Content of Capsaicinoids and Capsiate in “Filius” Pepper Varieties as Affected by Ripening
Abstract
1. Introduction
2. Results and Discussion
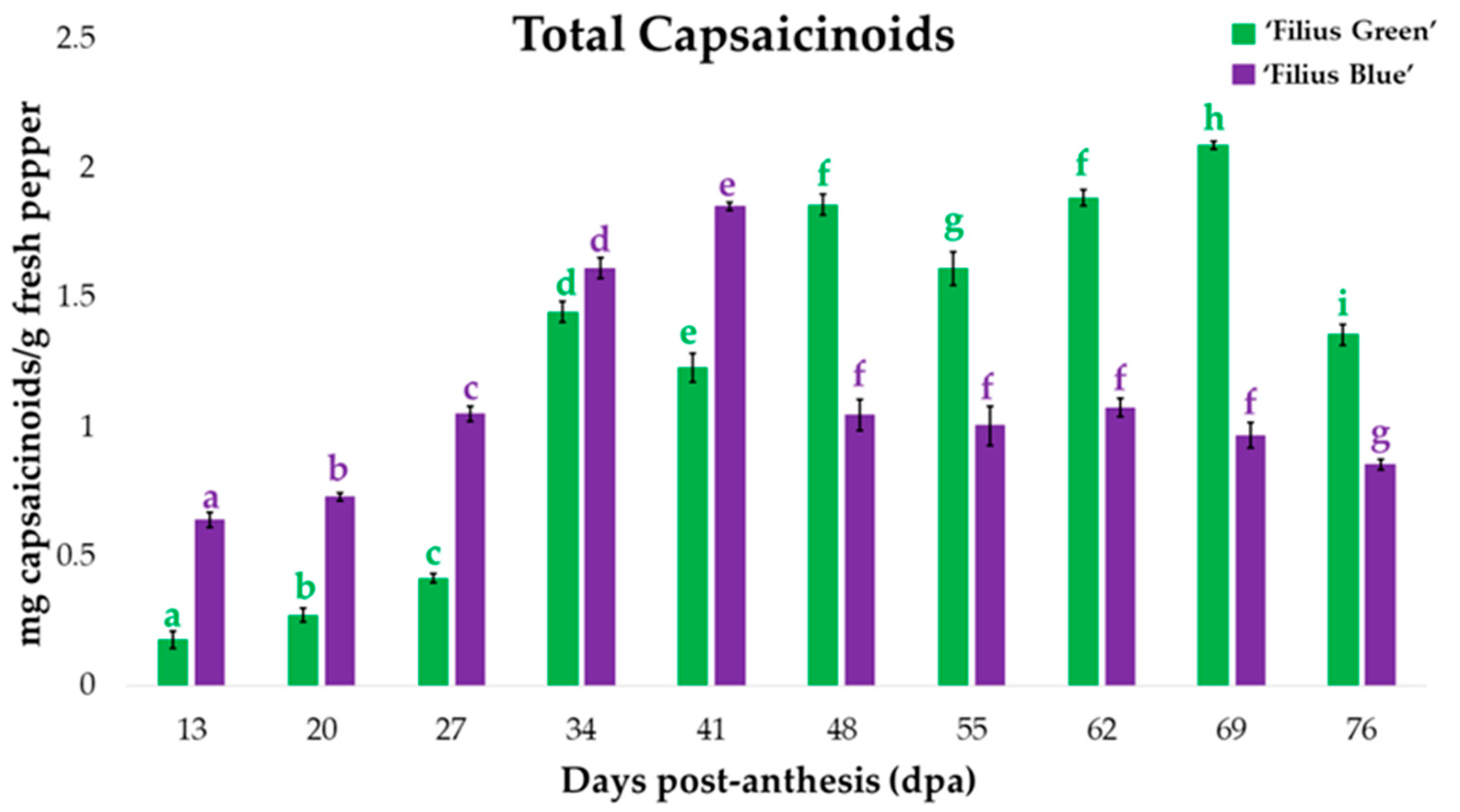
2.1. Changes in Peppers’ Total Capsaicinoids Content
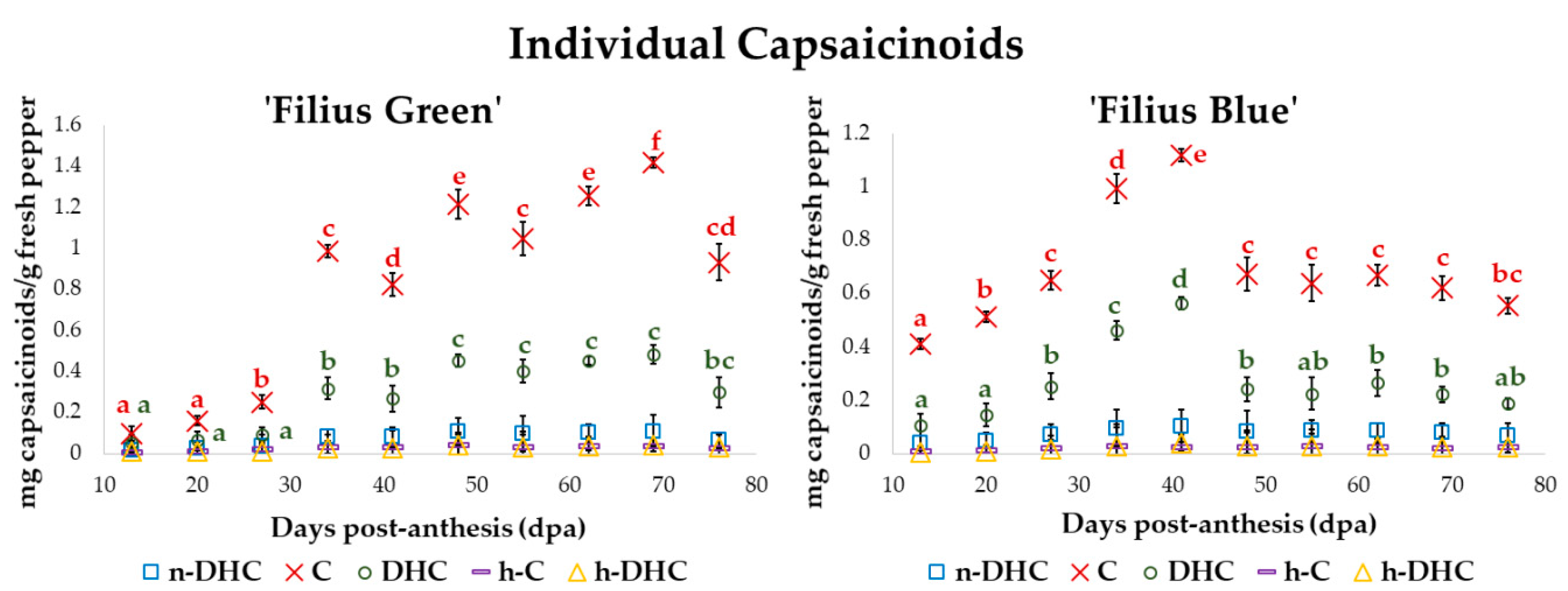
2.2. Changes in the Content of Individual Capsaicinoids
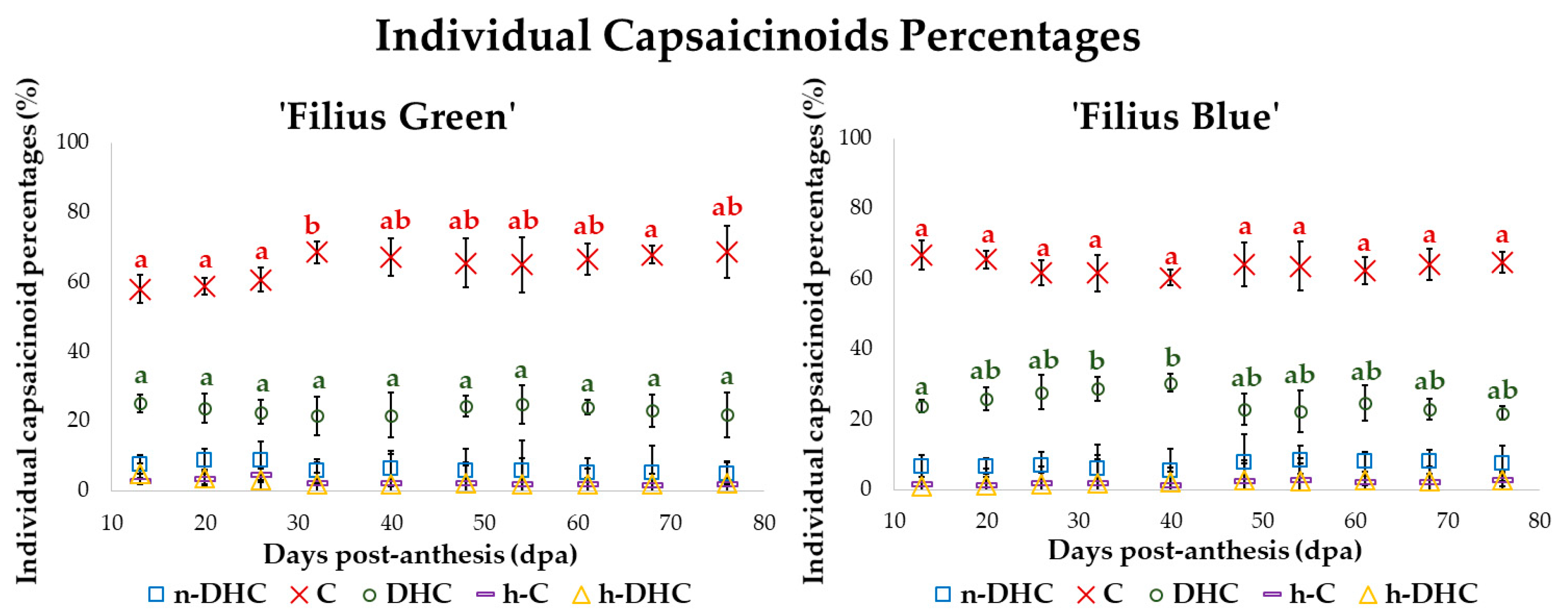
2.3. Changes in the Standardized Value of Capsaicinoids
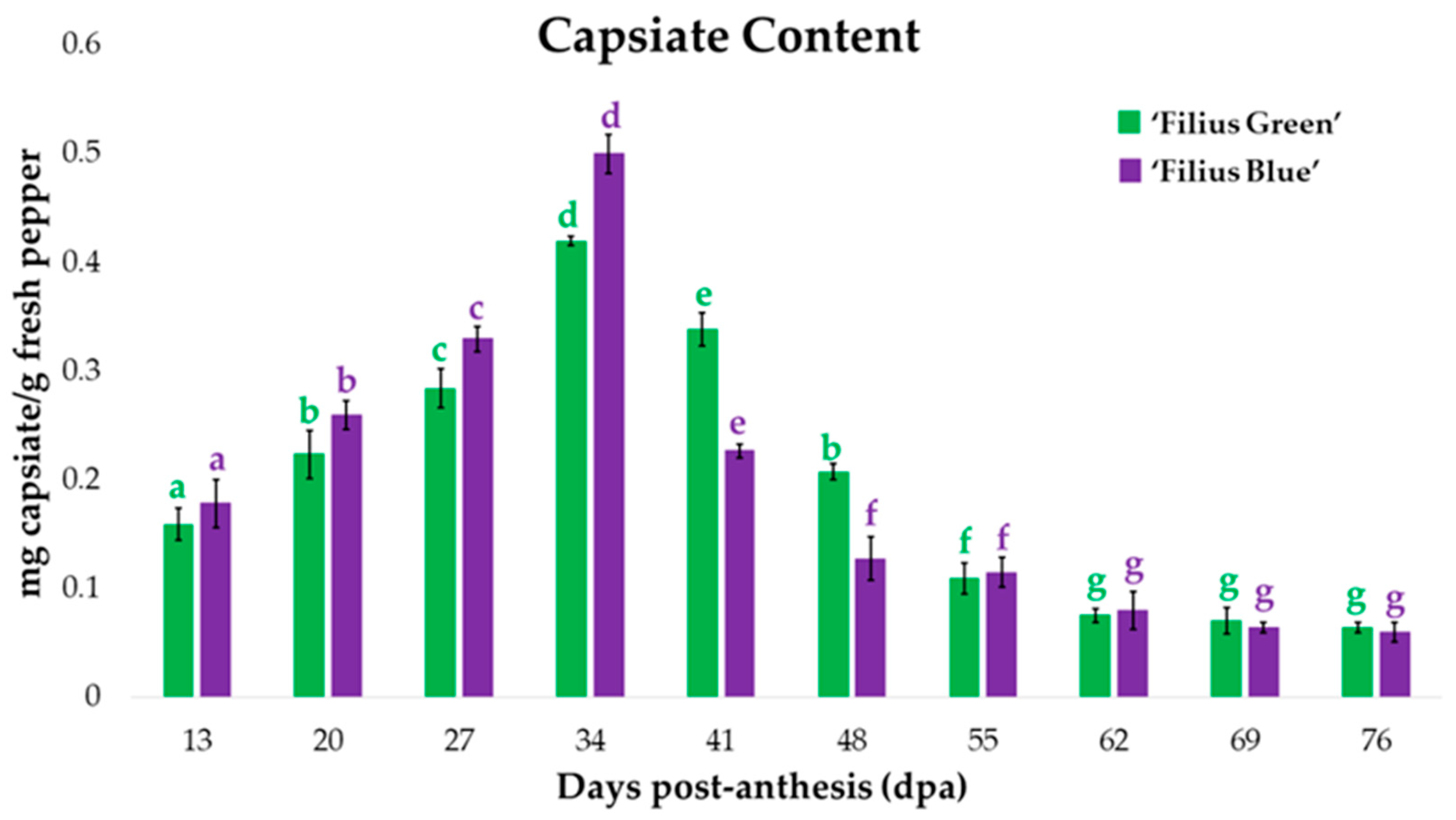
2.4. Changes in Capsiate Content during Fruit Ripening
3. Materials and Methods
3.1. Reagents
3.2. Pepper Cultivar and Growing Conditions
