Management of Infection by Parasitic Weeds: A Review
Abstract
:1. Introduction
2. Infection by Parasitic Weeds
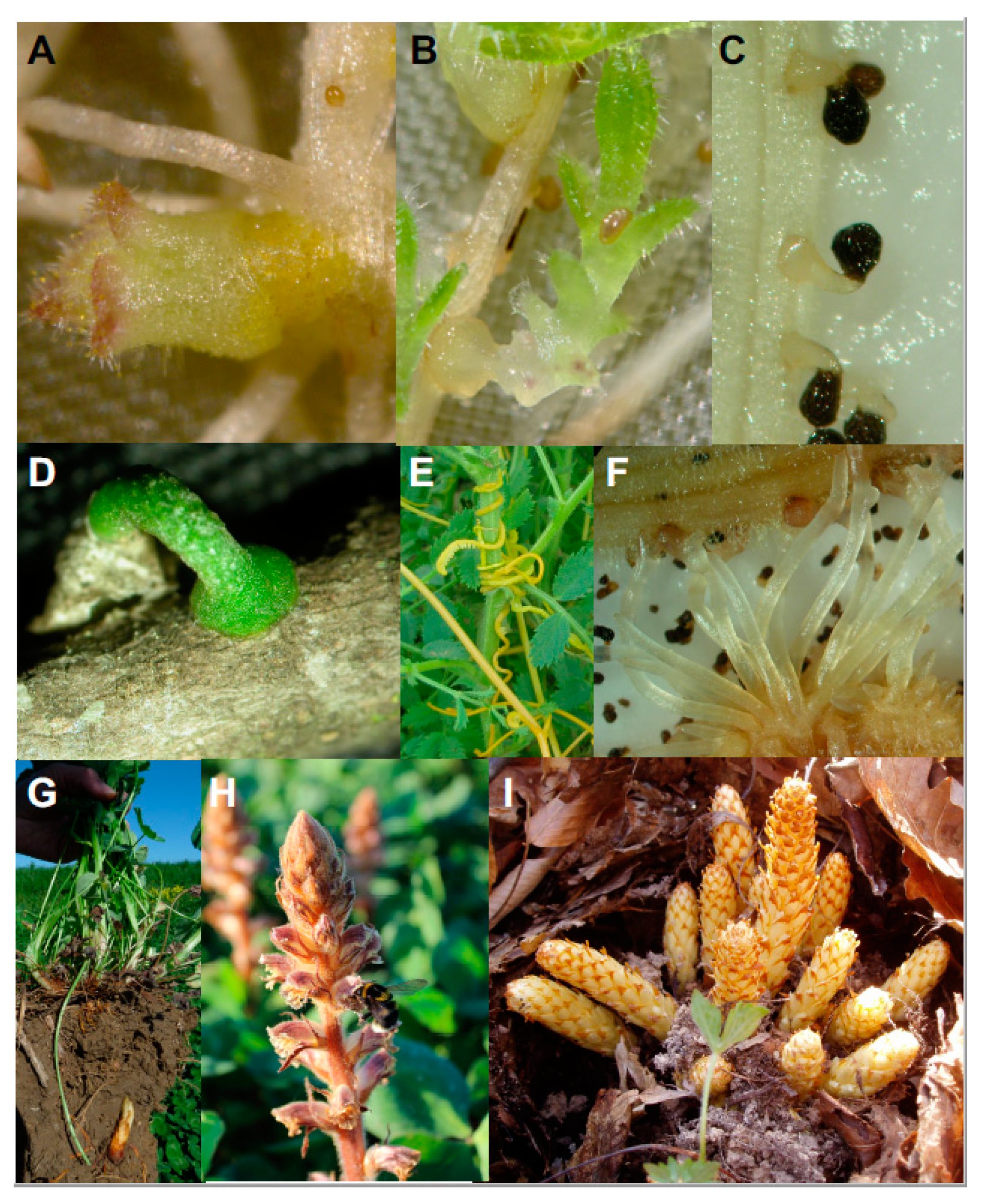
2.1. Crop Location for Germination, Host Trophic Growth and Haustorium Initiation
2.1.1. Germination
2.1.2. Host-Tropic Growth
2.1.3. Initiation of Haustorium
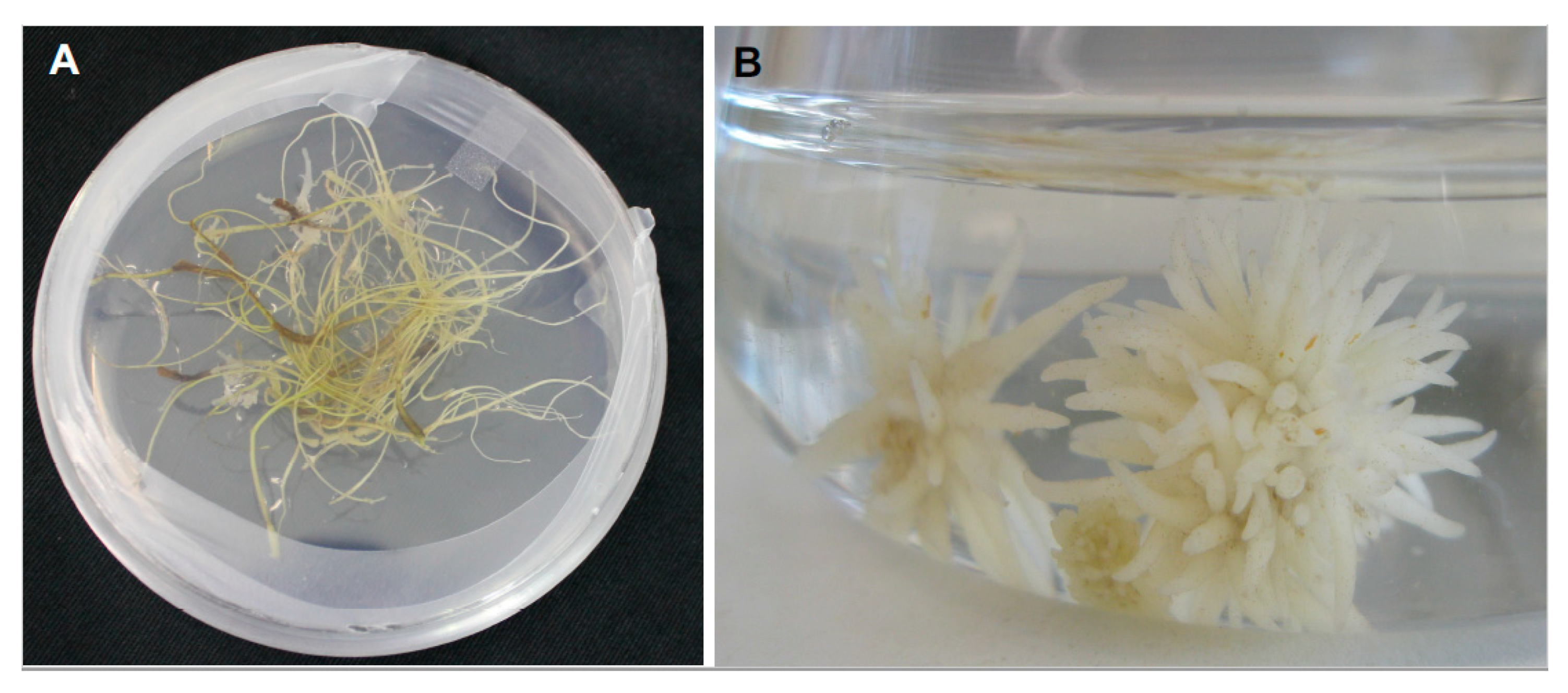
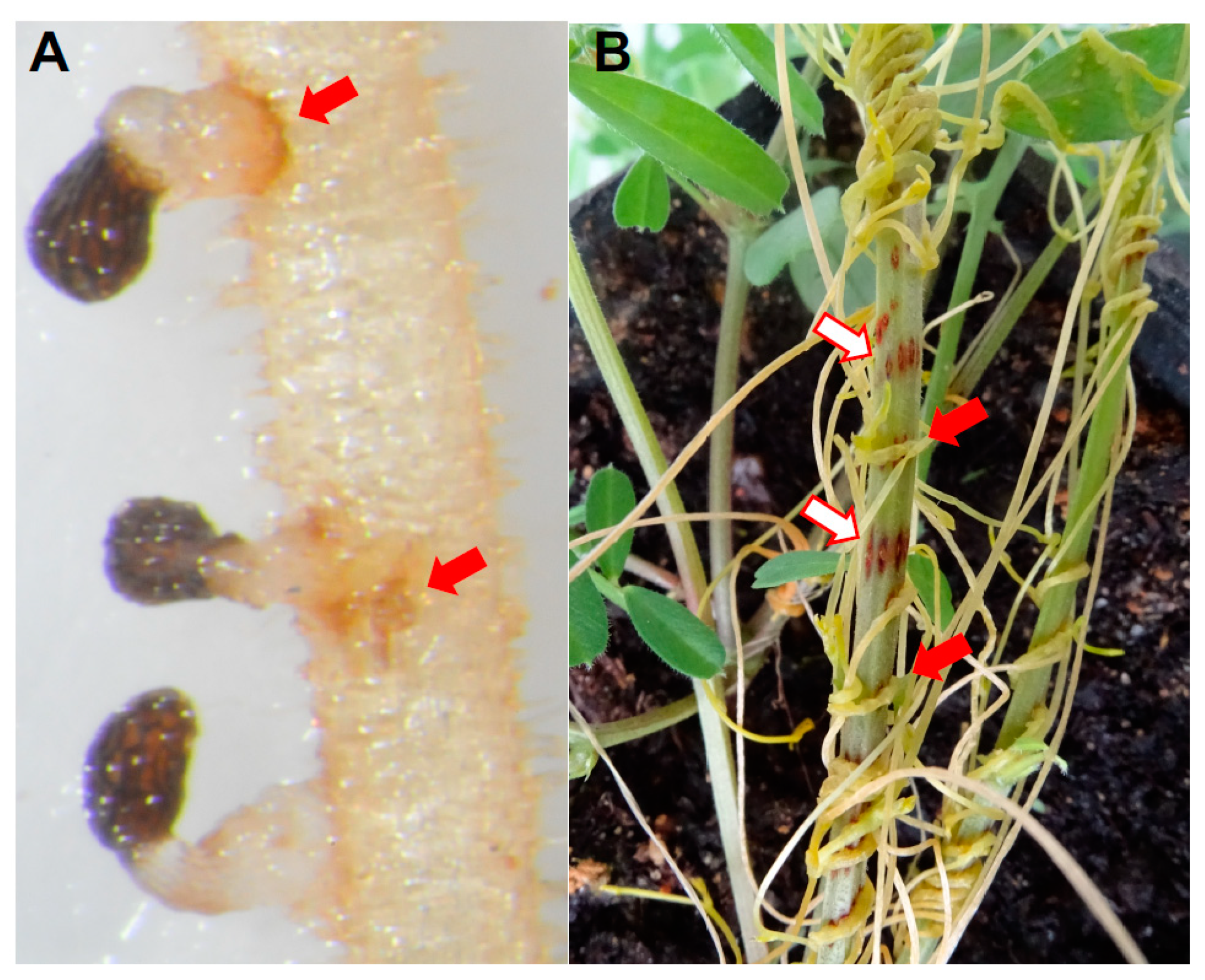
2.2. Host Invasion and Establishment of Vascular Connection
2.3. Maturation of Absorptive Sink
3. Effect of Parasitic Weed Infection on the Crop
4. Phenotypic Expression of Resistance
5. Evolution of Host Specificities and Races
6. Resistance Genes to Parasitic Plants
7. Parasite Effectors
8. Transcriptome and Proteome Analysis of Resistance
9. Strategies for Effective Parasitic Weed Management
9.1. Bioherbicides
9.2. Gene Pyramiding
9.3. Genetic Engineering
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kuijt, J. The Biology of Parasitic Plants; University of California Press: Berkeley, CA, USA, 1969. [Google Scholar]
- Parker, C.; Riches, C.R. Parasitic Weeds of the World: Biology and Control; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Nickrent, D.L.; Malécot, V.; Vidal-Russell, R.; Der, J.P. A revised classification of Santalales. Taxon 2010, 59, 538–558. [Google Scholar] [CrossRef]
- Westwood, J.H.; Yoder, J.I.; Timko, M.P.; de Pamphilis, C.W. The evolution of parasitism in plants. Trends Plant Sci. 2010, 15, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Heide-Jørgensen, H.S. Introduction: The parasitic syndrome in Higher Plants. In Parasitic Orobanchaceae; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–18. [Google Scholar]
- Westwood, J.H. The physiology of the established parasite-host association. In Parasitic Orobanchaceae; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 87–114. [Google Scholar]
- Riopel, J.L.; Timko, M.P. Haustorial initiation and differentiation. In Parasitic Plants; Press, M.C., Graves, J.D., Eds.; Chapman & Hall: London, UK, 1995; pp. 39–79. [Google Scholar]
- Joel, D.M. Functional structure of the mature haustorium. In Parasitic Orobanchaceae; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 25–60. [Google Scholar]
