fick, G.W. Quantifying Morphological Development of Alfalfa for Studies of Herbage Quality1. Crop Sci. 1981, 21, 267–271. [Google Scholar] [CrossRef]
- Van Soest, P.J. Acknowledgments. In Nutritional Ecology of the Ruminant; Cornell University Press: Ithaca, NY, USA, 1994; pp. ix–xii. [Google Scholar]
- Tariq, A.; Ahmed, A. Plant Phenolics Production: A Strategy for Biotic Stress Management. In Plant Phenolics in Biotic Stress Management; Springer Nature: Singapore, 2024; pp. 441–454. [Google Scholar] [CrossRef]
- Villalba, J.J.; Ramsey, R.D.; Athanasiadou, S. Review: Herbivory and the Power of Phytochemical Diversity on Animal Health. Animal 2024, 101287. [Google Scholar] [CrossRef]
- Rahman, A.; Albadrani, G.M.; Waraich, E.A.; Awan, T.H.; Yavaş, İ.; Hussain, S.; Rahman, A.; Albadrani, G.M.; Waraich, E.A.; Awan, T.H.; et al. Plant Secondary Metabolites and Abiotic Stress Tolerance: Overview and Implications. In Plant Abiotic Stress Responses and Tolerance Mechanisms; IntechOpen: London, UK, 2023. [Google Scholar]
- La, V.H.; Kim, T.H.; Calderon-Urrea, A.; Janda, T. Editorial: Plant Signaling in Response to Environmental Stresses. Front. Plant Sci. 2023, 14, 1282465. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Xu, Y.; Zang, F.; Li, D.; Liu, Z. The Evolutionary Trajectories of Specialized Metabolites towards Antiviral Defense System in Plants. Mol. Hortic. 2024, 4, 1–11. [Google Scholar] [CrossRef]
- Ray, R. Using Natural Variation in Nicotiana Attenuata to Elucidate Its Defense Response Against Herbivory. Doctoral Dissertation, Friedrich Schiller University Jena, Jena, Germany, 1990. [Google Scholar]
- Montesinos-Navarro, A.; López-Climent, M.F.; Pérez-Clemente, R.M.; Arenas-Sánchez, C.; Sánchez-Martín, R.; Gómez-Cadenas, A.; Verdú, M. Plant Metabolic Response to Stress in an Arid Ecosystem Is Mediated by the Presence of Neighbors. Ecology 2024, 105, e4247. [Google Scholar] [CrossRef] [PubMed]
- Waghorn, G. Beneficial and Detrimental Effects of Dietary Condensed Tannins for Sustainable Sheep and Goat Production—Progress and Challenges. Anim. Feed. Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. The Depth Distribution of Soil Organic Carbon in Relation to Land Use and Management and the Potential of Carbon Sequestration in Subsoil Horizons. Adv. Agron. 2005, 88, 35–66. [Google Scholar] [CrossRef]
- Ortiz-López, B.; Mariezcurrena-Berasain, M.D.; Barajas-Cruz, R.; Velázquez-Garduño, G.; Pliego, A.B.; Adegbeye, M.J.; Salem, A.Z.M.; Mariezcurrena-Berasain, M.A. Sustainable Evaluation of Tannin Extract Biomass as a Feed Product Additive: Effects on Growth Performance, Meat Fatty Acid Profile, and Lipid Oxidation in Bullocks. Biomass Convers. Biorefinery 2024, 14, 5101–5107. [Google Scholar] [CrossRef]
- Sun, Y.; Alseekh, S.; Fernie, A.R. Plant Secondary Metabolic Responses to Global Climate Change: A Meta-Analysis in Medicinal and Aromatic Plants. Glob. Change Biol. 2023, 29, 477–504. [Google Scholar] [CrossRef]
- Laftouhi, A.; Cordero, M.A.W.; Mahraz, M.A.; Zerkani, H.; Hmamou, A.; Idrissi, A.M.; Imane, T.; Eloutassi, N.; Rais, Z.; Taleb, A.; et al. Study of the Physiological Behavior of Some Plants in Response to Climate Change Conditions. Pol. J. Environ. Stud. 2024, 33, 3733–3745. [Google Scholar] [CrossRef]
- González-Teuber, M.; Palma-Onetto, V.; Aguirre, C.; Ibáñez, A.J.; Mithöfer, A. Climate Change-Related Warming-Induced Shifts in Leaf Chemical Traits Favor Nutrition of the Specialist Herbivore Battus Polydamas Archidamas. Front. Ecol. Evol. 2023, 11, 1152489. [Google Scholar] [CrossRef]
- Beale, P.K.; Foley, W.J.; Moore, B.D.; Marsh, K.J. Warmer Ambient Temperatures Reduce Protein Intake by a Mammalian Folivore. Philos. Trans. R. Soc. B 2023, 378, 20220543. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lv, C.; Miao, T.; Ma, X.; Xia, C. Interactive Effects of Rising Temperature, Elevated CO2 and Herbivory on the Growth and Stoichiometry of a Submerged Macrophyte Vallisneria Natans. Sustainability 2023, 15, 1200. [Google Scholar] [CrossRef]
- Balluffi-Fry, J.; Leroux, S.J.; Wiersma, Y.F.; Richmond, I.C.; Heckford, T.R.; Rizzuto, M.; Kennah, J.L.; Vander Wal, E. Integrating Plant Stoichiometry and Feeding Experiments: State-Dependent Forage Choice and Its Implications on Body Mass. Oecologia 2022, 198, 579–591. [Google Scholar] [CrossRef]
- Acamovic, T.; Brooker, J.D. Biochemistry of plant secondary metabolites and their effects in animals. Proc. Nutr. Soc. 2005, 64, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fajardo, R.A.; González-Pech, P.G.; Sandoval-Castro, C.A.; Torres-Acosta, J.F.d.J. Small Ruminant Production Based on Rangelands to Optimize Animal Nutrition and Health: Building an Interdisciplinary Approach to Evaluate Nutraceutical Plants. Animals 2020, 10, 1799. [Google Scholar] [CrossRef]
