Evidence of Climate Change and the Conservation Needed to Halt the Further Deterioration of Small Glacial Lakes
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Inventory of the High Alpine Lakes in the Albanian Upland Areas
3.2. Particularities of the Glacial Lakes, Distribution, and Specific Biodiversity Values of Selected Animal and Vegetation Components
- There was a statistically significant correlation between the vegetation cover of lakes and the water level oscillation (Pearson correlation 2-tailed: R2 = −0.666, α = 99%, p < 0.001).
3.3. Rapid Changes in Snow-Cover and Low-Temperature Days (−0 °C), Predicting Further Degradation Due to Climate Change and Anthropogenic Interventions
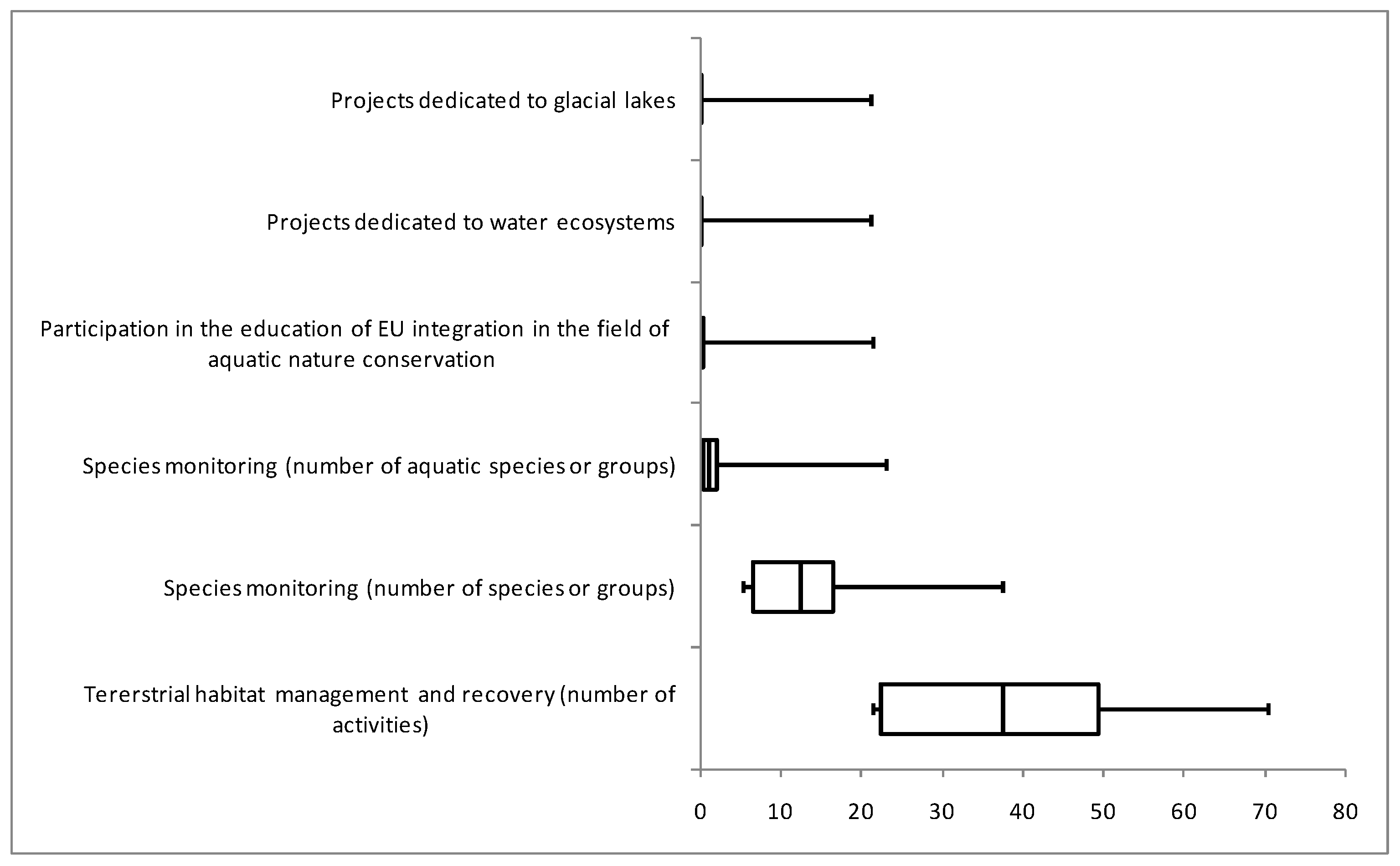
3.4. The State of Conservation and Bias with Protected Area Designation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cowie, R.H.; Bouchet, P.; Fontaine, B. The Sixth Mass Extinction: Fact, fiction or speculation? Biol. Rev. 2022, 97, 640–663. [Google Scholar] [CrossRef] [PubMed]
- WWF. Living Planet Report 2022—Building a Nature positive Society; Almond, R.E.A., Grooten, M., Bignoli, J., Petersen, T., Eds.; WWF Gland Switzerland: Gland, Switzerland, 2022; p. 118. [Google Scholar]
- Markovic, D.; Carrizo, S.; Carcher, O.; Walz, A.; David, J. Vulnerability of European freshwater catchments to climate change. Glob. Chang. Biol. 2017, 23, 3567–3580. [Google Scholar] [CrossRef]
- Shumka, L.; Papastefani, A.; Shumka, S.; Mali, S. The Potentials for the Ecological Management of Landscape Connectivity Including Aquatic Ecosystems in Northeast Albania. Hydrobiology 2023, 2, 44–54. [Google Scholar] [CrossRef]
- European Commission, Directorate-General for Environment. EU Biodiversity Strategy for 2030—Bringing Nature Back into Our Lives; Publications Office of the European Union: Luxembourg, 2021. [CrossRef]
- UN Environment Programme. Monitoring framework for the Kunming-Montreal Global Biodiversity Framework. In Proceedings of the Conference of the Parties to the Convention on Biological Diversity Fifteenth Meeting, Montreal, QC, Canada, 5–17 December 2022. [Google Scholar]
- Da Silva, J.P.; Hermoso, V.; Lopes-Lima, M.; Miranda, R.; Filipe, A.F.; Sousa, R. The role of connectivity in conservation planning for species with obligatory interactions: Prospects for future climates cenarios. Glob. Chang. Biol. 2024, 30, e17169. [Google Scholar] [CrossRef]
- Bocchiola, D.; Guglielmina, D. Evidence of climate change within the Adamello Glacier of Italy. Theor. Appl. Climatol. 2010, 100, 351–369. [Google Scholar] [CrossRef]
- Theurillat, J.P.; Guisan, A. Potential impact of climate change on vegetation in the European Alps: Areview. Clim. Chang. 2001, 50, 77–109. [Google Scholar] [CrossRef]
- Beniston, M.; Keller, F.; Goyette, S. Snow pack in the Swiss Alps under changing climatic conditions: An empirical approach for climate impacts studies. Theor. Appl. Climatol. 2003, 74, 19–31. [Google Scholar] [CrossRef]
- Paul, F.; Kääb, A.; Haeberli, W. Recent glacier changes in the Alps observed by satellite: Consequences forfuture monitoring strategies. Glob. Planet. Chang. 2007, 56, 111–122. [Google Scholar] [CrossRef]
- Buckel, J.; Otto, J.C.; Prasicek, G.; Keuschnig, M. Glacial lakes in Austria—Distribution and formation since the Little Ice Age. Glob. Planet. Chang. 2018, 164, 39–51. [Google Scholar] [CrossRef]
- Zemp, M.; Hoelzle, M.; Haeberli, W. Distributed modelling of the regional climatic equilibrium line altitude of glaciers in the EuropeanAlps. Glob. Planet. Chang. 2007, 56, 83–100. [Google Scholar] [CrossRef]
