Allelopathic Potential of Aqueous Extract from Acacia melanoxylon R. Br. on Lactuca sativa
Abstract
1. Introduction
2. Results
2.1. Effect of A. melanoxylon Foliage on Lettuce Growth and Biomass Accumulation
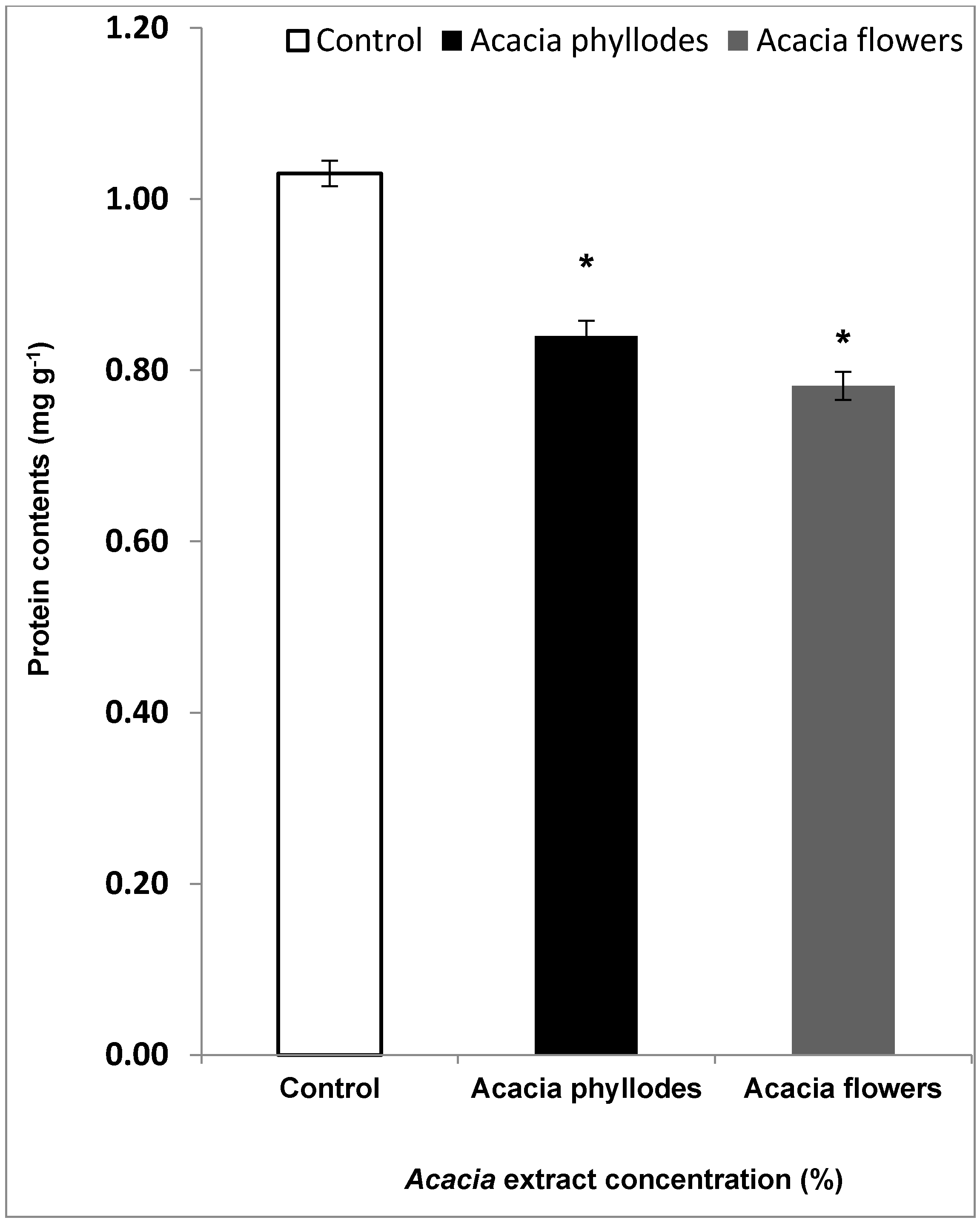
2.2. Effect of A. melanoxylon Foliage on Lettuce Leaf Protein Contents
2.3. Chemical Composition of the Acacia melanoxylon Aerial Foliage
3. Discussion
4. Methods and Materials
4.1. Plant Materials
4.2. Extraction of Polyphenols and HPLC Analysis
4.3. Extraction of Flavonoids from Flowers and Leaves of A. melanoxylon
4.4. UV-DIODE ARRAY Chemical Analyses
4.5. Acacia melanoxylon Flowers and Phyllodes Aqueous Extract Preparation for Bioassays
4.6. Plant Material and Growth Conditions
4.7. Plant Growth Measurements
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anaya, A.L.; Pelayo-Benavides, H.P. Allelopathic potential of Mirabilis jalapa L. (Nyctaginaceae): Effects on germination, growth and cell division of some plants. Allelop. J. 1997, 4, 57–68. [Google Scholar]
- Reigosa, M.J.; Sanchez-Moreiras, A.M.; Gonzalez, L. Ecophysiological approaches in allelopathy. Crit. Rev. Plant Sci. 1999, 18, 83–88. [Google Scholar] [CrossRef]
- Hussain, M.I.; Reigosa, M.J. Allelochemical stress inhibits growth, leaf water relations, PSII photochemistry, non-photochemical fluorescence quenching and heat energy dissipation in three C3 perennial species. J. Exp. Bot. 2011, 62, 4533–4545. [Google Scholar] [CrossRef]
- Lockwood, J.L.; Simberloff, D.; Mckinney, M.L.; Von Holle, B. How many, and which, plants will invade natural areas. Biol. Invasions 2001, 3, 1–8. [Google Scholar] [CrossRef]
- Hussain, M.I.; Reigosa, M.J. Characterization of xanthophyll pigments, photosynthetic performance, photon energy dissipation, reactive oxygen species generation and carbon isotope discrimination during artemisinin-induced stress in Arabidopsis thaliana. PLoS ONE 2015, 10, e0114826. [Google Scholar] [CrossRef][Green Version]
- Hussain, M.I.; El-Keblawy, A.; Tsombou, F.M. Leaf age, canopy position, and habitat affect the carbon isotope discrimination and water-use efficiency in three C3 leguminous Prosopis species from a hyper-arid climate. Plants 2019, 8, 402. [Google Scholar] [CrossRef]
- Hussain, M.I.; Tsombou, F.M.; El-Keblawy, A. Surface canopy position determines the photosystem II photochemistry in invasive and native Prosopis Congeners at Sharjah Desert, UAE. Forests 2020, 11, 740. [Google Scholar] [CrossRef]