- Reddy, P.R.K.; Elghandour, M.M.M.Y.; Salem, A.Z.M.; Yasaswini, D.; Reddy, P.P.R.; Reddy, A.N.; Hyder, I. Plant Secondary Metabolites as Feed Additives in Calves for Antimicrobial Stewardship. Anim. Feed. Sci. Technol. 2020, 264, 114469. [Google Scholar] [CrossRef]
- Beck, M.R.; Gregorini, P. How Dietary Diversity Enhances Hedonic and Eudaimonic Well-Being in Grazing Ruminants. Front. Vet. Sci. 2020, 7, 486377. [Google Scholar] [CrossRef] [PubMed]
- Villalba, J.J.; Manteca, X. A Case for Eustress in Grazing Animals. Front. Vet. Sci. 2019, 6, 303. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-Induced Responses of Photosynthesis and Antioxidant Metabolism in Higher Plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Gourlay, G.; Constabel, C.P. Condensed Tannins Are Inducible Antioxidants and Protect Hybrid Poplar against Oxidative Stress. Tree Physiol. 2019, 39, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Popović, B.M.; Štajner, D.; Ždero-Pavlović, R.; Tumbas-Šaponjac, V.; Čanadanović-Brunet, J.; Orlović, S. Water Stress Induces Changes in Polyphenol Profile and Antioxidant Capacity in Poplar Plants (Populus Spp.). Plant Physiol. Biochem. 2016, 105, 242–250. [Google Scholar] [CrossRef]
- Bryant, J.P.; Stuart Chapin, F.; Klein Bryant, D.R.; Bryant, J.P.; Chapin, F.S. Herbivore-Plant Interactions at Northern Latitudes. Oikos 1983, 40, 357–368. [Google Scholar] [CrossRef]
- Horner, J.D. Nonlinear Effects of Water Deficits on Foliar Tannin Concentration. Biochermcal Syst. Ecol. 1990, 18, 211–213. [Google Scholar] [CrossRef]
- Matyssek, R.; Schnyder, H.; Oßwald, W.; Ernst, D.; Munch, J.C.; Pretzsch, H. Ecological Studies 220 Growth and Defence in Plants Resource Allocation at Multiple Scales; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- AbdElgawad, H.; Peshev, D.; Zinta, G.; Van Den Ende, W.; Janssens, I.A.; Asard, H. Climate Extreme Effects on the Chemical Composition of Temperate Grassland Species under Ambient and Elevated CO2: A Comparison of Fructan and Non-Fructan Accumulators. PLoS ONE 2014, 9, e92044. [Google Scholar] [CrossRef]
- Anuraga, M.; Duarsa, P.; Hill, M.; Lovett, J. Soil Moisture and Temperature Affect Condensed Tannin Concentrations and Growth in Lotus Corniculatus and Lotus Pedunculatus. Aust. J. Agric. Res. 1993, 44, 1667–1681. [Google Scholar] [CrossRef]
- Windley, H.R.; Shimada, T. Cold Temperature Improves Tannin Tolerance in a Granivorous Rodent. J. Anim. Ecol. 2020, 89, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Beale, P.K.; Connors, P.K.; Dearing, M.D.; Moore, B.D.; Krockenberger, A.K.; Foley, W.J.; Marsh, K.J. Warmer Ambient Temperatures Depress Detoxification and Food Intake by Marsupial Folivores. Front. Ecol. Evol. 2022, 10, 888550. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P. Effects of Heat Stress on Postabsorptive Metabolism and Energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Lacetera, N.; Baumgard, L.H.; Rhoads, R.P.; Ronchi, B.; Nardone, A. Metabolic and Hormonal Acclimation to Heat Stress in Domesticated Ruminants. Animal 2010, 4, 1167–1183. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Thorup, V.M.; Kudahl, A.B.; Østergaard, S. Effects of Heat Stress on Feed Intake, Milk Yield, Milk Composition, and Feed Efficiency in Dairy Cows: A Meta-Analysis. J. Dairy. Sci. 2024, 107, 3207–3218. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.C.; Moraes, L.E.; Santos, J.E.P.; Baumgard, L.H.; Mueller, N.D.; Kebreab, E. Modeling the Relationship between Heat Stress, Feed Intake, and Day Relative to Calving in Nonlactating Dairy Cows. J. Dairy. Sci. 2023, 106, 8942–8952. [Google Scholar] [CrossRef]
- Sammad, A.; Wang, Y.J.; Umer, S.; Lirong, H.; Khan, I.; Khan, A.; Ahmad, B.; Wang, Y. Nutritional Physiology and Biochemistry of Dairy Cattle under the Influence of Heat Stress: Consequences and Opportunities. Animals 2020, 10, 793. [Google Scholar] [CrossRef] [PubMed]
- Safa, S.; Kargar, S.; Moghaddam, G.A.; Ciliberti, M.G.; Caroprese, M. Heat Stress Abatement during the Postpartum Period: Effects on Whole Lactation Milk Yield, Indicators of Metabolic Status, Inflammatory Cytokines, and Biomarkers of the Oxidative Stress. J. Anim. Sci. 2019, 97, 122–132. [Google Scholar] [CrossRef]
- Antanaitis, R.; Džermeikaitė, K.; Krištolaitytė, J.; Ribelytė, I.; Bespalovaitė, A.; Bulvičiūtė, D.; Palubinskas, G.; Anskienė, L. The Impacts of Heat Stress on Rumination, Drinking, and Locomotory Behavior, as Registered by Innovative Technologies, and Acid–Base Balance in Fresh Multiparous Dairy Cows. Animals 2024, 14, 1169. [Google Scholar] [CrossRef]
- Giannone, C.; Bovo, M.; Ceccarelli, M.; Torreggiani, D.; Tassinari, P. Review of the Heat Stress-Induced Responses in Dairy Cattle. Animals 2023, 13, 3451. [Google Scholar] [CrossRef]