- Hughes, P.D.; Woodward, J.C.; Gibbard, P.L. Relict rock glaciers as indicators of Mediterranean palaeo climate during the Last Glacial Maximum (Late Würmian) in northwest Greece. J. Quat. Sci. 2003, 18, 431–440. [Google Scholar] [CrossRef]
- Reuther, A.U.; Urdea, P.; Geiger, C.; Ivy-Ochs, S.; Niller, H.P.; Kubik, P.W.; Heine, K. Late Pleistocene glacial chronology of the Pietrele valley, Retezat mountains, southern Carpathians, constrained by 10 Beexposure ages and pedological investigations. Quat. Int. 2007, 164, 151–169. [Google Scholar] [CrossRef]
- Milivojević, M.; Menković, L.; Calić, C. Pleistocene glacial relief of the central part of Mt. Prokletije (Albanian Alps). Quat. Int. 2008, 190, 112–122. [Google Scholar] [CrossRef]
- Frasheri, A.; Bushati, S.; Bare, V. Geophysical outlook on structure of the Albanides. J. Balk. Geophys. Soc. 2009, 12, 9–30. [Google Scholar]
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Academic: San Diego, CA, USA, 2001; p. 1006. ISBN 9780127447605. [Google Scholar]
- Sharma, R.C. Habitat ecology and diversity of freshwater zooplankton of Uttarakh and Himalaya, India. Biodivers. Int. J. 2020, 5, 188–196. [Google Scholar]
- Gavrilko, D.; Zhikharev, V.; Zolotareva, T.; Kudrin, I.; Yakimov, B.; Erlashova, A. Biodiversity of zooplankton (Rotifera, Cladocera and Copepoda) in the tributaries of Cheboksary Reservoir (Middle Volga, Russia). Biodivers. Data J. 2024, 12, e116330. [Google Scholar] [CrossRef]
- Søndergaard, M.; Johansson, L.S.; Lauridsen, T.L.; Jørgensen, T.B.; Liboriussen, L.; Jeppesen, E. Submerged macrophytes as indicators of the ecological quality of lakes. Freshw. Biol. 2010, 55, 893–908. [Google Scholar] [CrossRef]
- Steffenhagen, P.; Zak, D.; Schulz, K.; Timmermann, T.; Zerbe, S. Biomass and nutrient stock of submersed and floating macrophytes in shallow lakes formed by rewetting of degraded fens. Hydrobiologia 2012, 692, 99–109. [Google Scholar] [CrossRef]
- EC(EuropeanCommission). Directive 2000/60/EC of the European Parliament and of the Council of 23rd October 2000 establishing a framework for Community action in the field of water policy. In Official Journal of the European Communities; L327/1; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Rigó, A.; Barina, Z. The Floristic Composition of Irrigation Ponds and Water Reservoirs in Albania after the Long Persistent Drought of 2016–2017. In Proceedings of the 1st International Electronic Conference on Biological Diversity, Ecology and Evolution, Basel, Switzerland, 15–31 March 2021; pp. 132–140. [Google Scholar] [CrossRef]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W.; et al. Freshwater Ecoregions of the World: A New Map of Biogeographic Units for Freshwater Biodiversity Conservation. BioScience 2008, 58, 403–414. [Google Scholar] [CrossRef]
- Shumka, S.; Berberi, E.; Kulici, M.; Mucaj, S.; Vladi, F. Assessing the relationship between biodiversity conservation and slow food culture in selected protected areas in Albania. Biodiversitas 2022, 23, 1319–1326. [Google Scholar] [CrossRef]
- Shumka, S.; Lalaj, S.; Šanda, R.; Shumka, L.; Meulenbroek, P. Recent data on the distribution of freshwater ichthyofauna in Albania. Croat. J. Fish. 2023, 81, 33–44. [Google Scholar] [CrossRef]
- UNESCO. Enhancing Our Heritage Toolkit Assessing Management Effectiveness of Natural World Heritage Sites; UNESCO World Heritage Centre: Paris, France, 2008; pp. 1–134.
