The Response of Daily Carbon Dioxide and Water Vapor Fluxes to Temperature and Precipitation Extremes in Temperate and Boreal Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Meteorological and CO2 and H2O Flux Data Sets
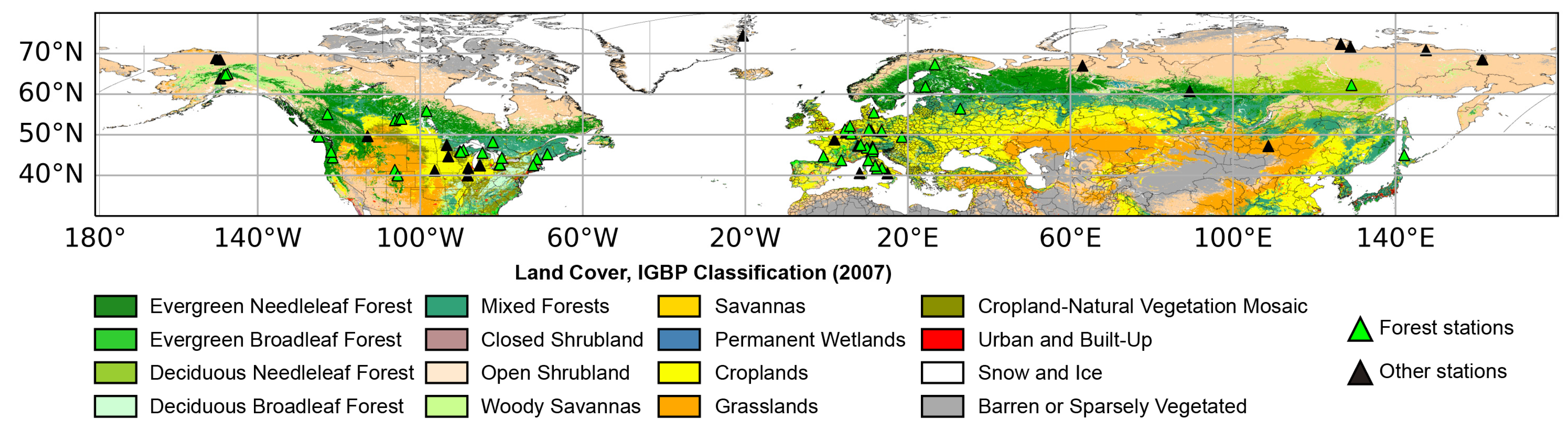
2.2. Selecting FLUXNET Stations for Flux Data Analysis
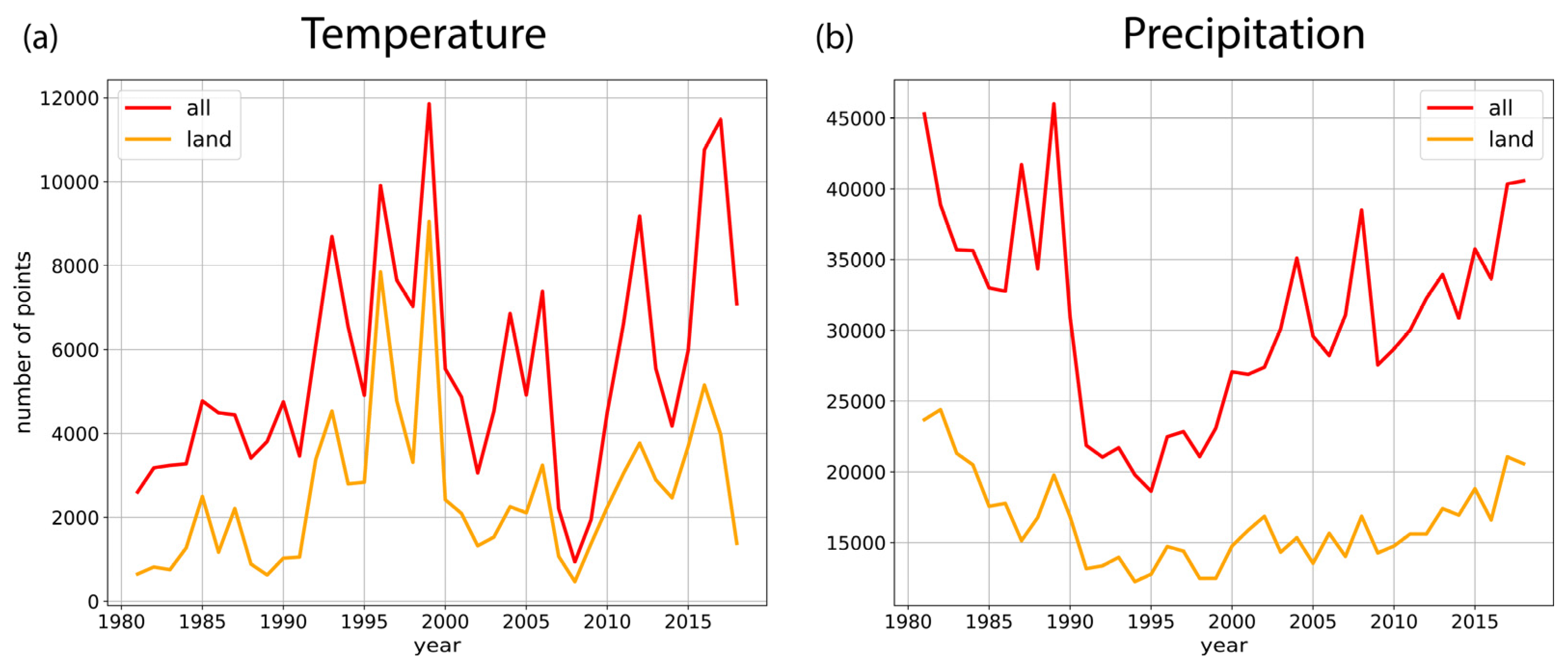