- Parker, C. Observations on the current status of Orobanche and Striga problems worldwide. Pest Manag. Sci. 2009, 65, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Scholes, J.D.; Press, M.C. Striga infestation of cereal crops—An unsolved problem in resource limited agriculture. Curr. Opin. Plant Biol. 2008, 11, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Delavault, P. Are root parasitic plants like any other plant pathogens? New Phytol. 2020, 226, 641–643. [Google Scholar] [CrossRef]
- Foy, C.L.; Jain, R.; Jacobsohn, R. Recent approaches for chemical control of broomrape (Orobanche spp.). In Reviews of Weed Science; Foy, C.L., Ed.; Weed Science Society of America: Champaign, IL, USA, 1989; Volume 4, pp. 123–152. [Google Scholar]
- Clarke, C.R.; Timko, M.P.; Yoder, J.I.; Axtell, M.J.; Westwood, J.H. Molecular Dialog Between Parasitic Plants and Their Hosts. Ann. Rev. Phytopathol. 2019, 57, 279–299. [Google Scholar] [CrossRef]
- Schneeweiss, G.M. Correlated evolution of life history and host range in the nonphotosynthetic parasitic flowering plants Orobanche and Phelipanche (Orobanchaceae). J. Evol. Biol. 2007, 20, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Estabrook, E.M.; Yoder, J.I. Plant-plant communications: Rhizosphere signalling between parasitic angiosperms and their hosts. Plant Physiol. 1998, 116, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bruce, T.B.A.; Gressel, J. Changing host specificities: By mutational changes or epigenetic reprogramming? In Parasitic Orobanchaceae; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 231–242. [Google Scholar]
- Parker, C. The parasitic weeds of the Orobanchaceae. In Parasitic Orobanchaceae; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 313–344. [Google Scholar]
- Joel, D.M. The seed and the seedling. In Parasitic Orobanchaceae; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 143–146. [Google Scholar]