- Nogrady, T.; Segers, H. Asplanchnidae, Gastropodidae, Lindiidae, Microcodidae, Synchaetidae, Trochosphaeridae and Filinia. In Rotifera. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; Backhuys Publishers: Leiden, The Netherlands, 2002; Volume 6. [Google Scholar]
- Ruttner-Kolisko, A. Plankton Rotifers, Biology and Taxonomy. English translation of Die Binnengenwasser; Schweizerbart: Sttutgart, Germany, 1974. [Google Scholar]
- Koste, W. Rotatoria, Die Radertiere Mitteleuropas. Ein Bestimmungswerk, be grundet von Max Voigt. In Uberordnung Monogononta; Gebruder Borntraeger: Berlin, Germany, 1978. (In German) [Google Scholar]
- Collin, A.; Dieffenbach, H.; Sachse, R.; Voigt, M. Rotatoria und Gastrotricha; Die Susswasser fauna Deutschlands: Lehre, Germany, 1961. [Google Scholar]
- Nogrady, T.; Pourriot, R.; Segers, H. Rotifera. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World. In The Notommatidae and the Scaridiidae; SPB Academic Publishing: The Hague, The Netherlands, 1995; Volume 3. [Google Scholar]
- Tutin, T.G.; Burges, N.A.; Chater, A.O.; Edmondsn, J.R.; Heywood, V.H.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europeae. I–V; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Barina, Z.; Somogyi, G.; Pifko, D.; Rakaj, M. Checklist of vascular plants of Albania. Phytotaxa 2018, 378, 1–339. [Google Scholar] [CrossRef]
- Raca, I.; Harpke, D.; Shuka, L.; Ranđelović, V. A new species of Crocus ser. Verni (Iridaceae) with 2n=12 chromosomes from the Balkans, Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2020, 156, 36–42. [Google Scholar] [CrossRef]
- Shuka, D.; Tan, K.; Hallaçi, B.; Shuka, L. Additions to the flora of North Albania. Phytol. Balc. 2020, 26, 517–522. [Google Scholar]
- Kohler, A. Methodender Kartierungv on Flora und Vegetation von Sus wasser biotopen. Landsch. Stadt. 1978, 10, 73–85. [Google Scholar]
- Trajanovska, S.; Talevska, M.; Schneider, S. Assessment of littoral eutrophication in LakeOhrid by submerged macrophytes. Biologia 2014, 69, 756–764. [Google Scholar] [CrossRef]
- Miho, A.; Kashta, L.; Beqiraj, S. Between the Land and the Sea—Ecoguide to Discover the Transitional Waters of Albania; Julvin2: Tirana, Albania, 2013; pp. 1–462. ISBN 978-9928-137-27-2. [Google Scholar]
- Hughes, P.D.; Woodward, J.C.; van Calsteren, P.C.; Thomas, L.E. The glacial history of the Dinaric Alps, Montenegro. Quat. Sci. Rev. 2011, 30, 3393–3412. [Google Scholar] [CrossRef]
- Hughes, P.D.; Gibbard, P.L.; Woodward, J.C. Geological controls on Pleistocene glaciation and cirque form in Greece. Geomorphology 2007, 88, 242–253. [Google Scholar] [CrossRef]
- Hughes, P.D. Twenty-first Century Glaciers and Climate in the Prokletije Mountains, Albania. Arct. Antarct. Alp. Res. 2009, 41, 455–459. [Google Scholar] [CrossRef]
- Shumka, S. Checklist of Rotifer Species from Albania (Phylum Rotifera). Opusc. Zool. 2021, 52, 99–109. [Google Scholar] [CrossRef]
- Špoljar, M.; Shumka, S.; Tasevska, O.; Tomljanovic, T.; Otoic, A.; Galir Balkic, A.; Lajtner, J.; Pepa, B.; Dražina, T.; Ternjej, I. Small Standing-Water Ecosystems in the Transitional Temperate Climate of the Western Balkans; Springer: Berlin/Heidelberg, Germany, 2021; pp. 21–51. [Google Scholar] [CrossRef]
- Obertegger, U.; Thaler, B.; Flaim, G. Rotifer species richness a longanaltitudinal gradient in the Alps. Glob. Ecol. Biogeogr. 2010, 19, 895–904. [Google Scholar] [CrossRef]
- Özdemir, M.D.; Ustaoğlu, M.R. Distribution of rotifers of high mountain lakes in the Eastern Black Sea Range of Turkey. Turk. J. Zool. 2017, 41, 674–685. [Google Scholar] [CrossRef]