- Ross, A.D.; Bryden, W.L.; Bakau, W.; Burgess, L.W. Induction of Heat Stress in Beef Cattle by Feeding the Ergots of Claviceps Purpurea. Aust. Vet. J. 1989, 66, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Idris, M.; Uddin, J.; Sullivan, M.; McNeill, D.M.; Phillips, C.J.C. Non-Invasive Physiological Indicators of Heat Stress in Cattle. Animals 2021, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Chakravarty, A.K.; Singh, A.; Upadhyay, A.; Singh, M.; Yousuf, S. Effect of Heat Stress on Reproductive Performances of Dairy Cattle and Buffaloes: A Review. Vet. World 2016, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Novo, A.; Pérez-Garnelo, S.S.; Villagrá, A.; Pérez-Villalobos, N.; Astiz, S. The Effect of Stress on Reproduction and Reproductive Technologies in Beef Cattle—A Review. Animals 2020, 10, 2096. [Google Scholar] [CrossRef]
- Hemantaranjan, A. Heat Stress Responses and Thermotolerance. Adv. Plants Agric. Res. 2014, 1, 1–10. [Google Scholar] [CrossRef]
- Alemu, T.W.; Pandey, H.O.; Salilew Wondim, D.; Gebremedhn, S.; Neuhof, C.; Tholen, E.; Holker, M.; Schellander, K.; Tesfaye, D. Oxidative and Endoplasmic Reticulum Stress Defense Mechanisms of Bovine Granulosa Cells Exposed to Heat Stress. Theriogenology 2018, 110, 130–141. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. A New Perspective on the Use of Plant Secondary Metabolites to Inhibit Methanogenesis in the Rumen. Phytochemistry 2010, 71, 1198–1222. [Google Scholar] [CrossRef]
- Chen, H.; Fan, W.; Zhang, H.; Yue, P.; Wang, R.; Zhang, W.; Mai, K. Effects of Dietary Methionine on Growth and Body Composition, Indicators of Digestion, Protein Metabolism and Immunity, and Resistance to Heat Stress of Abalone Haliotis Discus Hannai. Aquaculture 2023, 563, 738978. [Google Scholar] [CrossRef]
- Czech, B.; Wang, Y.; Szyda, J. Genome-Wide Association Study of Heat Stress Response in Bos taurus. bioRxiv 2023. [Google Scholar] [CrossRef]
- Lemal, P.; Tran, M.-N.; Schroyen, M.; Gengler, N. 143 Validation Strategies of Sensor Data in the Context of Heat Stress Detection. J. Anim. Sci. 2023, 101, 36–37. [Google Scholar] [CrossRef]
- Nickles, K.R.; Relling, A.E.; Garcia-Guerra, A.; Fluharty, F.L.; Parker, A.J. Environmental Stress during the Last Trimester of Gestation in Pregnant Cows and Its Effect on Offspring Growth Performance and Response to Glucose and Adrenocorticotropic Hormone. J. Anim. Sci. 2023, 101, skac332. [Google Scholar] [CrossRef] [PubMed]
- Chase, L.E. Cold Stress: Effects on Nutritional Requirements, Health and Performance. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Kim, W.-S.; Nejad, J.G.; Lee, H.-G. Impact of Cold Stress on Physiological, Endocrinological, Immunological, Metabolic, and Behavioral Changes of Beef Cattle at Different Stages of Growth. Animals 2023, 13, 1073. [Google Scholar] [CrossRef]
- Delfino, J.G.; Mathison, G.W. Effects of Cold Environment and Intake Level on the Energetic Efficiency of Feedlot Steers. J. Anim. Sci. 1991, 69, 4577–4587. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Q.; Peng, J.; Niu, H. Effects of Long-Term Cold Stress on Growth Performance, Behavior, Physiological Parameters, and Energy Metabolism in Growing Beef Cattle. Animals 2023, 13, 1619. [Google Scholar] [CrossRef] [PubMed]
- Nardone, A.; Ronchi, B.; Lacetera, N.; Ranieri, M.S.; Bernabucci, U. Effects of Climate Changes on Animal Production and Sustainability of Livestock Systems. Livest. Sci. 2010, 130, 57–69. [Google Scholar] [CrossRef]
- Coloma-García, W.; Mehaba, N.; Such, X.; Caja, G.; Salama, A.A.K. Effects of Cold Exposure on Some Physiological, Productive, and Metabolic Variables in Lactating Dairy Goats. Animals 2020, 10, 2383. [Google Scholar] [CrossRef] [PubMed]
- Berian, S. Effect of Cold Stress on Milk Yield, Physiological and Hemato-Biochemical Profile of Cross Bred Dairy Cattle. J. Anim. Res. 2019, 9, 335–338. [Google Scholar] [CrossRef]
- Sahib, Q.S.; Aafaq, I.; Ahmed, H.A.; Sheikh, G.G.; Ganai, I.A. Mitigating Cold Stress in Livestock by Nutritional Interventions: A Comprehensive Review. Indian. J. Anim. Res. 2024, 58, 353–363. [Google Scholar] [CrossRef]
- Wagner, D.G. Effects of Cold Stress on Cattle Performance and Management Factors to Reduce Cold Stress and Improve Performance. Bov. Pract. 1988, 23, 88–93. [Google Scholar] [CrossRef]
- Cantalapiedra-Hijar, G.; Abo-Ismail, M.; Carstens, G.E.; Guan, L.L.; Hegarty, R.; Kenny, D.A.; Mcgee, M.; Plastow, G.; Relling, A.; Ortigues-Marty, I. Review: Biological Determinants of between-Animal Variation in Feed Efficiency of Growing Beef Cattle. Animal 2018, 12, S321–S335. [Google Scholar] [CrossRef] [PubMed]
- Ames, D.R. Adjusting Rations for Climate. Vet. Clin. N. Am. Food Anim. Pract. 1988, 4, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Young, B.A. Cold Stress as It Affects Animal Production. J. Anim. Sci. 1981, 52, 154–163. [Google Scholar] [CrossRef]
- Lees, A.M.; Sejian, V.; Wallage, A.L.; Steel, C.C.; Mader, T.L.; Lees, J.C.; Gaughan, J.B. The Impact of Heat Load on Cattle. Animals 2019, 9. [Google Scholar] [CrossRef]