- Xie, X.; Yoneyama, K.; Yoneyama, K. The strigolactone story. Ann. Rev. Phytopathol. 2010, 48, 93–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechat, M.M.; Pouvreau, J.B.; Péron, T.; Gauthier, M.; Montiel, G.; Veronesi, C.; Todoroki, Y.; Le Bizec, B.; Monteau, F.; Macherel, D.; et al. PrCYP707A1, an ABA catabolic gene, is a key component of Phelipanche ramosa seed germination in response to the strigolactone analogue GR24. J. Exp. Bot. 2012, 63, 5311–5322. [Google Scholar] [CrossRef]
- Brun, G.; Thoiron, S.; Braem, L.; Pouvreau, J.B.; Montiel, G.; Lechat, M.M.; Simier, P.; Gevaert, K.; Goormachtig, S.; Delavault, P. CYP707As are effectors of karrikin and strigolactone signalling pathways in Arabidopsis thaliana and parasitic plants. Plant Cell Environ. 2019, 42, 2612–2626. [Google Scholar] [CrossRef] [PubMed]
- Musselman, L.J. The biology of Striga, Orobanche and other root parasitic weeds. Ann. Rev. Phytopathol. 1980, 18, 463–489. [Google Scholar] [CrossRef] [Green Version]
- López-Granados, F.; García-Torres, L. Effects of environmental factors on dormancy and germination of crenate broomrape (Orobanche crenata). Weed Sci. 1996, 44, 284–289. [Google Scholar]
- Kebreab, E.; Murdoch, A.J. A quantitative model for loss of primary dormancy and induction of secondary dormancy in imbibed seeds of Orobanche spp. J. Exp. Bot. 1999, 50, 211–219. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Roux, C.; Lopez-Raez, J.A.; Bécard, G. Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci. 2007, 12, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aparicio, M.; Flores, F.; Rubiales, D. Recognition of root exudates by seeds of broomrape (Orobanche and Phelipanche) species. Ann. Bot. 2009, 103, 423–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Aparicio, M.; Yoneyama, K.; Rubiales, D. The role of strigolactones in host specificity of Orobanche and Phelipanche seed germination. Seed Sci. Res. 2011, 21, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Aparicio, M.; Kisugi, T.; Xie, X.; Rubiales, D.; Yoneyama, K. Low strigolactone root exudation: A novel mechanism of broomrape (Orobanche and Phelipanche spp.) resistance available for faba bean breeding. J. Agric. Food Chem. 2014, 62, 7063–7071. [Google Scholar]