- Sturm, R. Freshwater molluscs in mountain lakes of the Eastern Alps (Austria): Relationship between environmental variables and lake colonization. J. Limnol. 2007, 66, 160–169. [Google Scholar] [CrossRef]
- Schneider, S.; Trajanovska, S.; Biberdžić, V.; Marković, A.; Talevska, M.; Imeri, A.; Cara, M. The Balkan macrophyte index (BMI) for assessment of eutrophication in lakes. Acta Zool. 2020, 72, 439–454. [Google Scholar]
- Mancinelli, G.; Mali, S.; Belmonte, G. Species richness and taxonomic distinctness of zooplankton in ponds and small lakes from Albania and North Macedonia: The role of bioclimatic factors. Water 2019, 11, 2384. [Google Scholar] [CrossRef]
- World Bank. Climate Risk Profile: Albania; The World Bank Group, World Bank Publications: Washington, DC, USA, 2021; p. 34. [Google Scholar]
- Hodenbrog, L.; Marelle, L.; Alterskjær, K.; Wood, R.R.; Ludwig, R.; Fischer, E.M.; Richardson, T.B.; Forster, P.M.; Sillman, J.; Myhre, G. Intensification of summer precipitation with shorter time-scales in Europe. Environ. Res. Lett. 2019, 14, 124050. [Google Scholar] [CrossRef]
- Grunewald, K.; Scheithauer, J. Europe’s southern most glaciers: Response and adaptation to climate change. J. Glaciol. 2010, 56, 129–142. [Google Scholar] [CrossRef]
- Ministry of Environment. Albania’s Second National Communication to the Conference of Parties under the UNFCCC; Ministry of Environment: Tirana, Albania, 2016; p. 296.
- Saura, S.; Bertzky, B.; Bastin, L.; Battistella, L.; Mandrici, A.; Dubois, A. Protected area connectivity: Shortfalls in global targets and country-level Priorities. Biol. Conserv. 2018, 219, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Beale, C.M.; Baker, N.E.; Brewer, M.J.; Lennon, J.J. Protected area networks and savannah bird biodiversity in the face of climate change and land degradation. Ecol. Lett. 2013, 16, 1061–1068. [Google Scholar] [CrossRef]
- Juffe-Bignoli, D.; Burgess, N.D.; Bingham, H.; Belle, E.M.S.; de Lima, M.G. Protected Planet Report; UNEP-WCMC: Cambridge, UK, 2014. [Google Scholar]
- Miszczak, S.; Shuka, D.; Shuka, L.; Migdalek, G.; Słomka, A. Low and high elevation Heliosperma species (Caryophyllaceae)—Insight based on chromosome number, pollen characters and seed micromorphology. Phytotaxa 2022, 554, 32–46. [Google Scholar] [CrossRef]
- Oikonomou, A.; Stefanidis, K. α-and β-diversity patterns of macrophytes and freshwater fishes are driven by different factors and processes in lakes of the unexplored southern Balkan biodiversity hot spot. Water 2020, 12, 1984. [Google Scholar] [CrossRef]
- Chappuis, E.; Gacia, E.; Ballesteros, E. Environmental factors explaining the distribution and diversity of vascular aquatic macrophytes in a highly heterogeneous Mediterranean region. Aquat. Bot. 2014, 113, 72–82. [Google Scholar] [CrossRef]
- Fernández-Aláez, C.; Fernández-Aláez, M.; García-Criado, F.; García-Girón, J. Environmental drivers of aquatic macrophyte assemblages in ponds a longanaltitudinal gradient. Hydrobiologia 2016, 812, 79–98. [Google Scholar] [CrossRef]
- Shuka, L.; Çullaj, A.; Shumka, S.; Miho, A.; Duka, S.; Bachofen, R. Response of Drinking-Water Reservoir Ecosystems to Anthropogenic Impacts in Albania: Trends of Interrelationship. J. Int. Environ. Appl. Sci. 2009, 4, 478–486. [Google Scholar]







| Group I | Group II | |
|---|---|---|
| Level 1 | Level 2 | Level 3 |
| Management plan | Yearly revision of management plan | Number of implemented projects, 2017–2023 |