3.3. Ultrasound-Assisted Extraction of Fresh Pepper
3.4. Capsaicinoids and Capsiate Content
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pickersgill, B. Genetic resources and breeding of Capsicum spp. Euphytica 1997, 96, 129–133. [Google Scholar] [CrossRef]
- Serrano, M.; Zapata, P.J.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Valero, D. Antioxidant and nutritive constituents during sweet pepper development and ripening are enhanced by nitrophenolate treatments. Food Chem. 2010, 118, 497–503. [Google Scholar] [CrossRef]
- Stommel, J.R.; Griesbach, R.J. Inheritance of fruit, foliar, and plant habit attributes in Capsicum. J. Am. Soc. Hortic. Sci. 2008, 133, 396–407. [Google Scholar] [CrossRef]
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2018, 274, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.H.; Ramya, M.; An, H.R.; Park, P.M.; Kim, Y.-J.; Lee, S.Y.; Jang, S. Progress and challenges in the improvement of ornamental plants by genome editing. Plants 2020, 9, 687. [Google Scholar] [CrossRef] [PubMed]
- Enciclopedia de Chiles. Una Colección de Todas las Variantes de Chiles por el Mundo. Available online: https://elholandespicante.com/enciclopedia-de-chiles/ (accessed on 2 August 2020).