- Yoneyama, K.; Xie, X.; Kim, H.I.; Kisugi, T.; Nomura, T.; Sekimoto, H.; Yokota, T.; Yoneyama, K. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 2012, 235, 1197–1207. [Google Scholar] [CrossRef] [Green Version]
- Auger, B.; Pouvreau, J.B.; Pouponneau, K.; Yoneyama, K.; Montiel, G.; Le Bizec, B.; Yoneyama, K.; Delavault, P.; Delourme, R.; Simier, P. Germination Stimulants of Phelipanche ramosa in the Rhizosphere of Brassica napus are Derived from the Glucosinolate Pathway. Mol. Plant Microbe Interact. 2012, 7, 993–1004. [Google Scholar] [CrossRef] [Green Version]
- Conn, C.E.; Bythell-Douglas, R.; Neumann, D.; Yoshida, S.; Whittington, B.; Westwood, J.H.; Shirasu, K.; Bond, C.S.; Dyer, K.A.; Nelson, D.C. Convergent evolution of strigolactone perception enabled host detection in parasitic plants. Science 2015, 349, 540–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, J.H.; Musselman, L.J.; Wolswinkel, P.; Dörr, I. Biology and control of Cuscuta. Rev. Weed Sci. 1994, 6, 265–317. [Google Scholar]
- Kaiser, B.; Vogg, G.; Fürst, U.B.; Albert, M. Parasitic plants of the genus Cuscuta and their interaction with susceptible and resistant host plants. Front. Plant Sci. 2015, 6, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabiri, S.; van Ast, A.; Rodenburg, J.; Bastiaans, L. Host influence on germination and reproduction of the facultative hemi-parasitic weed Rhamphicarpa fistulosa. Ann. Appl. Biol. 2016, 169, 144–154. [Google Scholar] [CrossRef]
- Saunders, A.R. Studies in phanerogamic parasitism, with particular reference to Striga lutea Lour. S. Afr. Dept. Agric. Sci. Bull. 1933, 128, 56. [Google Scholar]
- Williams, C.N. Tropism and morphogenesis of Striga seedlings in the host rhizosphere. Ann. Bot. 1961, 25, 406–415. [Google Scholar] [CrossRef]
- Riopel, J.L.; Baird, W.V. Morphogenesis of the early development of primary haustoria in Striga asiatica. In Parasitic Weeds in Agriculture; Musselman, L.J., Ed.; CRC: Boca Raton, FL, USA, 1987; pp. 107–125. [Google Scholar]
- Yoshida, S.; Shirasu, K. Multiple layers of incompatibility to the parasitic witchweed, Striga hermonthica. New Phytol. 2009, 183, 180–189. [Google Scholar] [CrossRef]