- Ma, H.; Chen, Y.; Chen, J.; Zhang, Y.; Zhang, T.; He, H. Comparison of allelopathic effects of two typical invasive plants: Mikania micrantha and Ipomoea cairica in Hainan island. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Chen, B.M.; Liao, H.X.; Chen, W.B.; Wei, H.J.; Peng, S.L. Role of allelopathy in plant invasion and control of invasive plants. Allelop. J. 2017, 41, 155–166. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Involvement of allelopathy in the invasive potential of Tithonia diversifolia. Plants 2020, 9, 766. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Blair, A.C.; Dayan, F.E.; Johnson, R.D.; Meepagala, K.M.; Cook, D.; Bajsa, J. Is (−)-Catechin a novel weapon of spotted knapweed (Centaurea stoebe)? J. Chem. Ecol. 2009, 35, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Knapic, S.; Tavares, F.; Pereira, H. Heartwood and sapwood variation in Acacia melanoxylon R. Br. trees in Portugal. Forest 2006, 79, 371–380. [Google Scholar] [CrossRef]
- Souto, X.C.; González, L.; Reigosa, M.J. Comparative analysis of the allelopathic effects produced by four forestry species during the decomposition process in their soils in Galicia (NW Spain). J. Chem. Ecol. 1994, 20, 3005–3015. [Google Scholar] [CrossRef] [PubMed]
- Souto, X.C.; Bolano, J.C.; González, L.; Reigosa, M.J. Allelopathic effects of tree species on some soil microbial populations and herbaceous plants. Biolog. Plant. 2001, 44, 269–275. [Google Scholar] [CrossRef]
- González, L.; Souto, X.C.; Reigosa, M.J. Allelopathic effects of Acacia melanoxylon R. Br. phyllodes during their decomposition. For. Ecol. Manag. 1995, 77, 53–63. [Google Scholar] [CrossRef]
- Hussain, M.I.; González, L.; Souto, C.; Reigosa, M.J. Ecophysiological responses of native plants to phytotoxic effect of Acacia melanoxylon R. Br. Agrofor. Syst. 2011, 83, 149–166. [Google Scholar] [CrossRef]
- Farooq, M.; Khan, I.; Nawaz, A.; Cheema, M.A.; Siddique, K.H. Using sorghum to suppress weeds in autumn planted maize. Crop Prot. 2020, 133, 105162. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Nawaz, A.; Farooq, M. Allelopathic crop water extracts application improves the wheat productivity under low and high fertilizer inputs in a semi-arid environment. Int. J. Plant Prod. 2020, 14, 23–35. [Google Scholar] [CrossRef]