2.2.1. Statistical Analysis of Temperature and Precipitation Trends
2.2.2. Selecting FLUXNET Stations for Analysis of Flux Response to Extreme Weather Conditions
2.3. Data Analysis
3. Results and Discussion
3.1. The Response of NEE and LE Fluxes to the Extreme Temperature Anomalies
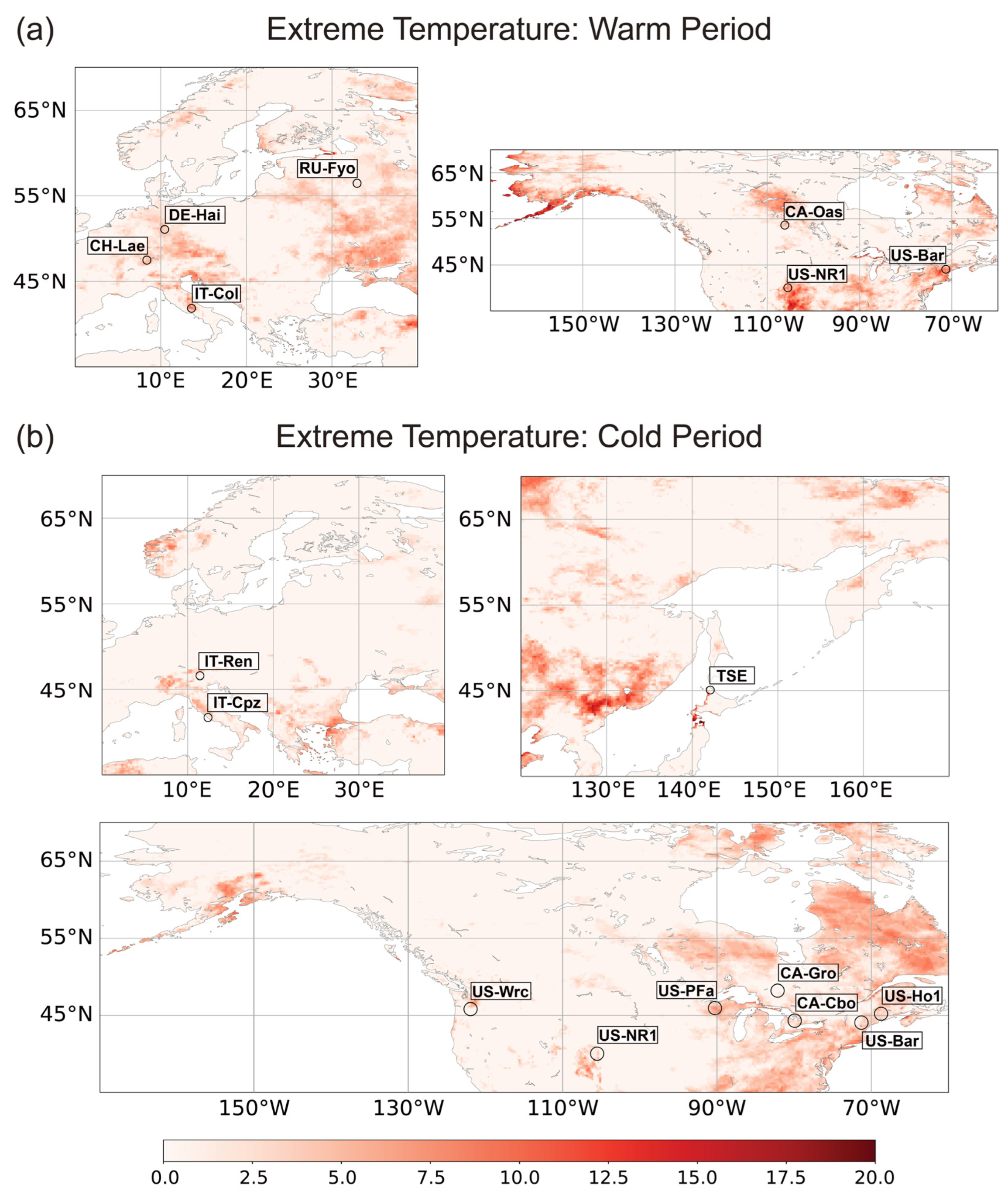
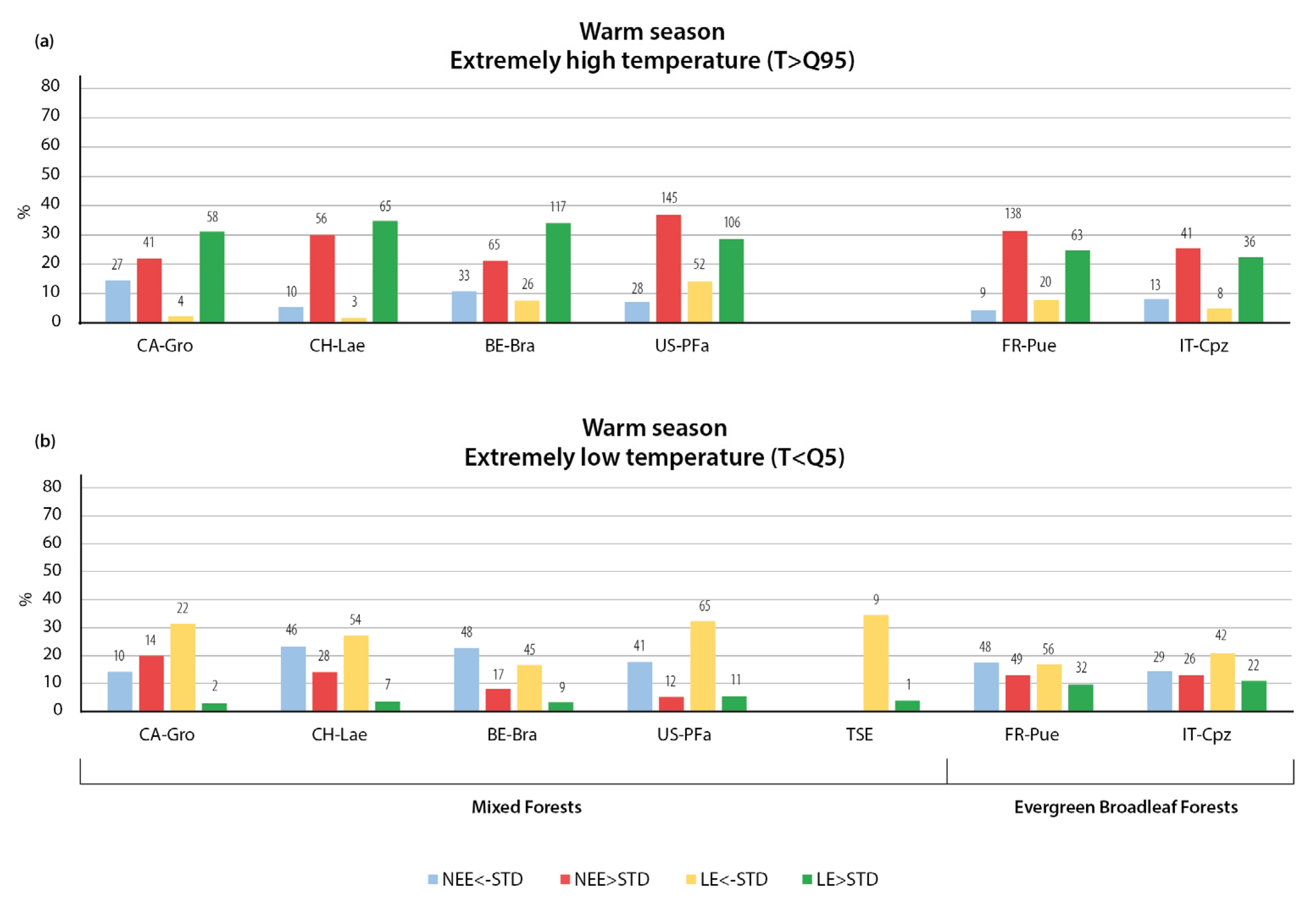
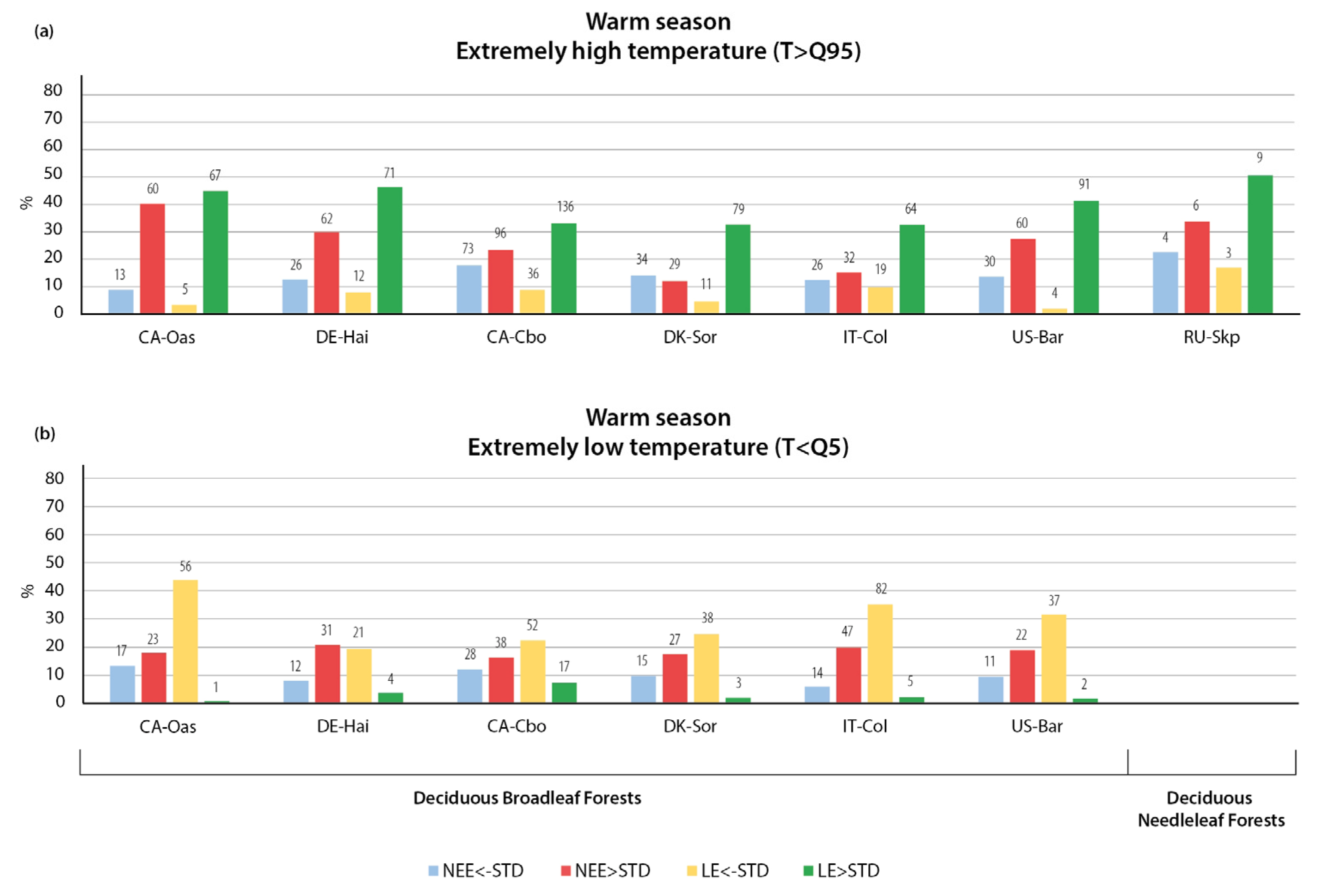