- Colditz, I.G.; Hine, B.C. Resilience in Farm Animals: Biology, Management, Breeding and Implications for Animal Welfare. Anim. Prod. Sci. 2016, 56, 1961–1983. [Google Scholar] [CrossRef]
- Paz, S. Climate Change Impacts on Vector-Borne Diseases in Europe: Risks, Predictions and Actions. Lancet Reg. Health—Eur. 2021, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Chikezie, F.M.; Opara, K.N.; Ubulom, P.M.E. Impacts of Changing Climate on Arthropod Vectors and Diseases Transmission. Niger. J. Entomol. 2024, 40, 179–192. [Google Scholar] [CrossRef]
- Bryer, K.E. Effects of Temperature and Humidity on the Mortality of the Tick Dermacentor Reticulatus in the UK. Doctoral Dissertation, University of Bristol, Bristol, UK, 2020. [Google Scholar]
- Deshpande, G.; Beetch, J.E.; Heller, J.G.; Naqvi, O.H.; Kuhn, K.G. Assessing the Influence of Climate Change and Environmental Factors on the Top Tick-Borne Diseases in the United States: A Systematic Review. Microorganisms 2023, 12, 50. [Google Scholar] [CrossRef]
- Domatskiy, V.N.; Sivkova, E.I. The Influence of Climatogeographic Conditions on the Expansion of the Range of Ixodes Ticks. Entomol. Appl. Sci. Lett. 2023, 10, 1–9. [Google Scholar] [CrossRef]
- Hoch, T.; Madouasse, A.; Jacquot, M.; Wongnak, P.; Beugnet, F.; Bournez, L.; Cosson, J.F.; Huard, F.; Moutailler, S.; Plantard, O.; et al. Seasonality of Host-Seeking Ixodes Ricinus Nymph Abundance in Relation to Climate. Peer Community J. 2024, 4, e2. [Google Scholar] [CrossRef]
- Brunner, J.L.; Killilea, M.; Ostfeld, R.S. Overwintering Survival of Nymphal Ixodes Scapularis (Acari: Ixodidae) Under Natural Conditions. J. Med. Entomol. 2012, 49, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Lemery, J.; Knowlton, K.; Sorensen, C. (Eds.) Global Climate Change and Human Health: From Science to Practice; John Wiley & Sons: Hoboken, NJ, USA, 2021; p. 636. [Google Scholar]
- Ogden, N.H.; Ben Beard, C.; Ginsberg, H.S.; Tsao, J.I. Possible Effects of Climate Change on Ixodid Ticks and the Pathogens They Transmit: Predictions and Observations. J. Med. Entomol. 2021, 58, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Dionizio, R.; Augusto, J.; Afonso, B.; Silmara, G.; Soares, L.; Pajeú E Silva, B.; Filipe, J.; Cajueiro, P.; Teles Coutinho, L.; et al. Occurrence of Foot Diseases in Cattle Attended at the Clínica de Bovinos de Garanhuns: Epidemiological, Clinical, Therapeutic and Economic Aspects. Ciência Anim. Bras. 2022, 23, e-72731. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Mudroň, P.; Abramowicz, B.; Kurek, Ł.; Stachura, R. Lameness in Cattle—Etiopathogenesis, Prevention and Treatment. Animals 2024, 14, 1836. [Google Scholar] [CrossRef] [PubMed]
- Chepkwony, R.; Castagna, C.; Heitkönig, I.; Van Bommel, S.; Van Langevelde, F. Associations between Monthly Rainfall and Mortality in Cattle Due to East Coast Fever, Anaplasmosis and Babesiosis. Parasitology 2020, 147, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Correia, L.; Loureiro, A.P.; Lilenbaum, W. Effects of Rainfall on Incidental and Host-Maintained Leptospiral Infections in Cattle in a Tropical Region. Vet. J. 2017, 220, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, O. Changes in the Mean and Variability of Temperature and Precipitation over Global Land Areas. Environ. Res. Clim. 2023, 2, 035006. [Google Scholar] [CrossRef]
- Luppichini, M.; Bini, M.; Giannecchini, R.; Zanchetta, G.; Luppichini, M.; Bini, M.; Giannecchini, R.; Zanchetta, G. The Effects of the Temperature Increase on the Rainfall Regimes in North-Central Italy. In Proceedings of the EGUGA, Vienna, Austria, 23–28 April 2023; p. EGU-5430. [Google Scholar] [CrossRef]
- Lall, U.; Josset, L.; Russo, T. A Snapshot of the World’s Groundwater Challenges. Annu. Rev. Environ. Resour. 2020, 45, 171–194. [Google Scholar] [CrossRef]
- Godde, C.M.; Mason-D’Croz, D.; Mayberry, D.E.; Thornton, P.K.; Herrero, M. Impacts of Climate Change on the Livestock Food Supply Chain; a Review of the Evidence. Glob. Food Sec. 2021, 28, 100488. [Google Scholar] [CrossRef] [PubMed]
- Maurya, V.P.; Sejian, V.; Kumar, K.; Singh, G.; Naqvi, S.M.K. Walking Stress Influence on Livestock Production. In Environmental Stress and Amelioration in Livestock Production; Springer: Berlin/Heidelberg, Germany, 2012; pp. 75–95. ISBN 9783642292057. [Google Scholar] [CrossRef]
- Sejian, V.; Maurya, V.P.; Naqvi, S.M.K. Effect of Walking Stress on Growth, Physiological Adaptability and Endocrine Responses in Malpura Ewes in a Semi-Arid Tropical Environment. Int. J. Biometeorol. 2012, 56, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Muzzo, B.I.; Maleko, D.D.; Thacker, E.; Provenza, F.D. Review: Rangeland Management in Tanzania: Opportunities, Challenges, and Prospects for Sustainability. Int. J. Trop. Drylands 2023, 7, 2. [Google Scholar] [CrossRef]
- He, T.; Yi, G.; Wang, X.; Sun, Y.; Li, J.; Wu, Z.; Guo, Y.; Sun, F.; Chen, Z. Effects of Heated Drinking Water during the Cold Season on Serum Biochemistry, Ruminal Fermentation, Bacterial Community, and Metabolome of Beef Cattle. Metabolites 2023, 13, 995. [Google Scholar] [CrossRef]