- Hussain, M.I.; González, L.; Reigosa, M.J. Phytotoxic effect of allelochemicals and herbicides on photosynthesis, growth and carbon isotope discrimination in Lactuca sativa. Allelop. J. 2010, 26, 157–174. [Google Scholar]
- Cheema, Z.A.; Khaliq, A.; Farooq, M. Sorghum allelopathy for weed management in wheat. In Allelopathy in Sustainable Agriculture and Forestry; Zeng, R.S., Mallik, A.U., Luo, S.M., Eds.; Springer: New York, NY, USA, 2008; pp. 255–270. [Google Scholar]
- Cheema, Z.A.; Khaliq, A.; Saeed, S. Weed control in maize (Zea mays L.) through sorghum allelopathy. J. Sustain. Agric. 2004, 23, 73–86. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E.; Romagni, J.G.; Rimando, A.M. Natural products as sources of herbicides: Current status and future trends. Weed Res. 2000, 40, 99–111. [Google Scholar] [CrossRef]
- Hussain, M.I.; Shackleton, R.T.; El-Keblawy, A.; Del Mar Trigo Pérez, M.; González, L. Invasive Mesquite (Prosopis juliflora), an allergy and health challenge. Plants 2020, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, S.W.; Shi, H.L.; Zhao, K.; Wang, J.; Liu, Y.; Liu, X.H.; Wang, W. Physiological and biochemical mechanisms mediated by allelochemical isoliquiritigenin on the growth of lettuce seedlings. Plants 2020, 9, 245. [Google Scholar] [CrossRef]
- Sánchez-Moreiras, M.A.; Reigosa, M.J. Whole plant response of lettuce after root exposure to BOA (2(3H)-benzoxazolinone). J. Chem. Ecol. 2005, 31, 2689–2703. [Google Scholar] [CrossRef] [PubMed]
- Farooq, N.; Abbas, T.; Tanveer, A.; Jabran, K. Allelopathy for weed management. In Co-Evolution of Secondary Metabolites; Mérillon, J.M., Ramawat, K., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2020; pp. 505–519. [Google Scholar] [CrossRef]
- AL-Wakeel, S.A.M.; Gabr, M.A.; Hamid, A.A.; Abu-EL-Soud, W.M. Allelopathic effects of Acacia nilotica leaf residue on Pisum sativun L. Allelop. J. 2007, 19, 23–34. [Google Scholar]
- Wang, S.; Wei, M.; Wu, B.; Cheng, H.; Wang, C. Combined nitrogen deposition and Cd stress antagonistically affect the allelopathy of invasive alien species Canada goldenrod on the cultivated crop lettuce. Sci. Hortic. 2020, 261, 108955. [Google Scholar] [CrossRef]
- EL-Khawas, S.; Shehata, M.M. The allelopathic potential of Acacia nilotica and Eucalyptus rostrata on monocot (Zea mays L.) and dicot (Phaseolus Vulgaris L.) plants. Biotechnology 2005, 4, 23–34. [Google Scholar]
- Lu, Y.; Wang, Y.; Wu, B.; Wang, S.; Wei, M.; Du, D.; Wang, C. Allelopathy of three Compositae invasive alien species on indigenous Lactuca sativa L. enhanced under Cu and Pb pollution. Sci. Hortic. 2020, 267, 109323. [Google Scholar] [CrossRef]