3.1.1. Variation in NEE and LE Flux in Warm Season
3.1.2. Variation in NEE and LE Flux in Cold Season
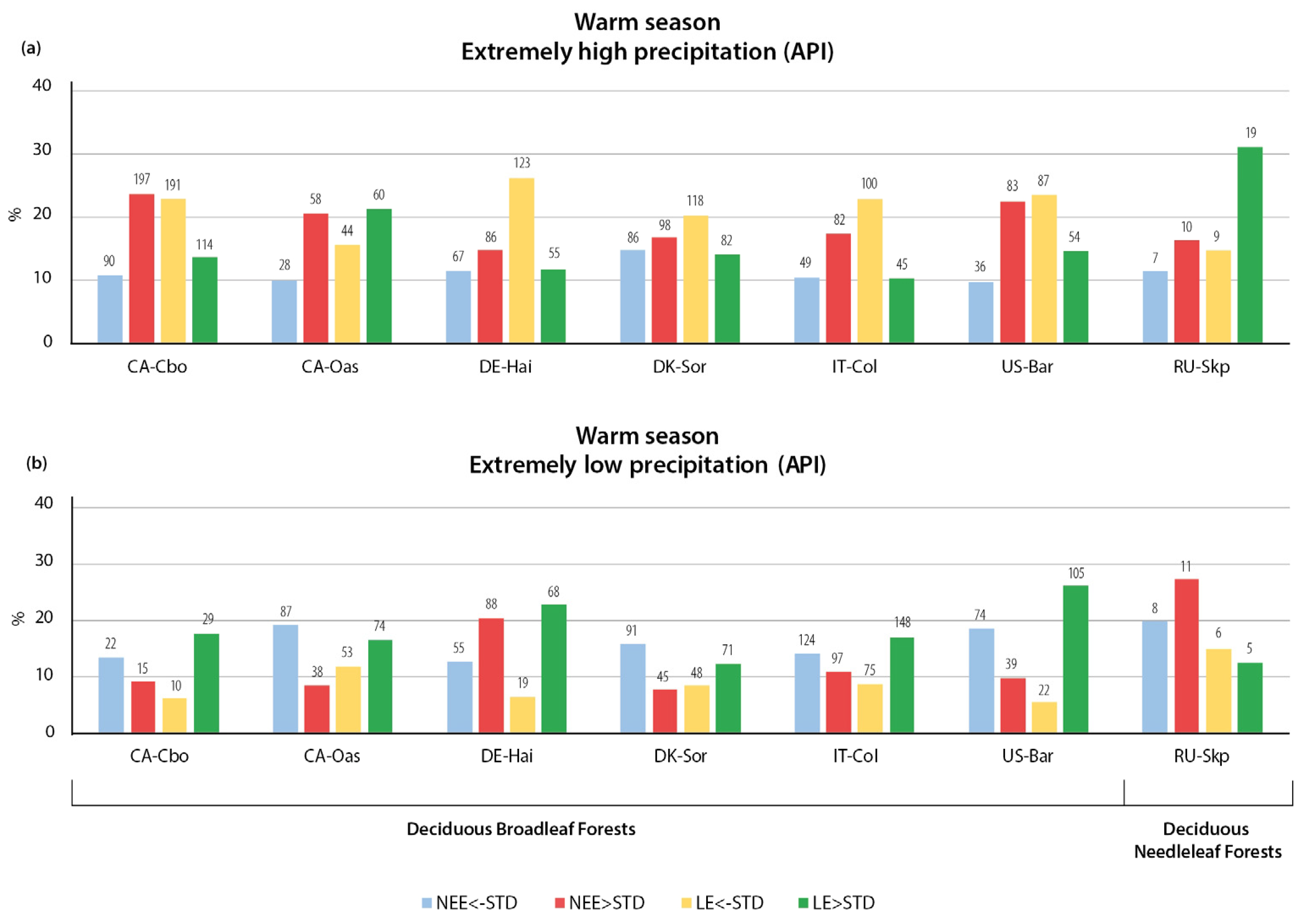
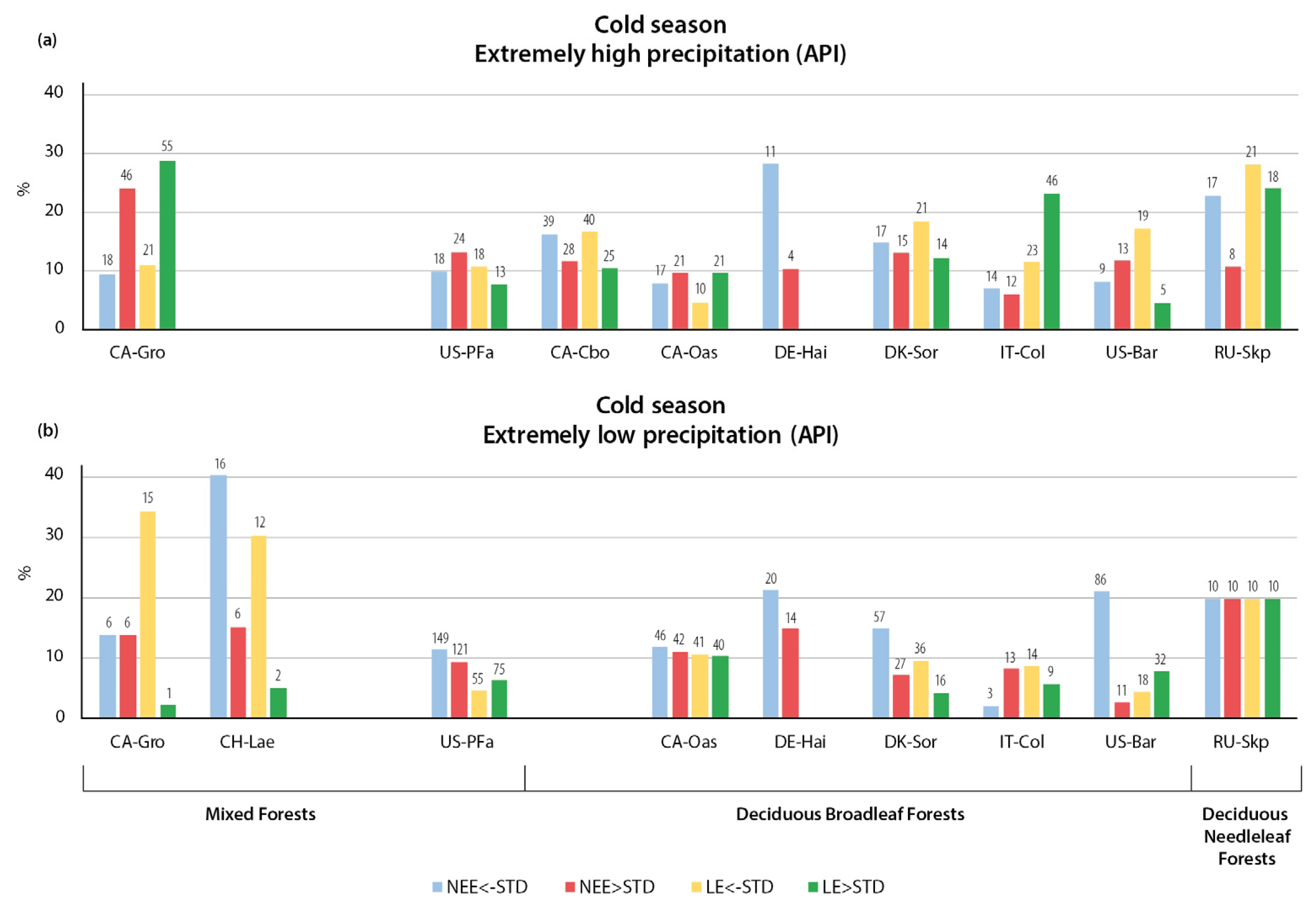
3.2. The Response of NEE and LE Fluxes to Extreme Precipitation
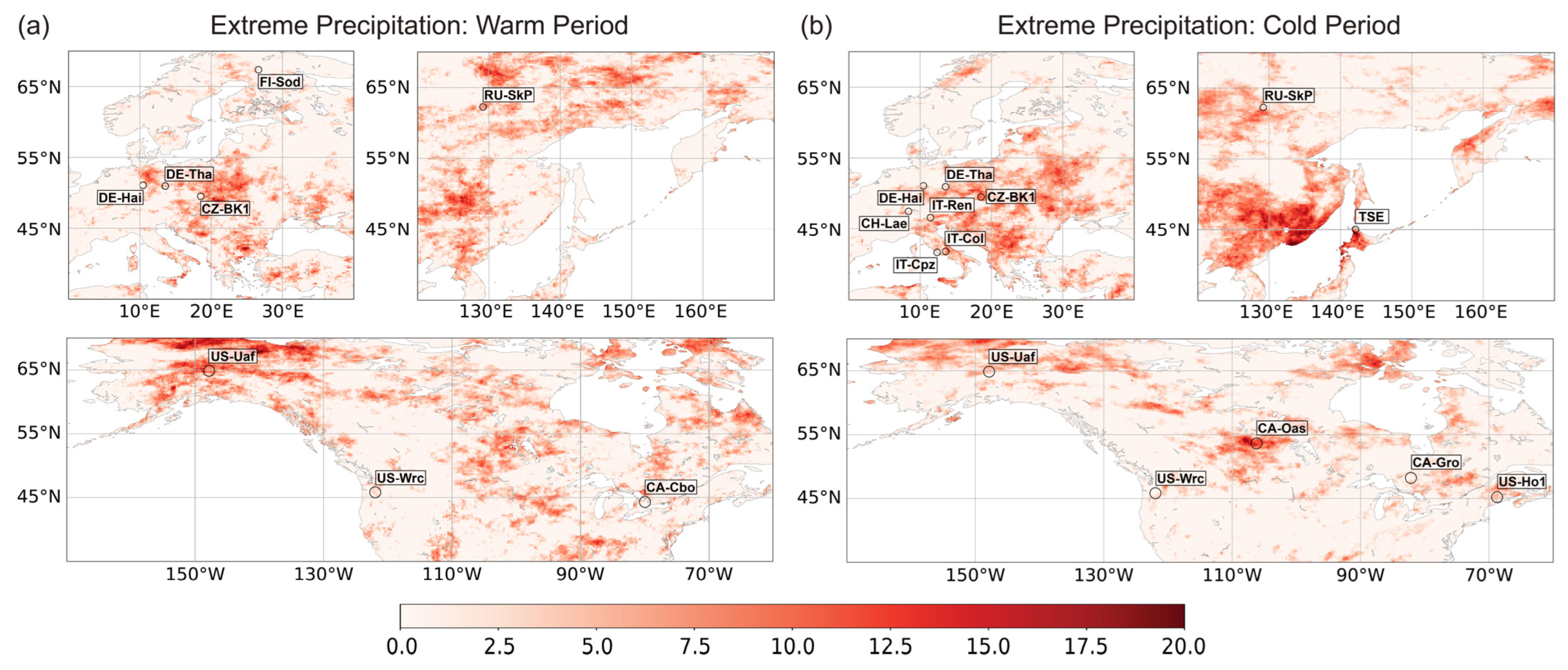
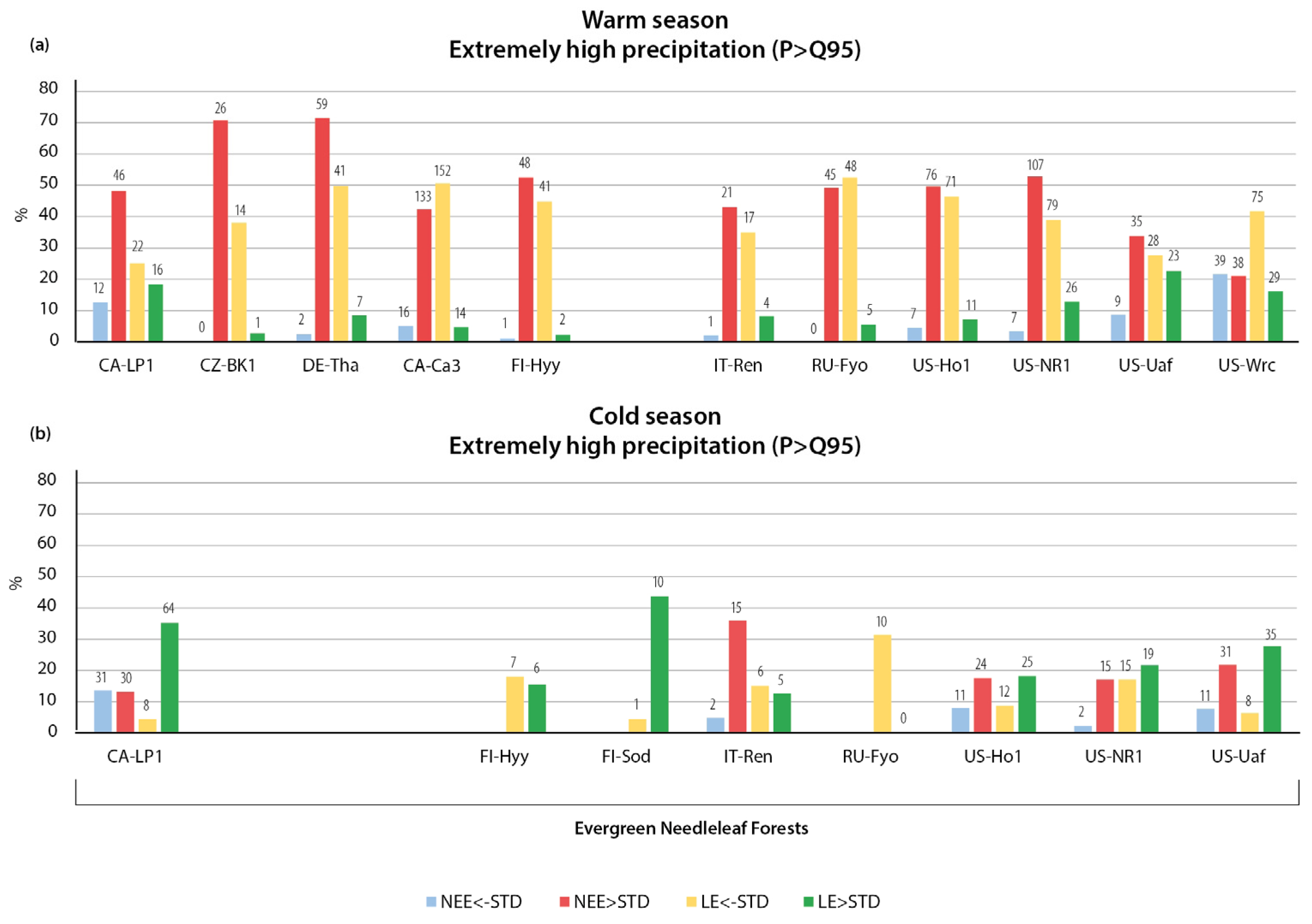
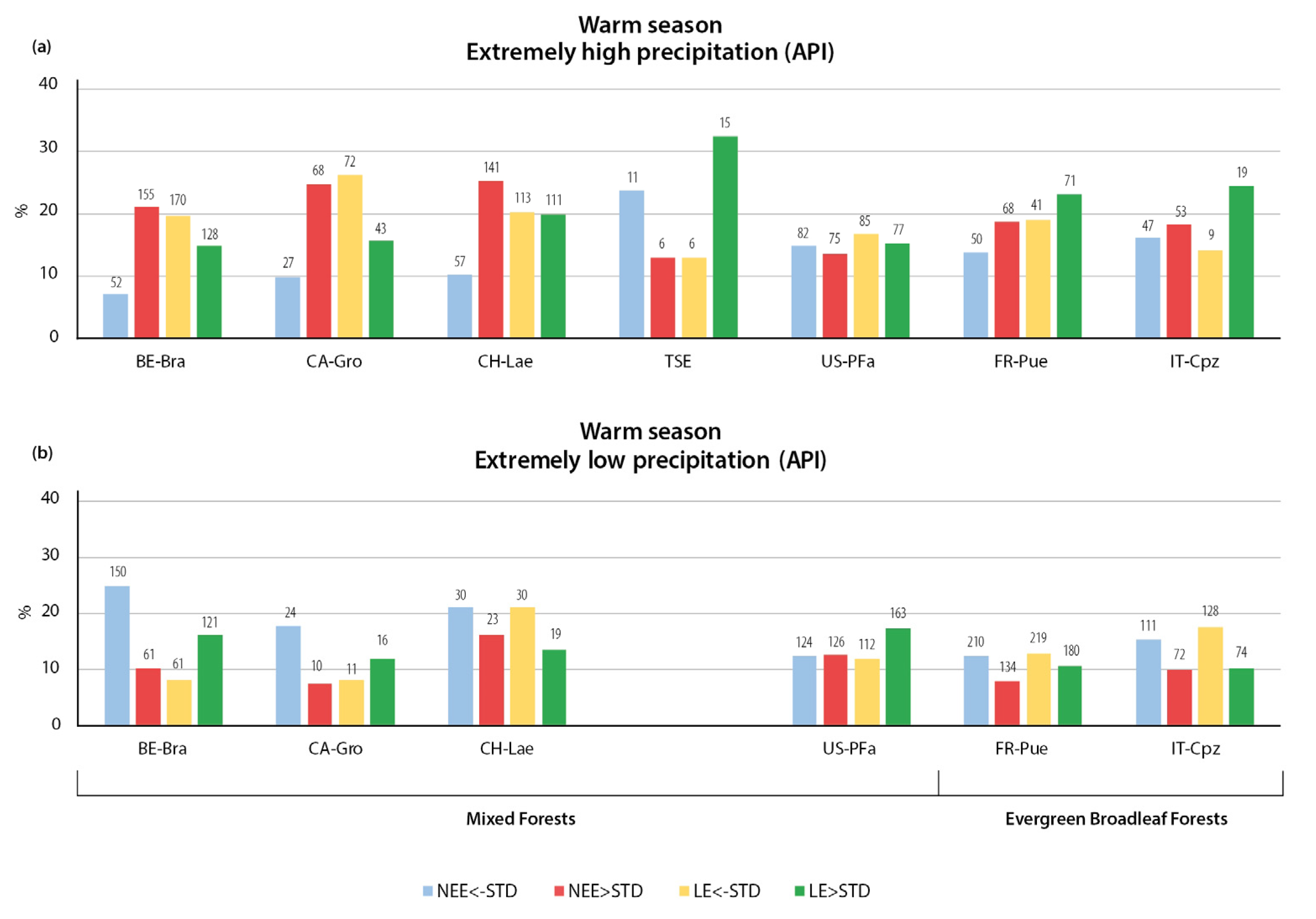
3.2.1. Variation in NEE and LE Flux in Warm Season
3.2.2. Variation in NEE and LE Flux in Cold Season
4. Conclusions
- The strong seasonal variability of atmospheric fluxes in response to weather extremes was found to be caused by differences in forest type, plant phenology, and functional traits, and the resilience of different tree species to atmospheric forcing.