| Trained staff | Database of performance | Glacial lake monitoring |
| Ranger service | Daily operational and guidance book | Education programs |
| Biodiversity inventory database | ||
| Hiking trails | ||
| Visitor center and guided tours | ||
| No. | Lake Name | Coordinates | Altitude (m) | Geomorphology of Watershed (According to [17]) | Vegetation Cover | Surface (ha) | Yearly Water Level Oscillation (m) | |
|---|---|---|---|---|---|---|---|---|
| 1 | Gramozi Lake | 40°21′52.17″ N | 20°47′25.62″ E | 2364 | Flysch alevrolitic | None | 0.48 | 1.50 |
| Jabllanica Mt | ||||||||
| 2 | Lake of Dragani | 41°16′54.71″ N | 20°27′2.33″ E | 1660 | Calcareous | Very dense | 7.1 | 3.50 |
| 3 | Lake of Kusar | 41°16′38.32″ N | 20°30′1.77″ E | 1862 | Mixed Calcareous and Granitic | None | 1.15 | 2.01 |
| 4 | Lake of Sal Xhyra | 41°15′56.87″ N | 20°29′59.60″ E | 1850 | Mixed Calcareous and Granitic | None | 0.77 | 2.10 |
| Shebeniku Mt | ||||||||
| 5 | Big lake of Dragostunja | 41°12′43.50″ N | 20°27′43.20″ E | 2054 | Ultramafic rocks | None | 1.56 | 1.75 |
| 6 | Great Lake of Dragostunja | 41°12′45.73″ N | 20°27′31.28″ E | 2005 | Ultramafic rocks | None | 1.15 | 1.50 |
| 7 | Shebenik Lake | 41°12′49.81″ N | 20°28′4.00″ E | 2006 | Ultramafic rocks | None | 1.52 | 1.40 |
| 8 | Great Lake of Likopatra, Rrajcë | 41°11′21.78″ N | 20°29′22.42″ E | 1905 | Ultramafic rocks | None | 3.54 | 1.20 |
| 9 | Small Lake of Likopatra, Rrajcë | 41°11′30.98″ N | 20°29′5.86″ E | 2007 | Ultramafic rocks | None | 1.54 | 1.20 |
| Valamara Mts | ||||||||
| 10 | Black Lake (Lenia) | 40°45′39.98″ N | 20°25′50.26″ E | 1698 | Ultramafic rocks | None | 3.25 | 2.30 |
| 11 | Black Lake of Valamara | 40°46′40.10″ N | 20°28′0.16″ E | 2003 | Ultramafic rocks | None | 1.93 | 1.71 |
| 12 | Lake in Valamara | 40°46′20.70″ N | 20°28′17.99″ E | 1950 | Ultramafic rocks | None | 0.8 | 1.81 |
| 13 | Lake of Lilies (Valamara) | 40°46′55.44″ N | 20°29′24.71″ E | 1865 | Ultramafic rocks | Dense | 2 | 1.75 |
| 14 | Lake in Valamara 3 | 40°46′51.03″ N | 20°29′4.37″ E | 1910 | Ultramafic rocks | None | 3.9 | 2.01 |
| 15 | Lake of Lenia | 40°47′17.35″ N | 20°28′16.41″ E | 2110 | Ultramafic rocks | None | 2.9 | 2.10 |
| 16 | Lake in Valamara 4 | 40°47′20.00″ N | 20°28′05.06″ E | 2121 | Ultramafic rocks | None | 2.37 | 1.40 |
| 17 | The Lake of the Spring of Shkumbin | 40°47′48.40″ N | 20°28′6.32″ E | 2147 | Ultramafic rocks | None | 2 | 1.31 |
| 18 | Lake in Valamara 5 | 40°47′36.96″ N | 20°28′38.06″ E | 2020 | Ultramafic rocks | None | 0.4 | 1.40 |
| 19 | Lake of Lukova 1 | 40°54′21.62″ N | 20°23′43.29″ E | 1855 | Ultramafic rocks | None | 6.53 | 1.91 |
| 20 | Lake of Lukova 2 | 40°53′53.80″ N | 20°24′21.60″ E | 1750 | Ultramafic rocks | None | 4.07 | 1.30 |
| Martanesh area | ||||||||
| 21 | Lake of Sopa | 41°26′5.87″ N | 20°17′16.73″ E | 1722 | Ultramafic rocks | Sparse | 3.22 | 1.35 |
| 22 | Lake of Hardha | 41°26′26.12″ N | 20°18′38.71″ E | 1725 | Ultramafic rocks | None | 7.3 | 1.90 |
| 23 | Lake of Sopoti | 41°27′6.15″ N | 20°18′51.51″ E | 1632 | Ultramafic rocks | Sparse | 7 | 2.10 |
| 24 | Black Lake (Bulqiza) | 41°27′15.16″ N | 20°18′7.29″ E | 1687 | Ultramafic rocks | None | 2 | 3.01 |
| 25 | White Lake | 41°27′41.28″ N | 20°17′43.43″ E | 1650 | Ultramafic rocks | Sparse | 7.4 | 3.012 |
| 26 | Lake Kocebse | 41°28′25.10″ N | 20°15′39.01″ E | 1798 | Ultramafic rocks | Sparse | 2.13 | 2.10 |
| 27 | Dry Lake | 41°28′15.67″ N | 20°15′10.82″ E | 1798 | Ultramafic rocks | Sparse | 3.8 | 2.01 |