- Chou, C.H.; Fu, C.Y.; Li, S.Y.; Wang, Y.F. Allelopathic potential of Acacia confusa and related species in Taiwan. J. Chem. Ecol. 1998, 24, 2131–2150. [Google Scholar] [CrossRef]
- Hoque, A.T.M.R.; Ahmad, R.; Uddin, M.B.; Hossain, M.K. Allelopathic effect of different concentration of water extracts of Acacia auriculiformis leaf on some initial growth parameters of five common agricultural crops. Pak. J. Agron. 2003, 2, 92–100. [Google Scholar]
- Oyun, M.B. Allelopathic potentialities of Gliricidia sepium and Acacia auriculiformis on the germination and seedling vigour of Maize (Zea maya L.). Amer. J. Agric. Biol. Sci. 2006, 1, 44–47. [Google Scholar] [CrossRef][Green Version]
- Roth, C.M.; Shroyer, J.P.; Paulsenice, G.M. Allelopathy of sorghum on wheat under several tillage systems. Agron. J. 2000, 92, 855–860. [Google Scholar] [CrossRef]
- Khaliq, A.; Matloob, A.; Irshad, M.S.; Tanveer, A.; Zamir, M.S.I. Organic weed management in maize (Zea mays L.) through integration of allelopathic crop residues. Pak. J. Weed Sci. Res. 2010, 16, 409–420. [Google Scholar]
- Djurdjevic, L.; Dinic, A.; Pavlovic, P.; Mitrovic, M.; Karadzic, B.; Tesevic, V. Allelopathic potential of Allium ursinum L. Biochem. Syst. Ecol. 2004, 32, 533–544. [Google Scholar] [CrossRef]
- Inderjit; Duke, S.O. Ecophysiological aspects of allelopathy. Planta 2003, 217, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Thiébaut, G.; Tarayre, M.; Rodríguez-Pérez, H. Allelopathic effects of native versus invasive plants on one major invader. Front. Plant Sci. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Rob, M.; Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity and identification of phytotoxic substances from Schumannianthus dichotomus. Plants 2020, 9, 102. [Google Scholar] [CrossRef]
- Hussain, M.I.; Reigosa, M.J. Higher peroxidase activity, leaf nutrient contents and carbon isotope composition changes in Arabidopsis thaliana are related to rutin stress. J. Plant Physiol. 2014, 171, 1325–1333. [Google Scholar] [CrossRef]
- Irimia, R.E.; Lopes, S.M.; Sotes, G.; Cavieres, L.A.; Eren, Ö.; Lortie, C.J.; French, K.; Hierro, J.L.; Rosche, C.; Callaway, R.M.; et al. Biogeographic differences in the allelopathy of leaf surface extracts of an invasive weed. Biol. Invasions 2019, 21, 3151–3168. [Google Scholar] [CrossRef]