- In the mid-latitudes, extreme temperature and precipitation had comparable effects on the NEE and LE fluxes, in contrast to the tropics, where the effect of precipitation on the fluxes dominated [45].
- Positive NEE anomalies were more frequent than negative anomalies in both the summer and the winter season. Extreme weather conditions were mostly associated with increased CO2 emissions rather than CO2 uptake, due to suppressed assimilation processes associated with extremely hot and dry periods, a greater reduction in GPP at lower temperatures, and higher decomposition rates of soil organic matter and autotrophic and heterotrophic respiration during wet periods.
- Extremely high temperatures had a stronger effect on NEE and LE fluxes than air temperature decreases, with a more pronounced difference during the warm season. During the warm season, extremely high temperatures usually led to increased CO2 emissions in all forest types, with the largest response in boreal coniferous forests, whereas air temperature decreases mostly led to an intensification of CO2 uptake. During the cold season, extremely low temperatures were not accompanied by significant NEE anomalies, and the thaws had a significant impact on NEE, mostly leading to increased CO2 emissions due to intensified ecosystem respiration. The response of LE fluxes to temperature variations did not change significantly over the year, with higher temperatures leading to an increase in LE and lower temperatures leading to a decrease in LE.
- The relationships of CO2 and LE fluxes with precipitation extremes were more heterogeneous than the temperature changes. The key finding of the study is the opposite immediate and delayed responses of NEE and LE fluxes to heavy precipitation, indicating a more important dependence of CO2 and LE on sufficient or deficient soil moisture than on the presence or absence of precipitation. The immediate response to heavy precipitation was, in most cases, an increase in CO2 emissions and a decrease in LE. In the warm season, the cumulative effect of heavy precipitation was the opposite of the immediate effect, resulting in enhanced CO2 uptake and higher LE. During the cold season, the cumulative effect of precipitation was similar to the immediate effect—increased CO2 emissions during wet periods.
- An unexpected type of relationship was detected for the precipitation deficit conditions: In most of the forest types considered, low API values (determined for 14 antecedent days) were associated with enhanced CO2 uptake during both seasons, indicating that soil moisture is not a limiting factor for photosynthesis in these ecosystems. In addition, the incoming solar radiation was greater during periods without cloud cover and associated precipitation, resulting in higher rates of plant photosynthesis.
- The response of LE fluxes to cumulative precipitation forcing could be divided into two types, with almost equal numbers of stations in each group. The first type of relationship consisted of increased evaporation during the wet periods and decreased evaporation during the precipitation deficit. The second type was characterized by a high API value with a decrease in evaporation and a low API value with an increase in evaporation.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Global Forest Resources Assessment 2020: Main Report; FAO: Rome, Italy, 2020; pp. 11–23. [Google Scholar]
- Ritter, F.; Berkelhammer, M.; Garcia-Eidell, C. Distinct response of gross primary productivity in five terrestrial biomes to precipitation variability. Commun. Earth Environ. 2020, 1, 34. [Google Scholar] [CrossRef]
- Frank, D.; Reichstein, M.; Bahn, M.; Thonicke, K.; Frank, D.; Mahecha, M.D.; Smith, P.; van der Velde, M.; Vicca, S.; Babst, F.; et al. Effects of climate extremes on the terrestrial carbon cycle: Concepts, processes and potential future impacts. Glob. Chang. Biol. 2015, 21, 2861–2880. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.D.; Ishii, H.R.; Carter, K.R.; Miyazaki, Y.; Cavaleri, M.A.; Araki, M.G.; Azuma, W.A.; Inoue, Y.; Hara, C. Predicting effects of climate change on productivity and persistence of forest trees. Ecol. Res. 2020, 35, 562–574. [Google Scholar] [CrossRef]
- Myhre, G.; Alterskjær, K.; Stjern, C.W.; Hodnebrog, Ø.; Marelle, L.; Samset, B.H.; Sillmann, J.; Schaller, N.; Fischer, E.; Schulz, M.; et al. Frequency of extreme precipitation increases extensively with event rareness under global warming. Sci. Rep. 2019, 9, 16063. [Google Scholar] [CrossRef]
- Ummenhofer, C.C.; Meeh, I.G.A. Extreme weather and climate events with ecological relevance: A review. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160135. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Vicente-Serrano, S.M.; Salguero, R.S.; Gutierrez, E.; de Luis, M.; Sanguesa-Barreda, G.; Novak, K.; Rozas, V.; Tıscar, P.A.; et al. Forest resilience to drought varies across biomes. Glob. Chang. Biol. 2018, 24, 2143–2158. [Google Scholar] [CrossRef] [PubMed]
- Forzieri, G.; Dakos, V.; McDowell, N.G.; Ramdane, A.; Cescatti, A. Emerging signals of declining forest resilience under climate change. Nature 2022, 608, 534–539. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Zhang, Z.; Ju, W.; Zhou, Y.; Li, X. Revisiting the cumulative effects of drought on global gross primary productivity based on new long-term series data (1982–2018). Glob. Chang. Biol. 2022, 28, 3620–3635. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xiao, J.; Zhang, Z. Heatwave effects on gross primary production of northern mid-latitude ecosystems. Environ. Res. Lett. 2020, 15, 074027. [Google Scholar] [CrossRef]
- Xu, B.; Arain, M.A.; Black, T.A.; Law, B.E.; Pastorello, G.Z.; Chu, H. Seasonal variability of forest sensitivity to heat and drought stresses: A synthesis based on carbon fluxes from North American forest ecosystems. Glob. Chang. Biol. 2020, 26, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Kljun, N.; Black, T.A.; Griffis, T.J.; Barr, A.G.; Gaumont-Guay, D.; Morgenstern, K.; McCaughey, J.H.; Nesic, Z. Response of net ecosystem productivity of three boreal forest stands to drought. Ecosystems 2006, 9, 1128–1144. [Google Scholar] [CrossRef]
- Reichstein, M.; Ciais, P.; Papale, D.; Valentini, R.; Running, S.; Viovy, N.; Cramer, W.; Granier, A.; Ogee, J.; Allard, A.; et al. Reduction of ecosystem productivity and respiration during the European summer 2003 climate anomaly: A joint flux tower, remote sensing and modelling analysis. Glob. Chang. Biol. 2007, 13, 634–651. [Google Scholar] [CrossRef]
- Welp, L.R.; Randerson, J.T.; Liu, H.P. The sensitivity of carbon fluxes to spring warming and summer drought depends on plant functional type in boreal forest ecosystems. Agric. For. Meteorol. 2007, 147, 172–185. [Google Scholar] [CrossRef]
- Thompson, R.L.; Broquet, G.; Gerbig, C.; Koch, T.; Lang, M.; Monteil, G.; Munassar, S.; Nickless, A.; Scholze, M.; Ramonet, M.; et al. Changes in net ecosystem exchange over Europe during the 2018 drought based on atmospheric observations. Philos. Trans. R. Soc. B 2020, 375, 20190512. [Google Scholar] [CrossRef] [PubMed]
- Lindroth, A.; Holst, J.; Linderson, M.-L.; Aurela, M.; Biermann, T.; Heliasz, M.; Chi, J.; Ibrom, A.; Kolari, P.; Klemedtsson, L.; et al. Effects of drought and meteorological forcing on carbon and water fluxes in Nordic forests during the dry summer of 2018. Philos. Trans. R. Soc. 2020, 375, 20190516. [Google Scholar] [CrossRef] [PubMed]