- Hamed, M.; Kalita, D.; Bartolo, M.E.; Jayanty, S.S. Capsaicinoids, polyphenols and antioxidant activities of Capsicum annuum: Comparative study of the effect of ripening stage and cooking methods. Antioxidants 2019, 8, 364. [Google Scholar] [CrossRef]
- Aiswarya, C.S.; Vijeth, S.; Sreelathakumary, I.; Prashant, K. Diallel analysis of Chili Pepper (Capsicum annuum L.) genotypes for morphological and fruit biochemical traits. Plants 2019, 9, 1. [Google Scholar] [CrossRef]
- Nelson, E.K.; Dawson, D.E. The constitution of capsaicin, the pungent principle of Capsicum. J. Am. Chem. Soc. 1923, 45, 2179–2181. [Google Scholar] [CrossRef]
- Fayos, O.; Mallor, C.; Garcés-Claver, A. Evolución del conocimiento sobre la pungencia de la cebolla (Allium cepa L.) y del pimiento (Capsicum spp.): Desde sus orígenes hasta el potencial nutracéutico actual. ITEA 2018, 114, 99–118. [Google Scholar] [CrossRef]
- Macho, A.; Sancho, R.; Minassi, A.; Appendino, G.; Lawen, A.; Muñoz, E. Involvement of reactive oxygen species in capsaicinoid-induced apoptosis in transformed cells. Free Radic. Res. 2003, 37, 611–619. [Google Scholar] [CrossRef]
- Rosa, A.; Deiana, M.; Casu, V.; Paccagnini, S.; Appendino, G.; Ballero, M.; Dessi, M.A. Antioxidant activity of capsinoids. J. Agric. Food Chem. 2002, 50, 7396–7401. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.I.; Kim, D.H.; Choi, J.W.; Yun, J.W. Proteomic analysis for antiobesity potential of capsaicin on white adipose tissue in rats fed with a high fat diet. J. Proteome Res. 2010, 9, 2977–2987. [Google Scholar] [CrossRef] [PubMed]
- Sancho, R.; Lucena, C.; Macho, A.; Calzado, M.A.; Blanco-Molina, M.; Minassi, A.; Appendino, G.; Muñoz, E. Immunosuppressive activity of capsaicinoids: Capsiate derived from sweet peppers inhibits NF-κB activation and is a potent antiinflammatory compound in vivo. Eur. J. Immunol. 2002, 32, 1753–1763. [Google Scholar] [CrossRef]
- Wong, G.Y.; Gavva, N.R. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res. Rev. 2009, 60, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Quian, Y.; Ge, Z.; Chen, H.; Qian, C.; Li, Y.; Chen, Z. Molecular basis and potential applications of capsaicinoids and capsinoids against the elongation of etiolated wheat (Triticum aestivum L.) coleoptiles in foods. Food Chem. 2019, 301, 125229. [Google Scholar] [CrossRef]
- Tanaka, Y.; Fukuta, S.; Koeda, S.; Goto, T.; Yoshida, Y.; Yasuba, K.I. Identification of a novel mutant pAMT allele responsible for low-pungency and capsinoid production in chili pepper: Accession ‘no. 4034′ (Capsicum chinense). Hortic. J. 2018, 87, 222–228. [Google Scholar] [CrossRef]
- Kobata, K.; Todo, T.; Yazawa, S.; Iwai, K.; Watanabe, T. Novel capsaicinoid-like substances, capsiate and dihydrocapsiate, from the fruits of a nonpungent cultivar, CH-19 Sweet, of pepper (Capsicum annuum L.). J. Agric. Food Chem. 1998, 46, 1695–1697. [Google Scholar] [CrossRef]
- Lang, Y.; Kisaka, H.; Sugiyama, R.; Nomura, K.; Morita, A.; Watanabe, T.; Tanaka, Y.; Yazawa, S.; Miwa, T. Functional loss of pAMT results in biosynthesis of capsinoids, capsaicinoid analogs, in Capsicum annuum cv. CH-19 Sweet. Plant J. 2009, 59, 953–961. [Google Scholar] [CrossRef]
- Sutoh, K.; Kobata, K.; Yazawa, S.; Watanabe, T. Capsinoid is biosynthesized from phenylalanine and valine in a non-pungent pepper, Capsicum annuum L. cv. CH-19 Sweet. Biosci. Biotechnol. Biochem. 2006, 70, 1513–1516. [Google Scholar] [CrossRef]