- Golher, D.M.; Patel, B.H.M.; Bhoite, S.H.; Syed, M.I.; Panchbhai, G.J.; Thirumurugan, P. Factors Influencing Water Intake in Dairy Cows: A Review. Int. J. Biometeorol. 2021, 65, 617–625. [Google Scholar] [CrossRef]
- Bewley, J.M.; Grott, M.W.; Einstein, M.E.; Schutz, M.M. Impact of Intake Water Temperatures on Reticular Temperatures of Lactating Dairy Cows. J. Dairy Sci. 2008, 91, 3880–3887. [Google Scholar] [CrossRef]
- Singh, A.K.; Bhakat, C.; Singh, P. A Review on Water Intake in Dairy Cattle: Associated Factors, Management Practices, and Corresponding Effects. Trop. Anim. Health Prod. 2022, 54, 1–13. [Google Scholar] [CrossRef]
- Grossi, S.; Rossi, L.; Dell’anno, M.; Biffani, S.; Sgoifo Rossi, C.A. Effects of Heated Drinking Water on the Growth Performance and Rumen Functionality of Fattening Charolaise Beef Cattle in Winter. Animals 2021, 11, 2218. [Google Scholar] [CrossRef] [PubMed]
- Valeria González Pereyra, A.; Maldonado May, V.; Catracchia, C.G.; Alejandra Herrero, M.; Flores, M.C.; Mazzini, M. Influence of water temperature and heat stress on drinking water intake in dairy cows. J. Agric. Res 2010, 70, 328–336. [Google Scholar] [CrossRef]
- Coffey, R.; Paul, M.J.; Stamp, J.; Hamilton, A.; Johnson, T. A Review of Water Quality Responses to Air Temperature and Precipitation Changes 2: Nutrients, Algal Blooms, Sediment, Pathogens. JAWRA J. Am. Water Resour. Assoc. 2019, 55, 844–868. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Bharti, V.K.; Kalia, S.; Arora, A.; Balaje, S.S.; Chaurasia, O.P. A Review on Water Quality and Dairy Cattle Health: A Special Emphasis on High-Altitude Region. Appl. Water Sci. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Wagner, J.J.; Engle, T.E. Invited Review: Water Consumption, and Drinking Behavior of Beef Cattle, and Effects of Water Quality. Appl. Anim. Sci. 2021, 37, 418–435. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, P.; Trivedi, M.M.; Modi, R.J.; Patel, Y.G. Article No.JSRR.116804 Review Article Arya et Al. J. Sci. Res. Rep. 2024, 30, 427–436. [Google Scholar] [CrossRef]
- Rexroad, C.; Vallet, J.; Matukumalli, L.K.; Reecy, J.; Bickhart, D.; Blackburn, H.; Boggess, M.; Cheng, H.; Clutter, A.; Cockett, N.; et al. Genome to Phenome: Improving Animal Health, Production, and Well-Being—A New USDA Blueprint for Animal Genome Research 2018–2027. Front. Genet. 2019, 10, 431609. [Google Scholar] [CrossRef]
- Nayak, S.S.; Panigrahi, M.; Rajawat, D.; Ghildiyal, K.; Sharma, A.; Jain, K.; Bhushan, B.; Dutt, T. Deciphering Climate Resilience in Indian Cattle Breeds by Selection Signature Analyses. Trop. Anim. Health Prod. 2024, 56, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Asadollahpour Nanaie, H.; Wang, X.; Dalai, B.; Zhao, M.; Wang, F.; Li, H.; Yang, D.; Zhang, H.; Li, Y.; et al. Genomic Adaptation to Extreme Climate Conditions in Beef Cattle as a Consequence of Cross-Breeding Program. BMC Genom. 2023, 24, 186. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Reinoso, M.A.; Aponte, P.M.; Garcia-Herreros, M. Genomic Analysis, Progress and Future Perspectives in Dairy Cattle Selection: A Review. Animals 2021, 11, 599. [Google Scholar] [CrossRef]
- de Brito, A. Effects of Dietary Forage and Protein Supplements on Production, Nitrogen Utilization and Microbial Protein Synthesis in Lactating Dairy Cows; The University of Wisconsin-Madison: Madison, WI, USA, 2004. [Google Scholar]
- Niyonzima, Y.B.; Strandberg, E.; Hirwa, C.D.; Manzi, M.; Ntawubizi, M.; Rydhmer, L. The Effect of High Temperature and Humidity on Milk Yield in Ankole and Crossbred Cows. Trop. Anim. Health Prod. 2022, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Castaño-Sánchez, J.P.; Rotz, C.A.; McIntosh, M.M.; Tolle, C.; Gifford, C.A.; Duff, G.C.; Spiegal, S.A. Grass Finishing of Criollo Cattle Can Provide an Environmentally Preferred and Cost Effective Meat Supply Chain from United States Drylands. Agric. Syst. 2023, 210, 103694. [Google Scholar] [CrossRef]
- Holliday, R. Epigenetics: A Historical Overview. Epigenetics 2006, 1, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Sevane, N.; Martínez, R.; Bruford, M.W. Genome-Wide Differential DNA Methylation in Tropically Adapted Creole Cattle and Their Iberian Ancestors. Anim. Genet. 2019, 50, 15–26. [Google Scholar] [CrossRef]
- Verma, S.; Taube, F.; Malisch, C.S. Examining the Variables Leading to Apparent Incongruity between Antimethanogenic Potential of Tannins and Their Observed Effects in Ruminants—A Review. Sustainability 2021, 13, 2743. [Google Scholar] [CrossRef]
- Kowalski, K.; Senf, C.; Okujeni, A.; Hostert, P. Large-Scale Remote Sensing Analysis Reveals an Increasing Coupling of Grassland Vitality to Atmospheric Water Demand. Glob. Chang. Biol. 2024, 30, e17315. [Google Scholar] [CrossRef] [PubMed]