- Arain, M.A.; Xu, B.; Brodeur, J.J.; Khomik, M.; Peichl, M.; Beamesderfer, E.; Restrepo-Couple, N.; Thorne, R. Heat and drought impact on carbon exchange in an age-sequence of temperate pine forests. Ecol. Process. 2022, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Mamkin, V.; Varlagin, A.; Yaseneva, I.; Kurbatova, J. Response of Spruce Forest Ecosystem CO2 Fluxes to Inter-Annual Climate Anomalies in the Southern Taiga. Forests 2022, 13, 1019. [Google Scholar] [CrossRef]
- Hoover, D.L.; Knapp, A.K.; Smith, M.D. The immediate and prolonged effects of climate extremes on soil respiration in a mesic grassland. J. Geophys. Res. Biogeosci. 2016, 121, 1034–1044. [Google Scholar] [CrossRef]
- Anjileli, H.; Huning, L.S.; Moftakhari, H.; Ashraf, S.; Asanjan, A.A.; Norouzi, H.; AghaKouchak, A. Extreme heat events heighten soil respiration. Sci. Rep. 2021, 11, 6632. [Google Scholar] [CrossRef]
- Sierra, C.A.; Harmon, M.E.; Thomann, E.; Perakis, S.S.; Loescher, H.W. Amplification and dampening of soil respiration by changes in temperature variability. Biogeosciences 2011, 8, 951–961. [Google Scholar] [CrossRef]
- Yang, Z.; Luo, X.; Shi, Y.; Zhou, T.; Luo, K.; Lai, Y.; Yu, P.; Liu, L.; Olchev, A.; Bond-Lamberty, B.; et al. Controls and variability of soil respiration temperature sensitivity across China. Sci. Total Environ. 2023, 871, 161974. [Google Scholar] [CrossRef] [PubMed]
- Flannigan, M.; Stocks, B.; Wotton, B. Climate change and forest fires. Sci. Total Environ. 2000, 262, 221–222. [Google Scholar] [CrossRef]
- Tchebakova, N.M.; Parfenova, E.; Soja, A.J. The effects of climate, permafrost and fire on vegetation change in Siberia in a changing climate. Environ. Res. Lett. 2009, 4, 045013. [Google Scholar] [CrossRef]
- Flower, C.E.; Gonzalez-Meler, M.A. Responses of Temperate Forest Productivity to Insect and Pathogen Disturbances. Annu. Rev. Plant Biol. 2015, 66, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Kirsanov, A.; Rozinkina, I.; Rivin, G.; Zakharchenko, D.; Olchev, A. Effect of natural forest fires on regional weather conditions in Siberia. Atmosphere 2020, 11, 1133. [Google Scholar] [CrossRef]
- Kirdyanov, A.V.; Saurer, M.; Siegwolf, R.; Knorre, A.A.; Prokushkin, A.S.; Sidorova, O.V.C.; Fonti, M.V.; Büntgen, U. Long-term ecological consequences of forest fires in the continuous permafrost zone of Siberia. Environ. Res. Lett. 2020, 15, 034061. [Google Scholar] [CrossRef]
- Charrier, G.; Martin-StPaul, N.; Damesin, C.; Delpierre, N.; Hänninen, H.; Torres-Ruiz, J.M.; Davi, H. Interaction of drought and frost in tree ecophysiology: Rethinking the timing of risks. Ann. For. Sci. 2021, 78, 40. [Google Scholar] [CrossRef]
- McCulley, R.L.; Boutton, T.W.; Archer, S.R. Soil Respiration in a Subtropical Savanna Parkland: Response to Water Additions. Soil Sci. Soc. Am. J. 2007, 7, 820–828. [Google Scholar] [CrossRef]
- Yuste, J.C.; Janssens, I.A.; Carrara, A.; Meiresonne, L.; Ceulemans, R. Interactive effects of temperature and precipitation on soil respiration in a temperate maritime pine forest. Tree Physiol. 2003, 23, 1263–1270. [Google Scholar] [CrossRef]
- Lei, N.; Han, J. Effect of precipitation on respiration of different reconstructed soils. Sci. Rep. 2020, 10, 7328. [Google Scholar] [CrossRef]
- Manzoni, S.; Chakrawal, A.; Fischer, T.; Schimel, J.P.; Porporato, A.; Vico, G. Rainfall intensification increases the contribution of rewetting pulses to soil heterotrophic respiration. Biogeosciences 2020, 17, 4007–4023. [Google Scholar] [CrossRef]
- Birch, H.F. Mineralisation of plant nitrogen following alternate wet and dry conditions. Plant Soil 1964, 20, 43–49. [Google Scholar] [CrossRef]
- Jarvis, P.; Rey, A.; Petsikos, C.; Wingate, L.; Rayment, M.; Pereira, J.; Banza, J.; David, J.; Miglietta, F.; Borghetti, M.; et al. Drying and Wetting of Mediterranean Soils Stimulates Decomposition and Carbon Dioxide Emission: The “Birch Effect”. Tree Physiol. 2007, 27, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Roby, M.C.; Scott, R.L.; Biederman, J.A.; Smith, W.K.; Moore, D.J.P. Response of soil carbon dioxide efflux to temporal repackaging of rainfall into fewer, larger events in a semiarid grassland. Front. Environ. Sci. 2022, 10, 940943. [Google Scholar] [CrossRef]
- Kramer, K.; Vreugdenhil, S.J.; van der Werf, D.C. Effects of flooding on the recruitment, damage and mortality of riparian tree species: A field and simulation study on the Rhine floodplain. For. Ecol. Manag. 2008, 255, 3893–3903. [Google Scholar] [CrossRef]
- Hilton, R.G.; Galy, A.; Hovius, N.; Chen, M.-C.; Horng, M.-J.; Chen, H. Tropical-cyclone-driven erosion of the terrestrial biosphere from mountains. Nat. Geosci. 2008, 1, 759–762. [Google Scholar] [CrossRef]
- Dinsmore, K.J.; Billett, M.F.; Dyson, K.E. Temperature and precipitation drive temporal variability in aquatic carbon and GHG concentrations and fluxes in a peatland catchment. Glob. Chang. Biol. 2013, 19, 2133–2148. [Google Scholar] [CrossRef] [PubMed]
- The Data Portal Serving the FLUXNET Community. Available online: https://fluxnet.org/data/ (accessed on 25 March 2023).
- Aubinet, M.; Vesala, T.; Papale, D. Eddy Covariance: A Practical Guide to Measurement and Data Analysis; Springer: Dordrecht, The Netherlands, 2012; p. 438. ISBN 9789400723504. [Google Scholar]
- Gushchina, D.; Tarasova, M.; Satosina, E.; Zheleznova, I.; Emelianova, E.; Novikova, E.; Olchev, A. Effects of Extreme Temperature and Precipitation Events on Daily CO2 Fluxes in the Tropics. Climate 2023, 11, 117. [Google Scholar] [CrossRef]
- The AmeriFlux Network. Available online: https://ameriflux.lbl.gov/ (accessed on 12 March 2023).
- The European Eddy Fluxes Database Cluster. Available online: http://www.europe-fluxdata.eu/home (accessed on 15 March 2023).
- The Regional Research Network AsiaFlux. Available online: https://www.asiaflux.net/ (accessed on 20 November 2022).
- Smith, M.D. An ecological perspective on extreme climatic events: A synthetic definition and framework to guide future research. J. Ecol. 2011, 99, 656–663. [Google Scholar] [CrossRef]
- Zheleznova, I.V.; Gushchina, D.Y. Variability of extreme air temperatures and precipitation in different natural zones in late XX and early XXI centuries according to ERA5 reanalysis data. Izv. Atmos. Ocean. Phys. 2023, 59, 479–488. [Google Scholar]
- Chatterjee, S.; Bisai, D.; Khan, A. Detection of Approximate Potential Trend Turning Points in Temperature Time Series (1941–2010) for Asansol Weather Observation Station, West Bengal, India. Atmos. Clim. Sci. 2014, 4, 64–69. [Google Scholar] [CrossRef]
- Sneyres, R. On the Statistical Analysis of Time Series of Observation. Tech. Note Word Meteorol. Organ. 1990, 143, 192–199. [Google Scholar]
- Mohsin, T.; Gough, W.A. Trend Analysis of Long Term Temperature Time series in the Greater Toronto Area (GTA). Theor. Appl. Climatol. 2009, 98, 311–327. [Google Scholar] [CrossRef]
- Loveland, T.R.; Reed, B.C.; Brown, J.F.; Ohlen, D.O.; Zhu, Z.; Yang, L.; Merchant, J.W. Development of a global land cover characteristics database and IGBP DISCover from 1 km AVHRR data. Int. J. Remote Sens. 2000, 21, 1303–1330. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Koppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Kohler, M.A.; Linsley, R.K. Predicting the runoff from storm rainfall. Weather Bur. Res. Pap. 1951, 30, 1–9. [Google Scholar]
- Li, X.; Wei, Y.; Li, F. Optimality of antecedent precipitation index and its application. J. Hydrol. 2021, 595, 126027. [Google Scholar] [CrossRef]
- Wutzler, T.; Lucas-Moffat, A.; Migliavacca, M.; Knauer, J.; Sickel, K.; Šigut, L.; Menzer, O.; Reichstein, M. Basic and extensible post-processing of eddy covariance flux data with REddyProc. Biogeosciences 2018, 15, 5015–5030. [Google Scholar] [CrossRef]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A.; et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Chang. Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Vekuri, H.; Tuovinen, J.P.; Kulmala, L.; Papale, D.; Kolari, P.; Aurela, M.; Laurila, T.; Liski, J.; Lohila, A. A widely-used eddy covariance gap-filling method creates systematic bias in carbon balance estimates. Sci. Rep. 2023, 13, 1720. [Google Scholar] [CrossRef]