- Luo, X.J.; Peng, J.; Li, Y.J. Recent advances in the study on capsaicinoids and capsinoids. Eur. J. Pharmacol. 2011, 650, 1–7. [Google Scholar] [CrossRef]
- Stewart, C., Jr.; Kang, B.-C.; Liu, K.; Mazourek, M.; Moore, S.L.; Eun, Y.Y.; Kim, B.-D.; Paran, I.; Jahn, M.M. The Pun1 gene for pungency in pepper encodes a putative acyltransferase. Plant J. 2005, 42, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Swint, J.M.; Beining, K.M.; Bryant, J.A.; Tucker, R.M.; Ludy, M.J. Comparison of Capsaicin and Capsiate’s Effects at a Meal. Chemosens. Percept. 2015, 8, 174–182. [Google Scholar] [CrossRef]
- Zewdie, Y.; Bosland, P.W. Evaluation of genotype, environment, and genotype-by-environment interaction for capsaicinoids in Capsicum annuum L. Euphytica 2000, 111, 185–190. [Google Scholar] [CrossRef]
- Harvell, K.P.; Bosland, P.W. The environment produces a significant effect on pungency of chiles. HortScience 1997, 32, 1292. [Google Scholar] [CrossRef]
- Guzman, I.; Bosland, P.W.; O’Connell, M.A. Heat, color, and flavor compounds in Capsicum fruit. Biol. Act. Phytochem. 2011, 41, 109–126. [Google Scholar] [CrossRef]
- Bae, H.; Jayaprakasha, G.K.; Crosby, K.; Yoo, K.S.; Leskovar, D.I.; Jifon, J.; Patil, B.S. Ascorbic acid, capsaicinoid, and flavonoid aglycone concentrations as a function of fruit maturity stage in greenhouse-grown peppers. J. Food Compos. Anal. 2014, 33, 195–202. [Google Scholar] [CrossRef]
- da Luz, P.B.; Brito dos Santos, A.A.; Ambrosio, V.C.; Neves, L.G.; Tavares, A.R. Selection of indexes to evaluate the genetic variability aiming ornamental use of peppers accessions. Ornam. Hortic. 2018, 24, 7–11. [Google Scholar] [CrossRef]
- Fortunato, F.L.G.; Do Rêgo, E.R.; De Carvalho, M.G.; Dos Santos, C.A.P.; Do Rêgo, M.M. Genetic diversity in ornamental pepper plants. Comun. Sci. 2019, 10, 364–375. [Google Scholar] [CrossRef]
- dos Santos Pessoa, A.M.; do Rêgo, E.R.; Carvalho, M.G.; dos Santos, C.A.P.; do Rêgo, M.M. Genetic diversity among accessions of Capsicum annuum L. through morphoagronomic characters. Genet. Mol. Res. 2018, 17, gmr16039883. [Google Scholar] [CrossRef]
- Rěgo, E.R.; Rěgo, M.M.; Finger, F.L.; Nascimento, N.F.F.; Nascimento, M.F.; Dos Santos, R.M.C. Phenotypic variability and importance of characters in a F2 segregating generation of ornamental Chili (Capsicum annuum). Acta Hortic. 2013, 1000, 493–498. [Google Scholar] [CrossRef]
- Stommel, J.R.; Bosland, P.W. Ornamental pepper: Capsicum annuum. In Flower Breeding Genetics: Challenges and Opportunities for the 21st Century; Springer Science & Business Media: Berlin, Germany, 2006; pp. 561–599. [Google Scholar] [CrossRef]
- Fayos, O.; de Aguiar, A.C.; Jiménez-Cantizano, A.; Ferreiro-González, M.; Garcés-Claver, A.; Martínez, J.; Mallor, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G.; et al. Ontogenetic variation of individual and total capsaicinoids in Malagueta peppers (Capsicum frutescens) during fruit maturation. Molecules 2017, 22, 736. [Google Scholar] [CrossRef] [PubMed]
- Olguín-Rojas, J.A.; Fayos, O.; Vázquez-León, L.A.; Ferreiro-González, M.; Rodríguez-Jiménes, G.C.; Palma, M.; Garcés-Claver, A.; Barbero, G.F. Progression of the total and individual capsaicinoids content in the fruits of three different cultivars of Capsicum chinense Jacq. Agronomy 2019, 9, 141. [Google Scholar] [CrossRef]