- Veronesi, C.; Bonnin, E.; Calvez, S.; Thalouarn, P.; Simier, P. Activity of secreted cell wall-modifying enzymes and expression of peroxidase-encoding gene following germination of Orobanche ramosa. Biol. Plant 2007, 51, 391–394. [Google Scholar] [CrossRef]
- Zhou, W.J.; Yoneyama, K.; Takeuchi, Y.; Iso, S.; Rungmekarat, S.; Chae, S.H.; Sato, D.; Joel, D.M. In vitro infection of host roots by differentiated calli of the parasitic plant Orobanche. J. Exp. Bot. 2004, 55, 899–907. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Aparicio, M.; Rubiales, D.; Bandaranayake, P.C.G.; Yoder, J.I.; Westwood, J.H. Transformation and regeneration of the holoparasitic plant Phelipanche aegyptiaca. Plant Methods 2011, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhang, Y.; Matusova, R.; Charnikhova, T.; Jamil, M.; Fernandez-Aparicio, M.; Huang, K.; Westwood, J.H.; Timko, M.; Ruyter-Spira, C.; et al. Striga hermonthica MAX2 restores branching but not the very low fluence response in the Arabidopsis thaliana max2 mutant. New Phytol. 2014, 202, 531–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billard, E.; Goyet, V.; Delavault, P.; Simier, S.; Montiel, G. Cytokinin treated microcalli of Phelipanche ramosa: An efficient model for studying haustorium formation in holoparasitic plants. Plant Cell Tissue Organ Cult. 2020, 141, 543–553. [Google Scholar] [CrossRef]
- Heide-Jørgensen, H.S. Parasitic Flowering Plants; Brill Leiden: Leiden, The Netherlands, 2008. [Google Scholar]
- Runyon, J.B.; Mescher, M.C.; De Moraes, C.M. Volatile chemical cues guide host location and host selection by parasitic plants. Science 2006, 313, 1964–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, M.G.; Smith, H. The function of phytochrome in plants growing in the natural environment. Nature 1975, 254, 512. [Google Scholar] [CrossRef]
- Orr, G.L.; Haidar, M.A.; Orr, D.A. Small seed dodder (Cuscuta planiflora) phototropism toward far-red when in white light. Weed Sci. 1996, 44, 233–240. [Google Scholar] [CrossRef]
- Bandaranayake, P.C.G.; Yoder, J.I. Haustorium initiation and early development. In Parasitic Orobanchaceae; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 61–74. [Google Scholar]