- Zhang, F.J.; Guo, J.Y.; Chen, F.X.; Liu, W.X.; Wan, F.H. Identification of volatile compounds released by leaves of the invasive plant croftonweed (Ageratina adenophora, compositae), and their inhibition of rice seedling growth. Weed Sci. 2012, 60, 205–211. [Google Scholar] [CrossRef]
- Shen, S.; Xu, G.; Li, D.; Clements, D.R.; Jin, G.; Liu, S.; Yang, Y.; Chen, A.; Zhang, F.; Kato-Noguchi, H. Allelopathic potential of sweet potato (Ipomoea batatas) germplasm resources of Yunnan Province in southwest China. Acta Ecol. Sin. 2018, 38, 444–449. [Google Scholar] [CrossRef]
- Freire, C.S.; Silvestre, A.J.; Neto, C.P. Demonstration of long-chain n-alkyl caffeates and Δ7-steryl glucosides in the bark of Acacia species by gas chromatography–mass spectrometry. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2007, 18, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Macheix, J.J.; Fleuriet, A.; Billot, Y. Fruit Phenolics; C.K.C. Press Inc.: Boca Ratón, FL, USA, 1990. [Google Scholar]
- Bolaño, J.C.; González, L.; Souto, X.C. Análisis de compuestos fenólicos de bajo peso molecular inducidos por condiciones de estrés, mediante HPLC. In Manual de Técnicas en Ecofisiología Vegetal; Pedrol, N., Reigosa, M.J., Eds.; Gamesal: Vigo, Spain, 1997; pp. 31–38. [Google Scholar]
- Molina, A.; Reigosa, M.J.; Carballeira, A. Release of allelochemical agents from litter, throughfall, and topsoil in plantations of Eucalyptus globulus Labill in Spain. J. Chem. Ecol. 1991, 17, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, P.; Pazos-Malvido, E.; Gonzalez, L.; Reigosa, M.J. Allelopathic interference of invasive Acacia dealbata: Physiological effects. Allelop. J. 2008, 22, 452–462. [Google Scholar]
- Macías, F.A.; Castellano, D.; Molinillo, J.M.G. Search for a standard phytotoxic bioassay for allelochemicals. Selection of standard target species. J. Agric. Food Chem. 2000, 48, 2512–2521. [Google Scholar] [CrossRef]
| Growth Characteristics | Treatments | 100% | 75% | 50% | 25% |
|---|---|---|---|---|---|
| Leaf fresh weight (g) | Control | 10.58 ± 0.60 | 10.58 ± 0.60 | 10.58 ± 0.60 | 10.58 ± 0.60 |
| Acacia phyllodes | 7.73 ± 0.3 * | 7.93 ± 0.5 * | 8.63 ± 0.2 * | 8.29 ± 1.0 * | |
| Acacia flowers | 5.10 ± 0.4 * | 5.62 ± 0.3 * | 7.25 ± 0.34 * | 6.85 ± 1.0 * | |
| Leaf dry weight (g) | Control | 1.63 ± 0.15 | 1.63 ± 0.15 | 1.63 ± 0.15 | 1.63 ± 0.15 |
| Acacia phyllodes | 1.78 ± 0.04 * | 1.77 ± 0.1 * | 1.90 ± 0.4 * | 1.98 ± 0.3 * | |