- Fayos, O.; Ochoa-Alejo, N.; Martínez de la Vega, O.; Savirón, M.; Orduna, J.; Mallor, C.; Barbero, G.F.; Garcés-Claver, A. Assessment of capsaicinoid and capsinoid accumulation patterns during fruit development in three chili pepper genotypes (Capsicum spp.) carrying Pun1 and pAMT alleles related to pungency. J. Agric. Food Chem. 2019, 67, 12219–12227. [Google Scholar] [CrossRef]
- Barbero, G.F.; de Aguiar, A.C.; Carrera, C.; Olachea, A.; Ferreiro-González, M.; Martínez, J.; Palma, M.; Barroso, C.G. Evolution of capsaicinoids in Peter Pepper (Capsicum annuum var. annuum) during fruit ripening. Chem. Biodivers. 2016, 13, 1068–1075. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum-Titze, P.; Mueller-Seitz, E.; Petz, M. Pungency in paprika (Capsicum annuum). 2. Heterogeneity of capsaicinoid content in individual fruits from one plant. J. Agric. Food Chem. 2002, 50, 1264–1266. [Google Scholar] [CrossRef]
- Sarpras, M.; Gaur, R.; Sharma, V.; Chhapekar, S.S.; Das, J.; Kumar, A.; Kumar Yadava, S.; Nitin, M.; Brahma, V.; Abraham, S.K.; et al. Comparative analysis of fruit metabolites and pungency candidate genes expression between Bhut jolokia and other Capsicum species. PLoS ONE 2016, 11, e0167791. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; Olguín-Rojas, J.A.; Fayos, O.; González-de-Peredo, A.V.; Espada-Bellido, E.; Ferreiro-González, M.; Barroso, C.G.; Barbero, G.F.; Garcés-Claver, A.; Palma, M. Influence of fruit ripening on the total and individual capsaicinoids and capsiate content in Naga Jolokia peppers (Capsicum chinense Jacq.). Agronomy 2020, 10, 252. [Google Scholar] [CrossRef]
- Barbero, G.F.; Ruiz, A.G.; Liazid, A.; Palma, M.; Vera, J.C.; Barroso, C.G. Evolution of total and individual capsaicinoids in peppers during ripening of the Cayenne pepper plant (Capsicum annuum L.). Food Chem. 2014, 153, 200–206. [Google Scholar] [CrossRef]
- Barrera, J.A.; Hernández, M.S.; Melgarejo, L.M.; Martínez, O.; Fernández-Trujillo, J.P. Physiological behavior and quality traits during fruit growth and ripening of four Amazonic hot pepper accessions. J. Sci. Food Agric. 2008, 88, 847–857. [Google Scholar] [CrossRef]
- Estrada, B.; Bernal, M.A.; Díaz, J.; Pomar, F.; Merino, F. Fruit development in Capsicum annuum: Changes in capsaicin, lignin, free phenolics, and peroxidase patterns. J. Agric. Food Chem. 2000, 48, 6234–6239. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Seitz, E.; Hiepler, C.; Petz, M. Chili pepper fruits: Content and pattern of capsaicinoids in single fruits of different ages. J. Agric. Food Chem. 2008, 56, 12114–12121. [Google Scholar] [CrossRef] [PubMed]
- Arce-Rodríguez, M.L.; Ochoa-Alejo, N. An R2R3-MYB transcription factor regulates capsaicinoid biosynthesis. Plant Physiol. 2017, 174, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.A.; Calderón, A.A.; Pedreño, M.A.; Muñoz, R.; Barceló, A.R.; de Cáceres, F.M. Capsaicin oxidation by peroxidase from Capsicum annuum (var. annuum) fruits. J. Agric. Food Chem. 1993, 41, 1041–1044. [Google Scholar] [CrossRef]
- Bernal, M.A.; Calderón, A.A.; Pedreño, M.A.; Muñoz, R.; Barceló, A.R.; de Cáceres, F.M. Dihydrocapsaicin oxidation by Capsicum annuum (var annuum) peroxidase. J. Food Sci. 1993, 58, 611–613. [Google Scholar] [CrossRef]
- Iwai, K.; Suzuki, T.; Fujiwake, H. Formation and accumulation of pungent principle of hot pepper fruits, capsaicin and its analogues, in Capsicum annuum var. annuum cv. Karayatsubusa at different growth stages after flowering. Agric. Biol. Chem. 1979, 43, 2493–2498. [Google Scholar] [CrossRef]