- Oltchev, A.; Ibrom, A.; Panferov, O.; Gushchina, D.; Kreilein, H.; Popov, V.; Propastin, P.; June, T.; Rauf, A.; Gravenhorst, G.; et al. Response of CO2 and H2O fluxes in a mountainous tropical rainforest in equatorial Indonesia to El Niño events. Biogeosciences 2015, 12, 6655–6667. [Google Scholar] [CrossRef]
- Burns, S.P.; Blanken, P.D.; Turnipseed, A.A.; Hu, J.; Monson, R.K. The Influence of Warm-Season Precipitation on the Diel Cycle of the Surface Energy Balance and Carbon Dioxide at a Colorado Subalpine Forest Site. Biogeosciences 2015, 12, 7349–7377. [Google Scholar] [CrossRef]
- Suni, T.; Rinne, J.; Reissell, A.; Altimir, N.; Keronen, P.; Rannik, Ü.; Maso, M.D.; Kulmala, M.; Vesala, T. Long-term measurements of surface fluxes above a Scots pine forest in Hyytiälä, southern Finland, 1996–2001. Boreal Environ. Res. 2003, 8, 287–301. [Google Scholar]
- Mensah, C.; Šigut, L.; Fischer, M.; Foltýnová, L.; Jocher, G.; Acosta, M.; Kowalska, N.; Kokrda, L.; Pavelka, M.; Marshall, J.D.; et al. Assessing the Contrasting Effects of the Exceptional 2015 Drought on the Carbon Dynamics in Two Norway Spruce Forest Ecosystems. Atmosphere 2021, 12, 988. [Google Scholar] [CrossRef]
- Acosta, M.; Pavelka, M.; Montagnani, L.; Kutsch, W.; Lindroth, A.; Juszczak, R.; Janouš, D. Soil surface CO2 efflux measurements in Norway spruce forests: Comparison between four different sites across Europe—From boreal to alpine forest. Geoderma 2013, 192, 295–303. [Google Scholar] [CrossRef]
- Vygodskaya, N.N.; Varlagin, A.V.; Kurbatova, Y.A.; Olchev, A.V.; Panferov, O.I.; Tatarinov, F.A.; Shalukhina, N.V. Response of taiga ecosystems to extreme weather conditions and climate anomalies. Dokl. Biol. Sci. 2009, 429, 571–574. [Google Scholar] [CrossRef]
- Oltchev, A.; Cermak, J.; Gurtz, J.; Kiely, G.; Nadezhdina, N.; Tishenko, A.; Zappa, M.; Lebedeva, N.; Vitvar, T.; Albertson, J.D.; et al. The response of the water fluxes of the boreal forest region at the Volga’s source area to climatic and land-use changes. J. Phys. Chem. Earth 2002, 27, 675–690. [Google Scholar] [CrossRef]
- Venäläinen, A.; Lehtonen, I.; Laapas, M.; Ruosteenoja, K.; Tikkanen, O.-P.; Viiri, H.; Ikonen, V.-P.; Peltola, H. Climate change induces multiple risks to boreal forests and forestry in Finland: A literature review. Glob. Chang. Biol. 2020, 26, 4178–4196. [Google Scholar] [CrossRef] [PubMed]
- Vacek, S.; Prokupková, A.; Vacek, Z.; Buluek, D.; Simunek, V.; Králícek, I.; Prausová, R.; Hájek, V. Growth response of mixed beech forests to climate change, various management and game pressure in Central Europe. J. For. Sci. 2019, 65, 116–129. [Google Scholar] [CrossRef]
- Pilegaard, K.; Ibrom, A.; Courtney, M.S.; Hummelshøj, P.; Jensen, N.O. Increasing net CO2 uptake by a Danish beech forest during the period from 1996 to 2009. Agric. For. Meteorol. 2011, 151, 934–946. [Google Scholar] [CrossRef]
- Valentini, R.; De Angelis, P.; Matteucci, G.; Monaco, R.; Dore, S.; Mugnozza, G.E.S. Seasonal net carbon dioxide exchange of a beech forest with the atmosphere. Glob. Chang. Biol. 1996, 2, 199–207. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, Q.; Batkhishig, O.; Ouyang, Z. Relationship between Evapotranspiration and Land Surface Temperature under Energy- and Water-Limited Conditions in Dry and Cold Climates. Adv. Meteorol. 2016, 1835487, 1–9. [Google Scholar] [CrossRef]
- Teskey, R.; Wertin, T.; Bauweraerts, I.; Ameye, M.; McGuire, M.A.; Steppe, K. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 2015, 38, 1699–1712. [Google Scholar] [CrossRef]
- Fu, Z.; Ciais, P.; Bastos, A.; Stoy, P.C.; Yang, H.; Green, J.K.; Wang, B.; Yu, K.; Huang, Y.; Knohl, A.; et al. Sensitivity of gross primary productivity to climatic drivers during the summer drought of 2018 in Europe. Philos. Trans. R. Soc. B 2020, 375, 20190747. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, L.; Zhou, G.; Wang, Y.; Song, J.; Zhang, S.; Zhou, M. Extreme temperature events reduced carbon uptake of a boreal forest ecosystem in Northeast China: Evidence from an 11-year eddy covariance observation. Front Plant Sci. 2023, 14, 1119670. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Ma, Y.; Liu, L. Effects of Low Temperature on the Relationship between Solar-Induced Chlorophyll Fluorescence and Gross Primary Productivity across Different Plant Function Types. Remote Sens. 2022, 14, 3716. [Google Scholar] [CrossRef]
- Natali, S.M.; Watts, J.D.; Rogers, B.M.; Potter, S.; Ludwig, S.M.; Selbmann, A.-K.; Sullivan, P.F.; Abbott, B.W.; Arndt, K.A.; Birch, L.; et al. Large loss of CO2 in winter observed across the northern permafrost region. Nat. Clim. Chang. 2019, 9, 852–857. [Google Scholar] [CrossRef]
- Schädel, C.; Bader, M.K.-F.; Schuur, E.A.G.; Biasi, C.; Bracho, R.; Čapek, P.; De Baets, S.; Diáková, K.; Ernakovich, J.; Estop-Aragones, C.; et al. Potential carbon emissions dominated by carbon dioxide from thawed permafrost soils. Nat. Clim. Chang. 2016, 6, 950–953. [Google Scholar] [CrossRef]
- Contosta, A.R.; Burakowski, E.A.; Varner, R.K.; Frey, S.D. Winter soil respiration in a humid temperate forest: The roles of moisture, temperature, and snowpack. J. Geophys. Res. Biogeosci. 2016, 121, 3072–3088. [Google Scholar] [CrossRef]
- Monson, R.K.; Lipson, D.L.; Burns, S.P.; Turnipseed, A.A.; Delany, A.C.; Williams, M.W.; Schmidt, S.K. Winter forest soil respiration controlled by climate and microbial community composition. Nature 2006, 439, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Zscheischler, J.; Reichstein, M.; Harmeling, S.; Rammig, A.; Tomelleri, E.; Mahecha, M.D. Extreme Events in Gross Primary Production: A Characterization across Continents. Biogeosciences 2014, 11, 2909–2924. [Google Scholar] [CrossRef]
- Ueyama, M.; Iwata, H.; Harazono, Y. Autumn warming reduces the CO2 sink of a black spruce forest in interior Alaska based on a nine-year eddy covariance measurement. Glob. Chang. Biol. 2014, 20, 1161–1173. [Google Scholar] [CrossRef]
- Wharton, S.; Falk, M.; Bible, K.; Schroeder, M.; Paw, U.K.T. Old-growth CO2 flux measurements reveal high sensitivity to climate anomalies across seasonal, annual and decadal time scales. Agric. For. Meteorol. 2012, 161, 1–14. [Google Scholar] [CrossRef]
- Oltchev, A.; Cermak, J.; Nadezhdina, N.; Tatarinov, F.; Tishenko, A.; Ibrom, A.; Gravenhorst, G. Transpiration of a mixed forest stand: Field measurements and simulation using SVAT models. J. Boreal Environ. Res. 2002, 7, 389–397. [Google Scholar]
- Pardos, M.; del Río, M.; Pretzsch, H.; Jactel, H.; Bielak, K.; Bravo, F.; Brazaitis, G.; Defossez, E.; Engel, M.; Godvod, K.; et al. The greater resilience of mixed forests to drought mainly depends on their composition: Analysis along a climate gradient across Europe. For. Ecol. Manag. 2021, 481, 118687. [Google Scholar] [CrossRef]
- Falge, E.; Reth, S.; Brüggemann, N.; Butterbach-Bahl, K.; Goldberg, V.; Oltchev, A.; Schaaf, S.; Spindler, G.; Stiller, B.; Queck, R.; et al. Comparison of surface energy exchange models with eddy flux data in forest and grassland ecosystems of Germany. J. Ecol. Model. 2005, 188, 174–216. [Google Scholar] [CrossRef]
- Ibrom, A.; Schütz, C.; Tworek, T.; Morgenstern, K.; Oltchev, A.; Falk, M.; Constantin, J.; Gravenhorst, G. Eddy-correlation measurements of fluxes of CO2 and H2O above a spruce forest. J. Phys. Chem. Earth 1996, 21, 409–414. [Google Scholar] [CrossRef]