- Fernández-Aparicio, M.; Masi, M.; Maddau, L.; Cimmino, A.; Evidente, M.; Rubiales, D.; Evidente, A. Induction of haustorium development by sphaeropsidones in radicles of the parasitic weeds Striga and Orobanche. A structure-activity relationship study. J. Agric. Food Chem. 2016, 64, 5188–5196. [Google Scholar]
- Hegenauer, V.; Körner, M.; Albert, M. Plants under stress by parasitic plants. Curr. Opin. Plant Biol. 2017, 38, 34–41. [Google Scholar] [CrossRef]
- Goyet, V.; Wada, S.; Cui, S.; Wakatake, T.; Shirasu, K.; Montiel, G.; Simier, P.; Yoshida, S. Haustorium Inducing Factors for Parasitic Orobanchaceae. Front. Plant Sci. 2019, 10, 1056. [Google Scholar] [CrossRef] [Green Version]
- Ishida, J.K.; Wakatake, T.; Yoshida, S.; Takebayashi, Y.; Kasahara, H.; Wafula, E.; de Pamphilis, C.W.; Namba, S.; Shirasu, K. Local auxin biosynthesis mediated by a YUCCA flavin monooxygenase regulates haustorium development in the parasitic plant Phtheirospermum japonicum. Plant Cell 2016, 28, 1795–1814. [Google Scholar] [CrossRef] [Green Version]
- Joel, D.M.; Losner-Goshen, D. The attachment organ of the parasitic angiosperms Orobanche cumana and O. aegyptiaca and its development. Can. J. Bot. 1994, 72, 564–574. [Google Scholar] [CrossRef]
- Hood, M.E.; Condom, J.M.; Timko, M.P.; Riopel, J.L. Primary haustorial development of Striga asiatica on host and nonhost species. Phytopathology 1998, 88, 70–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiss, G.C.; Bailey, J.A. Striga gesnerioides parasitizing cowpea: Development of infection structures and mechanisms of penetration. Ann. Bot. 1998, 81, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Baird, V.W.; Riopel, J. Experimental studies of the attachment of the parasitic angiosperm Agalinis purpurea to a host. Protoplasma 1983, 118, 206–218. [Google Scholar] [CrossRef]
- Heide-Jørgensen, H.S.; Kuijt, J. The haustorium of the root parasite Triphysaria (Scrophulariaceae), with special reference to xylem bridge ultrastructure. Am. J. Bot. 1995, 82, 782–797. [Google Scholar] [CrossRef]
- Musselman, L.J.; Dickinson, W.C. The structure and development of the haustorium in parasitic Scrophulariaceae. Bot. J. Linn. Soc. 1975, 70, 183–212. [Google Scholar] [CrossRef]