- Bernal, M.A.; Calderón, A.A.; Ferrer, M.A.; de Cáceres, F.M.; Barceló, A.R. Oxidation of capsaicin and capsaicin phenolic precursors by the basic peroxidase isoenzyme B6 from hot pepper. J. Agric. Food Chem. 1995, 43, 352–355. [Google Scholar] [CrossRef]
- Bernal, M.A.; De Cáceres, F.M.; Barceló, A.R. Histochemical localization of peroxidase in Capsicum fruits. LWT Food Sci. Technol. 1994, 27, 197–198. [Google Scholar] [CrossRef]
- Siddiqui, M.W.; Momin, C.M.; Acharya, P.; Kabir, J.; Debnath, M.K.; Dhua, R.S. Dynamics of changes in bioactive molecules and antioxidant potential of Capsicum chinense Jacq. cv. Habanero at nine maturity stages. Acta Physiol. Plant. 2013, 35, 1141–1148. [Google Scholar] [CrossRef]
- Sganzerla, M.; Coutinho, J.P.; de Melo, A.M.T.; Godoy, H.T. Fast method for capsaicinoids analysis from Capsicum chinense fruits. Food Res. Int. 2014, 64, 718–725. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Sales, L.P.; Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Martínez, J. Supercritical carbon dioxide extraction of Capsicum peppers: Global yield and capsaicinoid content. J. Supercrit. Fluids 2013, 81, 210–216. [Google Scholar] [CrossRef]
- Krajewska, A.M.; Powers, J.J. Sensory properties of naturally occurring capsaicinoids. J. Food Sci. 1988, 53, 902–905. [Google Scholar] [CrossRef]
- Barbero, G.F.; Liazid, A.; Azaroual, L.; Palma, M.; Barroso, C.G. Capsaicinoid contents in peppers and pepper-related spicy foods. Int. J. Food Prop. 2016, 19, 485–493. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Martínez, J. Comparative study of capsaicinoid composition in Capsicum Peppers grown in Brazil. Int. J. Food Prop. 2016, 19, 1292–1302. [Google Scholar] [CrossRef]
- Arce-Rodríguez, M.L.; Ochoa-Alejo, N. Silencing AT3 gene reduces the expression of pAmt, BCAT, Kas, and Acl genes involved in capsaicinoid biosynthesis in chili pepper fruits. Biol. Plant. 2015, 59, 477–484. [Google Scholar] [CrossRef]
- Lema, A.; Martínez-Cortés, T.; Garcés, A.; Mallor, C.; Fayos, O.; Barbero, G.F.; Silvar, C.; Pomar, F. 5,5′-dicapsiate: Product of the oxidation of capsiate by cationic peroxidases from pepper (Capsicum annuum L.). In Proceedings of the XVIth EUCARPIA Capsicum and Eggplant Working Group Meeting, Kecskemet, Hungary, 12–14 September 2016; pp. 500–505, ISBN 978-615-5270.27-7. [Google Scholar]
- Yazawa, S.; Suetome, N.; Okamoto, K.; Namiki, T. Content of capsaicinoids and capsaicinoid-like substances in fruit of pepper (Capsicum annuum L.) hybrids made with ‘CH-19 Sweet’ as a parent. J. Jpn. Soc. Hortic. Sci. 1989, 58, 601–607. [Google Scholar] [CrossRef]
- Jang, S.; Han, K.; Deuk Jo, Y.; Jeong, H.-J.; Irfan Siddique, M.; Kang, B.-C. Substitution of a dysfunctional pAMT allele results in low-pungency but high levels of capsinoid in Capsicum chinense ‘Habanero’. Plant Breed. Biotechnol. 2015, 3, 119–128. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakashima, F.; Kirii, E.; Goto, T.; Yoshida, Y.; Yasuba, K.-I. Difference in capsaicinoid biosynthesis gene expression in the pericarp reveals elevation of capsaicinoid contents in chili peppers (Capsicum chinense). Plant Cell Rep. 2017, 36, 267–279. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Ferreiro-González, M.; Barroso, C.G.; Palma, M.; Barbero, G.F.; Espada-Bellido, E. Optimizing and comparing ultrasound- and microwave-assisted extraction methods applied to the extraction of antioxidant capsinoids in peppers. Agronomy 2019, 9, 10. [Google Scholar] [CrossRef]