| 28 | Skënderi Lake | 41°28′27.02″ N | 20°14′45.74″ E | 1725 | Ultramafic rocks | Sparse | 3.2 | 1.80 |
| 29 | Lake without tracks | 41°28′4.39″ N | 20°14′42.34″ E | 1860 | Ultramafic rocks | None | 1.2 | 1.60 |
| 30 | Gatelli Lakes | 41°28′4.39″ N | 20°14′42.34″ E | 1810 | Ultramafic rocks | Sparse | 2.3 | 1.70 |
| 31 | Balgjai Lake | 41°33′16.53″ N | 20°12′49.33″ E | 1775 | Ultramafic rocks | None | 1 | 1.75 |
| 32 | Balgjai Lake 2 | 41°33′11.06″ N | 20°12′28.41″ E | 1808 | Ultramafic rocks | None | 4.5 | 1.80 |
| 33 | Lake of flowers (Kacni) | 41°33′37.55″ N | 20°13′25.69″ E | 1900 | Ultramafic rocks | Dense | 6.1 | 1.81 |
| 34 | Lake of Ksnika | 41°33′58.10″ N | 20°14′28.84″ E | 1855 | Ultramafic rocks | None | 1.44 | 1.75 |
| 35 | Black Lake of Kacnia | 41°34′6.51″ N | 20°13′55.42″ E | 1855 | Ultramafic rocks | None | 10 | 1.80 |
| 36 | Lake of Shtrunga | 41°34′23.00″ N | 20°14′17.30″ E | 1690 | Ultramafic rocks | None | 6 | 3.10 |
| 37 | Lake of Barzana | 41°34′23.75″ N | 20°13′52.16″ E | 1800 | Ultramafic rocks | Sparse | 2 | 1.50 |
| 38 | Lake of Kacnia 1 | 41°34′29.70″ N | 20°14′5.70″ E | 1740 | Ultramafic rocks | Dense | 1.72 | 1.70 |
| 39 | Lake of Kacnia 2 | 41°34′37.37″ N | 20°14′19.53″ E | 1665 | Ultramafic rocks | Sparse | 2 | 3.50 |
| 40 | Lake of Bruce | 41°34′18.16″ N | 20°15′15.14″ E | 1665 | Ultramafic rocks | None | 1.9 | 3.01 |
| 41 | Lake of Milloshi | 41°34′35.29″ N | 20°15′20.05″ E | 1636 | Ultramafic rocks | None | 2.5 | 2.40 |
| 42 | Lake of Kalia | 41°34′43.66″ N | 20°15′17.02″ E | 1655 | Ultramafic rocks | None | 2 | 2.20 |
| 43 | Lake of Batakëve 1 | 41°34′29.00″ N | 20°15′5.14″ E | 1686 | Ultramafic rocks | Sparse | 1.2 | 1.80 |
| 44 | Lake of Batakëve 2 | 41°34′19.41″ N | 20°15′0.57″ E | 1715 | Ultramafic rocks | Moderate | 1 | 1.60 |
| 45 | Lake of Batakëve 3 | 41°34′9.58″ N | 20°15′3.87″ E | 1773 | Ultramafic rocks | Sparse | 2.5 | 1.50 |
| Allamani Lakes | ||||||||
| 46 | Lake of Micekut | 41°34′10.54″ N | 20°13′2.00″ E | 1860 | Ultramafic rocks | None | 4.1 | 1.70 |
| 47 | Lake of Allamani | 41°34′27.30″ N | 20°12′46.85″ E | 1784 | Ultramafic rocks | None | 5.34 | 1.60 |
| 48 | Goat Lake | 41°34′41.09″ N | 20°12′49.19″ E | 1780 | Ultramafic rocks | Sparse | 1.7 | 1.30 |
| 49 | Lake of Flowers (Allaman) | 41°34′58.98″ N | 20°12′49.46″ E | 1805 | Ultramafic rocks | Dense | 1.22 | 1.35 |
| 50 | Lake of Kolë Madhi | 41°35′14.77″ N | 20°13′40.09″ E | 1715 | Ultramafic rocks | Moderate | 3 | 2.60 |
| Pas Deja Lura | ||||||||
| 51 | The stone of Virgo | 41°40′40.82″ N | 20°12′22.27″ E | 1555 | Ultramafic rocks | None | 3.15 | 3.01 |
| 52 | Lake of Pas Deja | 41°41′15.32″ N | 20°10′48.81″ E | 1892 | Ultramafic rocks | None | 0.44 | 2.01 |
| 53 | Lake of Flowers (Lura) | 41°44′23.01″ N | 20°11′55.45″ E | 1588 | Ultramafic rocks | None | 1.44 | 2.30 |
| 54 | Kallaba Lake | 41°44′32.19″ N | 20°11′49.31″ E | 1594 | Ultramafic rocks | Moderate | 4.16 | 2.20 |
| 55 | The Dryed Lake | 41°45′5.52″ N | 20°11′55.41″ E | 1636 | Ultramafic rocks | None | 2.64 | 1.91 |
| 56 | Black Lake, Lurë | 41°45′8.89″ N | 20°11′35.56″ E | 1743 | Ultramafic rocks | None | 2.56 | 2.20 |
| 57 | Hoti Lake | 41°45′51.55″ N | 20°11′36.86″ E | 1683 | Ultramafic rocks | None | 1.84 | 2.50 |
| 58 | Lake of Bruçi | 41°47′17.97″ N | 20°11′46.25″ E | 1724 | Ultramafic rocks | Dense | 0.7 | 2.01 |
| 59 | Kurti Lake | 41°47′17.89″ N | 20°12′0.08″ E | 1683 | Ultramafic rocks | Dense | 1.1 | 2.10 |
| 60 | Lake of Rrasave | 41°47′33.47″ N | 20°11′42.88″ E | 1710 | Ultramafic rocks | Sparse | 4 | 2.02 |