- Priya, M.V.; Kalpana, R.; Pazhanivelan, S.; Kumaraperumal, R.; Ragunath, K.P.; Vanitha, G.; Nihar, A.; Prajesh, P.J.; Vasumathi, V. Monitoring Vegetation Dynamics Using Multi-Temporal Normalized Difference Vegetation Index (NDVI) and Enhanced Vegetation Index (EVI) Images of Tamil Nadu. J. Appl. Nat. Sci. 2023, 15, 1170–1177. [Google Scholar] [CrossRef]
- Mallick, K.; Verfaillie, J.; Wang, T.; Ortiz, A.A.; Szutu, D.; Yi, K.; Kang, Y.; Shortt, R.; Hu, T.; Sulis, M.; et al. Net Fluxes of Broadband Shortwave and Photosynthetically Active Radiation Complement NDVI and near Infrared Reflectance of Vegetation to Explain Gross Photosynthesis Variability across Ecosystems and Climate. Remote Sens. Environ. 2024, 307, 114123. [Google Scholar] [CrossRef]
- Bhaga, T.D.; Dube, T.; Shekede, M.D.; Shoko, C. Impacts of Climate Variability and Drought on Surface Water Resources in Sub-Saharan Africa Using Remote Sensing: A Review. Remote Sens. 2020, 12, 4184. [Google Scholar] [CrossRef]
- Bellini, E. Assessing Grassland Development by Means of Modelling and Remote Sensing Approaches: Current Situation and Future Projections; University of Florence: Florence, Italy, 2023. [Google Scholar]
- König, S.; Thonfeld, F.; Förster, M.; Dubovyk, O.; Heurich, M. Assessing Combinations of Landsat, Sentinel-2 and Sentinel-1 Time Series for Detecting Bark Beetle Infestations. GISci. Remote Sens. 2023, 60, 2226515. [Google Scholar] [CrossRef]
- Poitras, T.B.; Villarreal, M.L.; Waller, E.K.; Nauman, T.W.; Miller, M.E.; Duniway, M.C. Identifying Optimal Remotely-Sensed Variables for Ecosystem Monitoring in Colorado Plateau Drylands. J. Arid. Environ. 2018, 153, 76–87. [Google Scholar] [CrossRef]
- Regos, A.; Gonçalves, J.; Arenas-Castro, S.; Alcaraz-Segura, D.; Guisan, A.; Honrado, J.P. Mainstreaming Remotely Sensed Ecosystem Functioning in Ecological Niche Models. Remote Sens. Ecol. Conserv. 2022, 8, 431–447. [Google Scholar] [CrossRef]
- Bibi, Z.; Maqsood, M.J.; Idrees, A.; Rafique, H.; Butt, A.A.; Ali, R.; Arif, Z.; Nabi, M.U. Retracted: Exploring the Role of Phenotypic Plasticity in Plant Adaptation to Changing Climate: A Review. Asian J. Res. Crop Sci. 2024, 9, 1–9. [Google Scholar] [CrossRef]
- Temperton, V.M.; Mwangi, P.N.; Scherer-Lorenzen, M.; Schmid, B.; Buchmann, N. Positive Interactions between Nitrogen-Fixing Legumes and Four Different Neighbouring Species in a Biodiversity Experiment. Oecologia 2007, 151, 190–205. [Google Scholar] [CrossRef]
- Pirhofer-Walzl, K.; Eriksen, J.; Rasmussen, J.; Høgh-Jensen, H.; Søegaard, K.; Rasmussen, J. Effect of Four Plant Species on Soil 15N-Access and Herbage Yield in Temporary Agricultural Grasslands. Plant Soil 2013, 371, 313–325. [Google Scholar] [CrossRef]
- Moorby, J.M.; Fraser, M.D. Review: New Feeds and New Feeding Systems in Intensive and Semi-Intensive Forage-Fed Ruminant Livestock Systems. Animal 2021, 15, 100297. [Google Scholar] [CrossRef]
- Lee, M.A. A Global Comparison of the Nutritive Values of Forage Plants Grown in Contrasting Environments. J. Plant Res. 2018, 131, 641–654. [Google Scholar] [CrossRef]
- Provenza, F.D.; Villalba, J.J.; Dziba, L.E.; Atwood, S.B.; Banner, R.E. Linking Herbivore Experience, Varied Diets, and Plant Biochemical Diversity. Small Rumin. Res. 2003, 49, 257–274. [Google Scholar] [CrossRef]
- Lagrange, S.; Villalba, J.J. Tannin-Containing Legumes and Forage Diversity Influence Foraging Behavior, Diet Digestibility, and Nitrogen Excretion by Lambs 1,2 3995 Tannin-Containing Legumes and Forage Intake. J. Anim. Sci. 2019, 97, 3994–4009. [Google Scholar] [CrossRef] [PubMed]
- Lagrange, S.; Beauchemin, K.A.; MacAdam, J.; Villalba, J.J. Grazing Diverse Combinations of Tanniferous and Non-Tanniferous Legumes: Implications for Beef Cattle Performance and Environmental Impact. Sci. Total Environ. 2020, 746, 140788. [Google Scholar] [CrossRef] [PubMed]
- Villalba, J.J.; Provenza, F.D.; K Clemensen, A.; Larsen, R.; Juhnke, J. Preference for Diverse Pastures by Sheep in Response to Intraruminal Administrations of Tannins, Saponins and Alkaloids. Grass Forage Sci. 2011, 66, 224–236. [Google Scholar] [CrossRef]
- Villalba, J.J.; Provenza, F.D.; Gibson, N.; López-Ortíz, S. Veterinary Medicine: The Value of Plant Secondary Compounds and Diversity in Balancing Consumer and Ecological Health. In Sustainable Food Production Includes Human and Environmental Health; Springer: Berlin/Heidelberg, Germany, 2014; pp. 165–190. [Google Scholar] [CrossRef]
- Garrett, K.; Beck, M.R.; Marshall, C.J.; Maxwell, T.M.R.; Logan, C.M.; Greer, A.W.; Gregorini, P. Varied Diets: Implications for Lamb Performance, Rumen Characteristics, Total Antioxidant Status, and Welfare. J. Anim. Sci. 2021, 99, skab334. [Google Scholar] [CrossRef]
- Jensen, T.L.; Provenza, F.D.; Villalba, J.J. Influence of Diet Sequence on Intake of Foods Containing Ergotamine d Tartrate, Tannins and Saponins by Sheep. Appl. Anim. Behav. Sci. 2013, 144, 57–62. [Google Scholar] [CrossRef]