- Krishnan, P.; Black, T.A.; Jassal, R.S.; Chen, B.; Nesic, Z. Interannual variability of the carbon balance of three different-aged Douglas-fir stands in the Pacific Northwest. J. Geophys. Res. Biogeosci. 2009, 114, G04011. [Google Scholar] [CrossRef]
- Montagnani, L.; Manca, G.; Canepa, E.; Georgieva, E.; Acosta, M.; Feigenwinter, C.; Janous, D.; Kerschbaumer, G.; Lindroth, A.; Minach, L. A new mass conservation approach to the study of CO2 advection in an alpine forest. J. Geophys. Res. 2009, 114, D07306. [Google Scholar]
- Kurbatova, J.; Li, C.; Varlagin, A.; Xiao, X.; Vygodskaya, N. Modeling carbon dynamics in two adjacent spruce forests with different soil conditions in Russia. Biogeosciences 2008, 5, 969–980. [Google Scholar] [CrossRef]
- Mamkin, V.; Kurbatova, J.; Avilov, V.; Ivanov, D.; Kuricheva, O.; Varlagin, A.; Yaseneva, I.; Olchev, A. Energy and CO2 exchange in an undisturbed spruce forest and clear-cut in the Southern Taiga. Agric. For. Meteorol. 2019, 265, 252–268. [Google Scholar] [CrossRef]
- Takagi, K.; Fukuzawa, K.; Liang, N.; Kayama, M.; Nomura, M.; Hojyo, H.; Sugata, S.; Shibata, H.; Fukazawa, T.; Nakaji, T.; et al. Change in the CO2 balance under a series of forestry activities in a cool-temperate mixed forest with dense undergrowth. Glob. Chang. Biol. 2012, 15, 1275–1288. [Google Scholar] [CrossRef]
- Chen, X.; Liang, S.; Cao, Y.; He, T.; Wang, D. Observed contrast changes in snow cover phenology in northern middle and high latitudes from 2001–2014. Sci. Rep. 2015, 19, 16820. [Google Scholar] [CrossRef] [PubMed]
- Knohl, A.; Schulze, E.-D.; Kolle, O.; Buchmann, N. Large carbon uptake by an unmanaged 250-year-old deciduous forest in Central Germany. Agric. For. Meteorol. 2003, 118, 151–167. [Google Scholar] [CrossRef]
- Etzold, S.; Ruehr, N.K.; Zweifel, R.; Dobbertin, M.; Zingg, A.; Pluess, P.; Häsler, R.; Eugster, W.; Buchmann, N. The Carbon Balance of Two Contrasting Mountain Forest Ecosystems in Switzerland: Similar Annual Trends, but Seasonal Differences. Ecosystems 2011, 14, 1289–1309. [Google Scholar] [CrossRef]






| Stations | Long, Lat | Elev. (m) | Vegetation Type IGBP | Climate Type | Forest Species Composition | Age (Years) | Height (m) | Period |
|---|---|---|---|---|---|---|---|---|
| (1) BE-Bra | 51.31° N, 4.52° E | 16 | Mixed forests | Cfb, temperate oceanic | Pinus sylvestris, Quercus robur | 94 | 21 | 1999–2014 |
| (2) CA- Gro | 48.22° N, 82.16° W | 340 | Dfb, warm summer humid continental | Populus tremuloides, Picea marian, Picea glauca, Betula papyrifer, Abies balsame | 93 | 31 | 2003–2014 | |
| (3) CH- Lae | 47.48° N, 8.36° E | 689 | Dfb, warm summer humid continental | Picea abies, Fagus sylvatica, Fraxinus excelsior, Acer pseudoplatanus | 52–185 | 30.6 | 2004–2014 | |
| (4) US- PFa | 45.95° N, 90.27° W | 470 | Dfb, warm summer humid continental | Populus grandidentata, Betula pendula, Acer rubrum, Tilia tomentosa, Alnus incana | 110– 120 | 24 | 1996–2022 | |
| (5) TSE: Teshio CC-LaG | 45.06° N, 142.11° E | 70 | Conifer–hardwood mixed forest | Dfb, warm summer humid continental | Quercus crispula, Betula ermanii, Betula platyphylla var. japonica, Abies sachalinensis, Picea jezoensis | 175 | 18–25 | 2001–2002 |
| (6) CA- Oas | 53.63° N, 106.20° W | 530 | Deciduous broadleaf forests | Dfc, subarctic | Populus tremuloides, Populus balsamifera, Corylus cornuta, Alnus crispa | 104 | 22 | 1996–2010 |
| (7) DE- Hai | 51.08° N, 10.45° E | 430 | Dfb, warm summer humid continental | Fagus sylvatica, Fraxinus excelsior, Acer pseudoplantanus, Acer plantanoides, Carpinus betulus | 250 | 33 | 2000–2012 | |
| (8) DK- Sor | 55.49° N, 11.64° E | 40 | Cfb, temperate oceanic | Fagus sylvatica | 102 | 25.8 | 1996–2014 | |
| (9) IT-Col | 41.85° N, 13.59° E | 1560 | Cwa, monsoon-influenced humid subtropical | Fagus sylvatica | 90 | 20.2 | 1996–2014 | |
| (10) US-Bar | 44.06° N, 71.29° W | 272 | Dfb, warm summer humid continental | Fagus grandifolia, Acer saccharum, Betula alleghaniensis, Betula papyrifera, Tsuga canadensis | 120 | 19 | 2004–2017 | |
| (11) CA- Cbo | 44.32° N, 79.93° W | 120 | Dfb, warm summer humid continental | Acer rubrum, Pinus strobus, Populus grandidentata, Fraxinus americana | 107 | 22 | 1995–2020 | |
| (12) CZ- BK1 | 49.50° N, 18.54° E | 875 | Evergreen needleleaf forests | Dfb, warm summer humid continental | Picea abies | 27 | 12 | 2004–2014 |
| (13) DE- Tha | 50.96° N, 13.56° E | 385 | Cfb, temperate oceanic | Picea abies, Betula pendula, Larix decidua, Pinus sylvestris | 136 | 25 | 1996–2014 | |
| (14) FI-Hyy | 61.85° N, 24.29° E | 181 | Dfc, subarctic | Pinus sylvestris | 80 | 14 | 1996–2014 | |
| (15) FI-Sod | 67.36° N, 26.64° E | 180 | Dfc, subarctic | Pinus sylvestris | 100 | 12.7 | 2001–2014 | |
| (16) IT-Ren | 46.59° N, 11.43° E | 1730 | Cfb, temperate oceanic | Picea abies, Pinus cembra, Larix decidua | 90 | 29 | 1998–2013 | |
| (17) RU- Fyo | 56.46° N, 32.92° E | 265 | Dfb, warm summer humid continental | Picea abies, Betula pubescens | 150 | 15 | 1998–2014 | |
| (18) US- NR1 | 40.03° N, 105.55° W | 3050 | Dfc, subarctic | Abies lasiocarpa, Picea engelmannii, Pinus contorta | 118 | 18 | 1998–2014 | |
| (19) CA- LP1 | 55.11° N, 122.84° W | 751 | Csa, hot summer Mediterranean | Pinus contorta | 97 | 15 | 2007–2021 | |
| (20) CA- Ca3 | 49.53° N, 124.90° W | 170 | Cfb, temperate oceanic | Pseudotsuga menziesii, Thuja plicata, Abies grandis | 35 | 8 | 2001–2021 | |
| (21) US- Ho1 | 45.20° N, 68.74° W | 60 | Dfb, warm summer humid continental | Picea rubens, Pinus strobus, Tsuga canadensis | 130 | 20 | 1996–2020 | |
| (22) US- Uaf | 64.87° N, 147.86° W | 155 | Dwc, monsoon-influenced subarctic | Picea mariana | 85 | 3 | 2003–2021 | |
| (23) US- Wrc | 45.82° N, 121.95° W | 371 | Csb, warm summer Mediterranean | Picea rubens, Tsuga canadensis | 500 | 60 | 1999–2015 | |
| (24) IT-Cpz | 41.70° N, 12.38° E | 68 | Evergreen broadleaf forests | Csb, warm summer Mediterranean | Quercus ilex | 100 | 10 | 1997–2009 |
| (25) FR- Pue | 43.74° N, 3.60° E | 270 | Csa, hot summer Mediterranean | Buxus sempervirens, Quercus ilex | 129 | 19 | 2000–2014 | |
| (26) RU- SkP | 62.26° N, 129.17° E | 246 | Deciduous needleleaf forests | Dfc, subarctic | Larix, Salix, Betula pendula | 190 | 20 | 2012–2014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gushchina, D.; Tarasova, M.; Satosina, E.; Zheleznova, I.; Emelianova, E.; Gibadullin, R.; Osipov, A.; Olchev, A. The Response of Daily Carbon Dioxide and Water Vapor Fluxes to Temperature and Precipitation Extremes in Temperate and Boreal Forests. Climate 2023, 11, 206. https://doi.org/10.3390/cli11100206
Gushchina D, Tarasova M, Satosina E, Zheleznova I, Emelianova E, Gibadullin R, Osipov A, Olchev A. The Response of Daily Carbon Dioxide and Water Vapor Fluxes to Temperature and Precipitation Extremes in Temperate and Boreal Forests. Climate. 2023; 11(10):206. https://doi.org/10.3390/cli11100206
Chicago/Turabian StyleGushchina, Daria, Maria Tarasova, Elizaveta Satosina, Irina Zheleznova, Ekaterina Emelianova, Ravil Gibadullin, Alexander Osipov, and Alexander Olchev. 2023. "The Response of Daily Carbon Dioxide and Water Vapor Fluxes to Temperature and Precipitation Extremes in Temperate and Boreal Forests" Climate 11, no. 10: 206. https://doi.org/10.3390/cli11100206
APA StyleGushchina, D., Tarasova, M., Satosina, E., Zheleznova, I., Emelianova, E., Gibadullin, R., Osipov, A., & Olchev, A. (2023). The Response of Daily Carbon Dioxide and Water Vapor Fluxes to Temperature and Precipitation Extremes in Temperate and Boreal Forests. Climate, 11(10), 206. https://doi.org/10.3390/cli11100206