| 61 | Lake in Lura | 41°47′31.59″ N | 20°11′31.77″ E | 1730 | Ultramafic rocks | Sparse | 0.4 | 2.50 |
| 62 | Lake of Cows | 41°47′58.60″ N | 20°11′20.81″ E | 1620 | Ultramafic rocks | Dense | 1.5 | 2.50 |
| Korabi Mts | ||||||||
| 63 | Lake of Ladys | 41°45′22.79″ N | 20°30′29.15″ E | 1884 | Granitic rocks | Dense | 1.7 | 1.80 |
| 64 | Grama Lake | 41°45′34.20″ N | 20°29′35.71″ E | 1754 | Granitic rocks | None | 5 | 1.75 |
| 65 | Lakes of Steps | 41°47′58.12″ N | 20°30′4.53″ E | 1786 | Calcareous | Moderate | 0.7 | 1.70 |
| 66 | Black Lake, Radomira | 41°49′12.26″ N | 20°29′13.54″ E | 1470 | Granitic rocks | Moderate | 0.8 | 2.80 |
| Sylbicë-Doberdol | ||||||||
| 67 | Lake of Zanave | 42°30′59.12″ N | 20° 5′4.61″ E | 2207 | Granitic rocks | None | 0.9 | 0.80 |
| 68 | Lake of Black Peak | 42°31′9.03″ N | 20° 5′0.44″ E | 2215 | Granitic rocks | None | 0.7 | 1.10 |
| 69 | Sylbica Lake | 42°31′34.13″ N | 20° 5′23.25″ E | 2090 | Granitic rocks | Sparse | 3 | 1.51 |
| 70 | Yellow Lake | 42°31′15.59″ N | 20° 5′20.66″ E | 2087 | Granitic rocks | None | 1.1 | 1.10 |
| 71 | Lake of Dogs | 42°31′21.56″ N | 20° 5′6.77″ E | 2135 | Granitic rocks | None | 1.1 | 1.21 |
| 72 | Southern Lake | 42°30′29.75″ N | 20° 5′47.21″ E | 2000 | Granitic rocks | None | 0.5 | 1.75 |
| 73 | Lake of Sheep | 42°30′16.59″ N | 20° 6′4.01″ E | 2010 | Granitic rocks | None | 1 | 1.80 |
| 74 | Lake of Dashi | 42°31′47.24″ N | 20° 4′36.82″ E | 2180 | Granitic rocks | None | 3.5 | 2.01 |
| 75 | Beri Lake | 42°32′21.87″ N | 20° 4′25.22″ E | 1994 | Granitic rocks | Moderate | 0.7 | 2.01 |
| Albanian Alps | ||||||||
| 76 | Lake of Lulashi | 42°28′5.23″ N | 19°49′0.61″ E | 1665 | Calcareous | None | 1.3 | 1.90 |
| 77 | Lake of Shalë | 42°28′11.16″ N | 19°48′44.75″ E | 1755 | Calcareous | None | 1.47 | 2.50 |
| 78 | Lake of Lohjanit | 42°28′4.94″ N | 19°48′40.08″ E | 1757 | Calcareous | None | 2.63 | 2.60 |
| 79 | Lake of Mjelsave | 42°27′55.40″ N | 19°48′31.38″ E | 1806 | Calcareous | None | 1.2 | 2.70 |
| 80 | Great Lake of Jezerca | 42°27′39.34″ N | 19°48′23.68″ E | 1795 | Calcareous | None | 4.3 | 2.80 |
| 81 | Lake of SheuiBardhë | 42°27′27.42″ N | 19°46′22.64″ E | 1672 | Calcareous | None | 0.7 | 2.90 |
| 82 | Lake of Peshkeqes | 42°26′51.13″ N | 19°46′14.50″ E | 1616 | Calcareous | None | 0.7 | 3.10 |
| 83 | Ponari Lake | 42°22′12.03″ N | 22°00′50.08″ E | 1363 | Calcareous | Moderate | 2.2 | 2.80 |
| 84 | Lake of Kelmendi fortress | 42°27′5.22″ N | 19°42′45.38″ E | 1757 | Calcareous | None | 1.2 | 1.80 |
| Variables | Vegetation Cover | Frequency (N) | Mean ± Std. Deviation |
|---|---|---|---|
| Lakes Altitude | none | 54 | 1870.45 ± 187.16 |
| sparse | 13 | 1761.85 ± 113.60 | |
| moderate | 8 | 1638.50 ± 154.24 | |
| dense | 8 | 1777.62 ± 102.00 | |
| very dense | 1 | 1660.00 ± 0.0 | |
| Total | 84 | 1820.19 ± 181.56 | |
| Geomorphology Watershed | none | 54 | 3.06 ± 0.74 |
| sparse | 13 | 3.08 ± 0.28 | |
| moderate | 8 | 2.88 ± 0.64 | |
| dense | 8 | 3.13± 0.36 | |
| very dense | 1 | 2.00 ± 0.0 | |
| Total | 84 | 3.04 ± 0.65 | |
| Water Level Oscillation of the Lakes | none | 54 | 1.95± 0.58 |
| sparse | 13 | 1.90 ± 0.48 | |
| moderate | 8 | 2.43 ± 0.81020 | |
| dense | 8 | 1.88 ± 0.34 | |
| very dense | 1 | 3.50± 0.0 | |
| Total | 84 | 1.99 ± 0.60 |
| Variables | Sum of Squares | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|---|
| Altitude | Between Groups | 484774.18 | 4 | 121193.55 | 4.254 | 0.004 |
| Within Groups | 2250632.77 | 79 | 28489.02 | |||
| Total | 2735406.95 | 83 | ||||
| Geomorphology Watershed | Between Groups | 1.39 | 4 | 0.35 | 0.817 | 0.518 |