- Dörr, I. Feinstruktur intrazellular wachsender Cuscuta-Hyphen. Protoplasma 1969, 67, 123–137. [Google Scholar] [CrossRef]
- Haidar, M.A.; Orr, G.L.; Westra, P. Effects of light and mechanical stimulation on coiling and pre-haustoria formation in Cuscuta spp. Weed Res. 1997, 37, 219–228. [Google Scholar] [CrossRef]
- Vaughn, K.C. Attachment of the parasitic weed dodder to the host. Protoplasma 2002, 219, 227–237. [Google Scholar] [CrossRef]
- Honaas, L.; Wafula, E.; Yang, Z.; Der, J.; Wickett, N.; Altman, N.S.; Taylor, C.G.; Yoder, J.I.; Timko, M.P.; Westwood, J.H.; et al. Functional genomics of a generalist parasitic plant: Laser microdissection of host-parasite interface reveals host-specific patterns of parasite gene expression. BMC Plant Biol. 2013, 13, 9. [Google Scholar] [CrossRef] [Green Version]
- Ranjan, A.; Ichihashi, Y.; Farhi, M.; Zumstein, K.; Townsley, B.; David-Schwartz, R.; Sinha, N.R. De novo assembly and characterization of the transcriptome of the parasitic weed Cuscuta pentagona identifies genes associated with plant parasitism. Plant Physiol. 2014, 166, 1186–1199. [Google Scholar] [CrossRef] [Green Version]
- Ichihashi, Y.; Mutuku, J.M.; Yoshida, S.; Shirasu, K. Transcriptomics exposes the uniqueness of parasitic plants. Brief Funct. Genom. 2015, 14, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wafula, E.K.; Honaas, L.A.; Zhang, H.; Das, M.; Fernández-Aparicio, M.; Huang, K.; Bandaranayake, P.C.G.; Wu, B.; Der, J.P.; et al. Comparative transcriptome analyses reveal core parasitism genes and suggest gene duplication and repurposing as sources of structural novelty. Mol. Biol. Evol. 2014, 32, 767–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, H.C. Anatomische Studien an den Haustorien einiger parasitischer Scrophulariaceen Mitteleuropas. Ber. Dtsch. Bot. Ges. 1976, 89, 57–84. [Google Scholar]
- Alexander, T.; Weber, H.C. Zur parasitischen Lebensweise von Parentucellia latifolia (L.) Caruel (Scrophulariaceae). Beitr. Biol. Pflanz. 1985, 60, 23–34. [Google Scholar]