- Kamler, J.F.; Ballard, W.B.; Gilliland, R.L.; Mote, K. Coyote (Canis Latrans) Movements Relative to Cattle (Bos Taurus) Carcass Areas. West. N. Am. Nat. 2004, 64, 53–58. [Google Scholar]
- Villalba, J.J.; Provenza, F.D.; Manteca, X. Links between Ruminants’ Food Preference and Their Welfare. Animal 2010, 4, 1240–1247. [Google Scholar] [CrossRef]
- Tilman, D. Plant Strategies and the Dynamics and Structure of Plant Communities; Princeton University Press: Princeton, NJ, USA, 1988; Volume 26. [Google Scholar]
- Gregorini, P.; Villalba, J.J.; Chilibroste, P.; Provenza, F.D. Grazing Management: Setting the Table, Designing the Menu and Influencing the Diner. Anim. Prod. Sci. 2017, 57, 1248–1268. [Google Scholar] [CrossRef]
- Distel, R.A.; Arroquy, J.I.; Lagrange, S.; Villalba, J.J. Designing Diverse Agricultural Pastures for Improving Ruminant Production Systems. Front. Sustain. Food Syst. 2020, 4, 596869. [Google Scholar] [CrossRef]
- Jordán, M.J.; Martínez-Conesa, C.; Bañón, S.; Otal, J.; Quílez, M.; García-Aledo, I.; Romero-Espinar, P.; Sánchez-Gómez, P. The Combined Effect of Mediterranean Shrubland Pasture and the Dietary Administration of Sage By-Products on the Antioxidant Status of Segureña Ewes and Lambs. Antioxidants 2020, 9, 938. [Google Scholar] [CrossRef]
- Athanasiadou, S.; Tzamaloukas, O.; Kyriazakis, I.; Jackson, F.; Coop, R.L. Testing for Direct Anthelmintic Effects of Bioactive Forages against Trichostrongylus Colubriformis in Grazing Sheep. Vet. Parasitol. 2005, 127, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Saed-Moucheshi, A.; Shekoofa, A.; Pessarakli, M. Reactive Oxygen Species (ROS) Generation and Detoxifying in Plants. J. Plant Nutr. 2014, 37, 1573–1585. [Google Scholar] [CrossRef]
- Monjardino, M.; Revell, D.; Pannell, D.J. The Potential Contribution of Forage Shrubs to Economic Returns and Environmental Management in Australian Dryland Agricultural Systems. Agric. Syst. 2010, 103, 187–197. [Google Scholar] [CrossRef]
- Revell, C.K.; Ewing, M.A.; Nutt, B.J. Breeding and Farming System Opportunities for Pasture Legumes Facing Increasing Climate Variability in the South-West of Western Australia. Crop Pasture Sci. 2012, 63, 840–847. [Google Scholar] [CrossRef]
- Revell, D.K.; Norman, H.C.; Vercoe, P.E.; Phillips, N.; Toovey, A.; Bickell, S.; Hulm, E.; Hughes, S.; Emms, J. Australian Perennial Shrub Species Add Value to the Feed Base of Grazing Livestock in Low- to Medium-Rainfall Zones. Anim. Prod. Sci. 2013, 53, 1221–1230. [Google Scholar] [CrossRef]
- Robertson, M.; Revell, C. Perennial Pastures in Cropping Systems of Southern Australia: An Overview of Present and Future Research. Crop Pasture Sci. 2014, 65, 1084–1090. [Google Scholar] [CrossRef]
- Landeen, M.; Jones, C.; Jensen, S.; Whittaker, A.; Summers, D.D.; Eggett, D.; Petersen, S.L. Establishing Seed Islands for Native Forb Species on Rangelands Using N-Sulate Ground Cover Fabric. Nativ. Plants J. 2021, 22, 51–63. [Google Scholar] [CrossRef]
- Santos-Gally, R.; Boege, K. Biodiversity Islands: The Role of Native Tree Islands Within Silvopastoral Systems in a Neotropical Region. In Biodiversity Islands: Strategies for Conservation in Human-Dominated Environments; Springer International Publishing: Cham, Switzerland, 2022; pp. 117–138. [Google Scholar] [CrossRef]
- Schrader, J.; Wright, I.J.; Kreft, H.; Westoby, M. A Roadmap to Plant Functional Island Biogeography. Biol. Rev. 2021, 96, 2851–2870. [Google Scholar] [CrossRef]
- Kim, N.H.; Cho, T.J.; Rhee, M.S. Current Interventions for Controlling Pathogenic Escherichia coli. Adv. Appl. Microbiol. 2017, 100, 1–47. [Google Scholar] [CrossRef]
- Charles, A.S.; Baskaran, A.; Murcott, C.; Schreiber, D.; Hoagland, T.; Venkitanarayanan, K. Reduction of Escherichia coli O157:H7 in Cattle Drinking-Water by Trans-Cinnamaldehyde. Foodborne Pathog. Dis. 2008, 5, 763–771. [Google Scholar] [CrossRef]
- Zhao, T.; Zhao, P.; West, J.W.; Bernard, J.K.; Cross, H.G.; Doyle, M.P. Inactivation of Enterohemorrhagic Escherichia Coli in Rumen Content- or Feces-Contaminated Drinking Water for Cattle. Appl. Environ. Microbiol. 2006, 72, 3268–3273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Richards, S.; Rao, L.; Connelly, S.; Raj, A.; Raveendran, L.; Shirin, S.; Jamwal, P.; Helliwell, R. Sustainable Water Resources through Harvesting Rainwater and the Effectiveness of a Low-Cost Water Treatment. J. Environ. Manag. 2021, 286, 112223. [Google Scholar] [CrossRef]
- Sobsey, M.D.; Khatib, L.A.; Hill, V.R.; Alocilja, E.; Pillai, S. Pathogens in Animal Wastes and the Impacts of Waste Management Practices on Their Survival, Transport and Fate; ASABE: St. Joseph, MI, USA, 2006; pp. 609–666. [Google Scholar] [CrossRef]