| Within Groups | 33.51 | 79 | 0.43 | |||
| Total | 34.90 | 83 | ||||
| Water Oscillation | Between Groups | 4.12 | 4 | 1.03 | 3.206 | 0.017 |
| Within Groups | 25.38 | 79 | 0.32 | |||
| Total | 29.50 | 83 | ||||
| Variables | Rotifer Species (n) | Frequency N | Mean Std. Deviation |
|---|---|---|---|
| Lakes Altitude | 3 | 1 | 2090.00 ± 0.0 |
| 4 | 2 | 2217.00 ± 207.89 | |
| 5 | 1 | 2100.00 ± 0.0 | |
| 6 | 1 | 2006.00 ± 0.0 | |
| 7 | 2 | 1842.50 ± 88.39 | |
| 9 | 1 | 1710.00 ± 0.0 | |
| 10 | 1 | 1740.00 ± 0.0 | |
| 13 | 1 | 1610.00 ± 0.0 | |
| 15 | 1 | 1470.00 ± 0.0 | |
| Total | 11 | 1895.00 ± 260.07 | |
| Lake Vegetation Cover | 3 | 1 | 0.00 ± 0.0 |
| 4 | 2 | 0.00 ± 0.0 | |
| 5 | 1 | 0.00 ± 0.0. | |
| 6 | 1 | 0.00 ± 0.0 | |
| 7 | 2 | 0.50 ± 0.707 | |
| 9 | 1 | 2.00 ± 0.0 | |
| 10 | 1 | 2.00 ± 0.0 | |
| 13 | 1 | 4.00 ± 0.0 | |
| 15 | 1 | 3.00 ± 0.0 | |
| Total | 11 | 1.09 ± 1.45 |
| Sum of Squares | df | Mean Square | F | Sig. | ||
|---|---|---|---|---|---|---|
| Lakes’ Altitude | Between Groups | 625,351.50 | 8 | 78,168.94 | 3.064 | 0.269 |
| Within Groups | 51,030.50 | 2 | 25,512.25 | |||
| Total | 676,382.00 | 10 | ||||
| Vegetation Cover of Lakes | Between Groups | 20.41 | 8 | 2.55 | 10.205 | 0.0092 |
| Within Groups | 500 | 2 | 0.25 | |||
| Total | 20.91 | 10 | ||||
| Name of the Lake | Altitude (m) | Geomorphology of the Watershed | Vegetation Cover | Surface (ha) | Species Richness |
|---|---|---|---|---|---|
| Lake of Dragani | 1660 | Calcareous | Very dense | 7.1 | 4 |
| Lake of Lilies (Valamara) | 1865 | Ultramafic rocks | Dense | 2 | 3 |
| Lake of Flowers (Kacni) | 1900 | Ultramafic rocks | Dense | 6.1 | 5 |
| Goat Lake | 1780 | Ultramafic rocks | Sparse | 1.7 | 5 |
| Lake of Kacnia 1 | 1740 | Ultramafic rocks | Dense | 1.72 | 6 |
| Lake of Flowers (Allaman) | 1805 | Ultramafic rocks | Dense | 1.22 | 5 |
| Lake of Flowers (Lurë) | 1588 | Ultramafic rocks | Very dense | 1.44 | 7 |
| Lake of Bruçi | 1724 | Ultramafic rocks | Dense | 0.7 | 5 |
| Kurti Lake | 1683 | Ultramafic rocks | Dense | 1.1 | 5 |
| Lake of Cows | 1620 | Ultramafic rocks | Dense | 1.5 | 6 |
| Lake of Ladies | 1884 | Granitic rocks | Dense | 1.7 | 6 |
| Variable Pairs | N Valid Samples | Spearman Coefficient R | t(N-2) Statistics | p-Value |
|---|---|---|---|---|
| S and area | 14 | 0.2341 * | 1.6543 | 0.0154 |
| S and % of trained staff | 14 | 0.3323 * | 2.1234 | 0.0321 |
| S and ranger numbers per ha | 14 | 0.0110 | 0.7654 | 0.4321 |
| S and national category | 14 | 0.2876 * | 2.212 | 0.0245 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumka, S.; Shumka, L.; Špoljar, M.; Shuka, L. Evidence of Climate Change and the Conservation Needed to Halt the Further Deterioration of Small Glacial Lakes. Climate 2024, 12, 124. https://doi.org/10.3390/cli12080124
Shumka S, Shumka L, Špoljar M, Shuka L. Evidence of Climate Change and the Conservation Needed to Halt the Further Deterioration of Small Glacial Lakes. Climate. 2024; 12(8):124. https://doi.org/10.3390/cli12080124
Chicago/Turabian StyleShumka, Spase, Laura Shumka, Maria Špoljar, and Lulëzim Shuka. 2024. "Evidence of Climate Change and the Conservation Needed to Halt the Further Deterioration of Small Glacial Lakes" Climate 12, no. 8: 124. https://doi.org/10.3390/cli12080124
APA StyleShumka, S., Shumka, L., Špoljar, M., & Shuka, L. (2024). Evidence of Climate Change and the Conservation Needed to Halt the Further Deterioration of Small Glacial Lakes. Climate, 12(8), 124. https://doi.org/10.3390/cli12080124