- Sutoh, K.; Kobata, K.; Watanabe, T. Stability of Capsinoid in Various Solvents. J. Agric. Food Chem. 2001, 49, 4026–4030. [Google Scholar] [CrossRef]
- Barbero, G.F.; Molinillo, J.M.G.; Varela, R.M.; Palma, M.; Macías, F.A.; Barroso, C.G. Application of Hansch’s Model to capsaicinoids and capsinoids: A study using the quantitative structure—Activity relationship. A novel method for the synthesis of capsinoids. J. Agric. Food Chem. 2010, 58, 3342–3349. [Google Scholar] [CrossRef] [PubMed]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of capsaicinoids from peppers. Talanta 2008, 75, 1332–1337. [Google Scholar] [CrossRef] [PubMed]

| Start of Fruit Development | dpa | Color of the Fruits | |
|---|---|---|---|
| ‘Filius Green’ | ‘Filius Blue’ | ||
| 17/09 | 13 | Green | Brown/Purple |
| 10/09 | 20 | Green | Purple |
| 03/09 | 27 | Green | Purple |
| 27/08 | 34 | Green/Red | Purple/Red |
| 20/08 | 41 | Green/Red | Red |
| 13/08 | 48 | Red | Red |
| 06/08 | 55 | Red | Red |
| 30/07 | 62 | Red | Red |
| 23/07 | 69 | Red | Red |
| 16/07 | 76 | Over-ripeness | Over-ripeness |
| (dpa) | 13 | 20 | 27 | 34 | 41 | 48 | 55 | 62 | 69 | 76 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘Filius Green’ | n-DHC | 12.49 | 25.66 | 33.77 | 76.11 | 73.54 | 98.58 | 89.03 | 94.43 | 100.00 | 63.49 |
| C | 6.96 | 11.27 | 17.78 | 69.48 | 58.12 | 85.76 | 73.82 | 88.54 | 100.00 | 65.75 | |
| DHC | 10.33 | 13.43 | 19.58 | 65.83 | 55.50 | 93.78 | 83.52 | 94.20 | 100.00 | 61.76 | |
| h-C | 12.62 | 22.15 | 45.57 | 74.49 | 68.94 | 100.00 | 77.35 | 85.56 | 87.04 | 66.92 | |
| h-DHC | 20.83 | 24.34 | 30.84 | 62.77 | 61.29 | 95.43 | 77.14 | 84.74 | 100.00 | 69.98 | |
| ‘Filius Blue’ | n-DHC | 37.41 | 47.41 | 69.05 | 92.43 | 100.00 | 77.56 | 82.14 | 83.57 | 74.46 | 62.53 |
| C | 36.81 | 45.91 | 58.07 | 88.85 | 100.00 | 60.13 | 57.19 | 59.84 | 55.56 | 49.59 | |
| DHC | 19.12 | 25.77 | 44.81 | 82.24 | 100.00 | 42.80 | 39.81 | 46.94 | 39.47 | 33.25 | |
| h-C | 32.64 | 36.08 | 67.50 | 98.33 | 88.68 | 86.53 | 100.00 | 83.88 | 73.67 | 86.17 | |
| h-DHC | 13.40 | 24.78 | 45.22 | 75.21 | 100.00 | 70.37 | 66.47 | 72.12 | 62.67 | 59.93 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Espinosa, M.; Fayos, O.; V. González-de-Peredo, A.; Espada-Bellido, E.; Ferreiro-González, M.; Palma, M.; Garcés-Claver, A.; F. Barbero, G. Content of Capsaicinoids and Capsiate in “Filius” Pepper Varieties as Affected by Ripening. Plants 2020, 9, 1222. https://doi.org/10.3390/plants9091222
Vázquez-Espinosa M, Fayos O, V. González-de-Peredo A, Espada-Bellido E, Ferreiro-González M, Palma M, Garcés-Claver A, F. Barbero G. Content of Capsaicinoids and Capsiate in “Filius” Pepper Varieties as Affected by Ripening. Plants. 2020; 9(9):1222. https://doi.org/10.3390/plants9091222
Chicago/Turabian StyleVázquez-Espinosa, Mercedes, Oreto Fayos, Ana V. González-de-Peredo, Estrella Espada-Bellido, Marta Ferreiro-González, Miguel Palma, Ana Garcés-Claver, and Gerardo F. Barbero. 2020. "Content of Capsaicinoids and Capsiate in “Filius” Pepper Varieties as Affected by Ripening" Plants 9, no. 9: 1222. https://doi.org/10.3390/plants9091222
APA StyleVázquez-Espinosa, M., Fayos, O., V. González-de-Peredo, A., Espada-Bellido, E., Ferreiro-González, M., Palma, M., Garcés-Claver, A., & F. Barbero, G. (2020). Content of Capsaicinoids and Capsiate in “Filius” Pepper Varieties as Affected by Ripening. Plants, 9(9), 1222. https://doi.org/10.3390/plants9091222