- Hunt, L.P.; Mcivor, J.G.; Grice, A.C.; Bray, S.G. Principles and Guidelines for Managing Cattle Grazing in the Grazing Lands of Northern Australia: Stocking Rates, Pasture Resting, Prescribed Fire, Paddock Size and Water Points—A Review. Rangel. J. 2014, 36, 105–119. [Google Scholar] [CrossRef]
- Muzzo, B.I.; Provenza, F.D. Review of strategies for overcoming challenges of beef production in Tanzania. Livestock Res. Rural Development 2018, 30, 199. [Google Scholar]
- Masters, D.G.; Blache, D.; Lockwood, A.L.; Maloney, S.K.; Norman, H.C.; Refshauge, G.; Hancock, S.N. Shelter and Shade for Grazing Sheep: Implications for Animal Welfare and Production and for Landscape Health. Anim. Prod. Sci. 2023, 63, 623–644. [Google Scholar] [CrossRef]
- Valtorta, S.E.; Leva, P.E.; Gallardo, M.R. Evaluation of Different Shades to Improve Dairy Cattle Well-Being in Argentina. Int. J. Biometeorol. 1997, 41, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Titto, C.G.; Titto, E.A.L.; Titto, R.M.; Mourão, G.B. Heat Tolerance and the Effects of Shade on the Behavior of Simmental Bulls on Pasture. Anim. Sci. J. 2011, 82, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Van Laer, E.; Moons, C.P.H.; Ampe, B.; Sonck, B.; Vandaele, L.; De Campeneere, S.; Tuyttens, F.A.M. Effect of Summer Conditions and Shade on Behavioural Indicators of Thermal Discomfort in Holstein Dairy and Belgian Blue Beef Cattle on Pasture. Animal 2015, 9, 1536–1546. [Google Scholar] [CrossRef]
- Davison, T.M.; Silver, B.A.; Lisle, A.T.; Orr, W.N. The Influence of Shade on Milk Production of Holstein-Friesian Cows in a Tropical Upland Environment. Aust. J. Exp. Agric. 1988, 28, 149–154. [Google Scholar] [CrossRef]
- McIlvain, E.H.; Shoop, M.C. Shade for Improving Cattle Gains and Rangeland Use (El Uso de Sombreadores Para Mejorar Las Ganancias de Novillas y Pastoreo de Animales). J. Range Manag. 1971, 24, 181. [Google Scholar] [CrossRef]
- Moreno García, C.A.; Maxwell, T.M.R.; Hickford, J.; Gregorini, P. On the Search for Grazing Personalities: From Individual to Collective Behaviors. Front. Vet. Sci. 2020, 7, 502292. [Google Scholar] [CrossRef]
- Gregorini, P.; Tamminga, S.; Gunter, S.A. Behavior and Daily Grazing Patterns of Cattle. Prof. Anim. Sci. 2006, 22, 201–209. [Google Scholar] [CrossRef]
- Lyons, R.K.; Machen, R.V. Livestock Grazing Distribution: Considerations and Management; Texas AgriLife Extension: College Station, TX, USA, 2023. [Google Scholar]
- Heins, B.J.; Pereira, G.M.; Sharpe, K.T. Precision Technologies to Improve Dairy Grazing Systems. JDS Commun. 2023, 4, 318–323. [Google Scholar] [CrossRef]
- Bello, R.-W.; Talib, A.Z.H.; Mohamed, A.S.A. A Framework for Real-Time Cattle Monitoring Using Multimedia Networks. Int. J. Recent Technol. Eng. (IJRTE) 2020, 8, 974–979. [Google Scholar] [CrossRef]
- Ramezani Gardaloud, N.; Guse, C.; Lidauer, L.; Steininger, A.; Kickinger, F.; Öhlschuster, M.; Auer, W.; Iwersen, M.; Drillich, M.; Klein-Jöbstl, D. Short Communications: An Ear-Attached Accelerometer Detects Effects of Regrouping on Lying, Rumination, and Activity Times in Calves. Vet. Res. Commun. 2023, 47, 2333–2337. [Google Scholar] [CrossRef] [PubMed]
- Versluijs, E.; Niccolai, L.J.; Spedener, M.; Zimmermann, B.; Hessle, A.; Tofastrud, M.; Devineau, O.; Evans, A.L. Classification of Behaviors of Free-Ranging Cattle Using Accelerometry Signatures Collected by Virtual Fence Collars. Front. Anim. Sci. 2023, 4, 1083272. [Google Scholar] [CrossRef]
- Džermeikaitė, K.; Bačėninaitė, D.; Antanaitis, R. Innovations in Cattle Farming: Application of Innovative Technologies and Sensors in the Diagnosis of Diseases. Animals 2023, 13, 780. [Google Scholar] [CrossRef]
- Sprinkle, J.E.; Sagers, J.K.; Hall, J.B.; Ellison, M.J.; Yelich, J.V.; Brennan, J.R.; Taylor, J.B.; Lamb, J.B. Predicting Cattle Grazing Behavior on Rangeland Using Accelerometers. Rangel. Ecol. Manag. 2021, 76, 157–170. [Google Scholar] [CrossRef]
- Riaboff, L.; Shalloo, L.; Smeaton, A.F.; Couvreur, S.; Madouasse, A.; Keane, M.T. Predicting Livestock Behaviour Using Accelerometers: A Systematic Review of Processing Techniques for Ruminant Behaviour Prediction from Raw Accelerometer Data. Comput. Electron. Agric. 2022, 192, 106610. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzzo, B.I.; Ramsey, R.D.; Villalba, J.J. Changes in Climate and Their Implications for Cattle Nutrition and Management. Climate 2025, 13, 1. https://doi.org/10.3390/cli13010001
Muzzo BI, Ramsey RD, Villalba JJ. Changes in Climate and Their Implications for Cattle Nutrition and Management. Climate. 2025; 13(1):1. https://doi.org/10.3390/cli13010001
Chicago/Turabian StyleMuzzo, Bashiri Iddy, R. Douglas Ramsey, and Juan J. Villalba. 2025. "Changes in Climate and Their Implications for Cattle Nutrition and Management" Climate 13, no. 1: 1. https://doi.org/10.3390/cli13010001
APA StyleMuzzo, B. I., Ramsey, R. D., & Villalba, J. J. (2025). Changes in Climate and Their Implications for Cattle Nutrition and Management. Climate, 13(1), 1. https://doi.org/10.3390/cli13010001