| Acacia flowers | 1.23 ± 0.2 * | 1.21 ± 0.4 * | 1.45 ± 0.8 * | 1.64 ± 0.2 | |
| LDFW ratio | Control | 0.154 ± 0.07 | 0.154 ± 0.07 | 0.154 ± 0.07 | 0.154 ± 0.07 |
| Acacia phyllodes | 0.230 ± 0.0 * | 0.223 ± 0.00 * | 0.221 ± 0.0 * | 0.238 ± 0.05 * | |
| Acacia flowers | 0.272 ± 0.1 * | 0.215 ± 0.0 * | 0.200 ± 0.0 * | 0.240 ± 0.01 * |
| Growth Characteristics | Treatments | 100% | 75% | 50% | 25% |
|---|---|---|---|---|---|
| Root fresh weight (g) | Control | 6.31 ± 1.1 | 6.31 ± 1.0 | 6.31 ± 1.1 | 6.31 ± 1.2 |
| Acacia phyllodes | 4.57 ± 0.3 * | 3.9 ± 0.6 * | 4.11 ± 0.13 * | 4.5 ± 0.4 * | |
| Acacia flowers | 3.82 ± 0.1 * | 3.44 ± 1.3 * | 3.79 ± 1.3 * | 3.07 ± 0.6 * | |
| Rood dry weight (g) | Control | 0.40 ± 0.02 | 0.40 ± 0.03 | 0.40 ± 0.04 | 0.40 ± 0.05 |
| Acacia phyllodes | 0.27 ± 0.04 * | 0.32 ± 0.01 * | 0.29 ± 0.09 * | 0.37 ± 0.04 * | |
| Acacia flowers | 0.28 ± 0.2 * | 0.28 ± 0.04 * | 0.23 ± 0.001 * | 0.27 ± 0.09 * | |
| RDFW ratio | Control | 0.064 ± 0.02 | 0.064 ± 0.02 | 0.064 ± 0.02 | 0.064 ± 0.02 |
| Acacia phyllodes | 0.059 ± 0.01 | 0.082 ± 0.01 * | 0.071 ± 0.01 * | 0.082 ± 0.01 * | |
| Acacia flowers | 0.073 ± 0.14 * | 0.081 ± 0.01 * | 0.061 ± 0.01 * | 0.088 ± 0.01 * |
| Growth Characteristics | Treatments | 100% | 75% | 50% | 25% |
|---|---|---|---|---|---|
| Shoot length (cm) | Control | 15.30 ± 0.23 | 15.30 ± 0.23 | 15.30 ± 0.23 | 15.30 ± 0.23 |
| Acacia phyllodes | 7.53 ± 0.5 * | 8.96 ± 1.5 * | 7.66 ± 0.5 * | 9.16 ± 1.6 * | |
| Acacia flowers | 8.96 ± 2.2 * | 8.0 ± 0.5 * | 8.16 ± 2.0 * | 9.66 ± 0.5 * | |
| Root length (cm) | Control | 27.77 ± 0.7 | 27.77 ± 0.7 | 27.77 ± 0.7 | 27.77 ± 0.7 |
| Acacia phyllodes | 21.33 ± 2.3 * | 19.0 ± 1.5 * | 22.66 ± 2.5 * | 21.5 ± 2.7 * | |
| Acacia flowers | 19.66 ± 2.0 * | 17.3 ± 2.0 * | 22.0 ± 1.7 * | 21.3 ± 2.0 * | |
| RS ratio | Control | 1.82 ± 0.03 | 1.82 ± 0.03 | 1.82 ± 0.03 | 1.82 ± 0.03 |
| Acacia phyllodes | 2.83 ± 0.02 * | 2.12 ± 0.02 * | 2.95 ± 0.02 * | 2.34 ± 0.02 * | |
| Acacia flowers | 2.19 ± 0.01 * | 2.16 ± 0.02 * | 2.69 ± 0.01 * | 2.20 ± 0.02 * |
| Growth Characteristics | Treatments | 100% | 75% | 50% | 25% |
|---|---|---|---|---|---|
| Root fresh weight (g) | Control | 6.31 ± 0.34 | 6.31 ± 0.34 | 6.31 ± 0.34 | 6.31 ± 0.34 |
| Acacia phyllodes | 4.57 ± 0.7 * | 3.90 ± 0.07 * | 4.12 ± 0.3 * | 4.49 ± 0.4 * | |
| Acacia flowers | 3.82 ± 0.1 * | 3.44 ± 0.29 * | 3.79 ± 0.6 * | 3.07 ± 0.6 * | |
| Root dry weight (g) | Control | 0.45 ± 0.042 | 0.45 ± 0.042 | 0.45 ± 0.042 | 0.45 ± 0.042 |
| Acacia phyllodes | 0.27 ± 0.02 * | 0.323 ± 0.04 * | 0.290 ± 0.09 * | 0.373 ± 0.04 * | |
| Acacia flowers | 0.28 ± 0.06 * | 0.286 ± 0.04 * | 0.273 ± 0.01 * | 0.270 ± 0.09 * | |
| RDWFW ratio | Control | 0.071 ± 0.02 | 0.071 ± 0.02 | 0.071 ± 0.02 | 0.071 ± 0.02 |
| Acacia phyllodes | 0.059 ± 0.01 * | 0.083 ± 0.01 * | 0.070 ± 0.01ns | 0.083 ± 0.01 * | |
| Acacia flowers | 0.073 ± 0.04ns | 0.083 ± 0.04 * | 0.072 ± 0.01ns | 0.088 ± 0.01 * |
| Sr. No. | Common Name | Scientific Name | Flowers mg/L | Phyllodes mg/L | RT |
|---|---|---|---|---|---|
| PHENOLICS | |||||
| 1 | Gallic acid | 3,4,5-trihydroxy benzoic acid | 4.4 | 3.41 | 6.5 |
| 2 | Protocatechuic acid | 3,4-dihydroxybenzoic acid | 5.06 | 12.9 | |
| 3 | p-Hydroxybenzoic acid | 4-hydroxybenzoic acid | 12.33 | 1.46 | 21.9 |
| 4 | p-Hydroxybenzaldehyde | 0.91 | 0.16 | 28.6 | |
| 5 | Vanillic acid | 4-hydroxy-3-methoxybenzoic acid | 9.7 | 1.64 | 32.2 |
| 6 | Syringic acid | 4-hydroxy-3,5-dimethoxybenzoic acid | 2.64 | 40.1 | |
| 7 | p-Coumaric acid | 4-hydroxycinnamic acid | 3.19 | 3.6 | 49.7 |
| 8 | Ferulic acid | 4-hydroxy-3-methoxycinnamic acid | 3.87 | 57.9 | |
| FLAVONOIDS | |||||
| 9 | Rutin | 5342.39 | 5032.87 | 20.4 | |
| 10 | Quercetin | 3,3′,4′,5,7-Pentahydroxyflavone | 326.4 | 25.2 | |
| 11 | Luteolin | 3′,4′,5,7-Tetrahydroxyflavone | 388.89 | 706.89 | 26.2 |
| 12 | Apigenin | 4′,5,7-Trihydroxyflavone | 85.55 | 28.5 | |
| 13 | Catechin | (±)-3,3′,4′,5,7-Flavanpentol | 765.44 | 7.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, M.I.; El-Sheikh, M.A.; Reigosa, M.J. Allelopathic Potential of Aqueous Extract from Acacia melanoxylon R. Br. on Lactuca sativa. Plants 2020, 9, 1228. https://doi.org/10.3390/plants9091228
Hussain MI, El-Sheikh MA, Reigosa MJ. Allelopathic Potential of Aqueous Extract from Acacia melanoxylon R. Br. on Lactuca sativa. Plants. 2020; 9(9):1228. https://doi.org/10.3390/plants9091228
Chicago/Turabian StyleHussain, M. Iftikhar, Mohamed A. El-Sheikh, and Manuel J. Reigosa. 2020. "Allelopathic Potential of Aqueous Extract from Acacia melanoxylon R. Br. on Lactuca sativa" Plants 9, no. 9: 1228. https://doi.org/10.3390/plants9091228
APA StyleHussain, M. I., El-Sheikh, M. A., & Reigosa, M. J. (2020). Allelopathic Potential of Aqueous Extract from Acacia melanoxylon R. Br. on Lactuca sativa. Plants, 9(9), 1228. https://doi.org/10.3390/plants9091228