- Riopel, J.; Musselman, L. Experimental initiation of haustoria in Agalinis purpurea. Am. J. Bot. 1979, 66, 570–575. [Google Scholar] [CrossRef]
- Krause, D. Vergleichende Morphologisch/Anatomische Untersuchungen an Striga-Arten (Scrophulariaceae). Ph.D. Dissertation, Philipps-University, Marburg, Germany, 1990. [Google Scholar]
- Kim, D.; Kocz, R.; Boone, L.; Keyes, W.J.; Lynn, D.G. On becoming a parasite: Evaluating the role of wall oxidases in parasitic plant development. Chem. Biol. 1998, 5, 103–117. [Google Scholar] [CrossRef] [Green Version]
- Losner-Goshen, D.; Portnoy, V.H.; Mayer, A.M.; Joel, D.M. Pectolytic activity by the haustorium of the parasitic plant Orobanche L. (Orobanchaceae) in host roots. Ann. Bot. 1998, 81, 319–326. [Google Scholar] [CrossRef]
- Dörr, I. How Striga parasitizes its host: A TEM and SEM study. Ann. Bot. 1997, 79, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Bar-Nun, N.; Sachs, T.; Mayer, A.M. A role for IAA in the infection of Arabidopsis thaliana by Orobanche aegyptiaca. Ann. Bot. 2008, 101, 261–265. [Google Scholar] [CrossRef] [Green Version]
- Ekawa, M.; Aoki, K. Phloem-Conducting Cells in Haustoria of the Root-Parasitic Plant Phelipanche aegyptiaca Retain Nuclei and Are Not Mature Sieve Elements. Plants 2017, 6, 60. [Google Scholar] [CrossRef] [Green Version]
- Dörr, I.; Kollmann, R. Symplasmic sieve element continuity between Orobanche and its host. Bot. Acta 1995, 108, 47–55. [Google Scholar] [CrossRef]
- Lee, K.B. Structure and development of the upper haustorium in the parasitic flowering plant Cuscuta japonica (Convolvulaceae). Am. J. Bot. 2007, 94, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Ahkami, A.H.; Lischewski, S.; Haensch, K.T.; Porfirova, S.; Hofmann, J.; Rolletschek, H.; Melzer, M.; Franken, P.; Hause, B.; Druege, U.; et al. Molecular physiology of adventitious root formation in Petunia hybrida cuttings: Involvement of wound response and primary metabolism. New Phytol. 2009, 181, 613–625. [Google Scholar] [CrossRef]
- Alakonya, A.; Kumar, R.; Koenig, D.; Kimura, S.; Townsley, B.; Runo, S.; Garces, H.M.; Kang, J.; Yanez, A.; David-Schwartz, R.; et al. Interspecific RNA interference of SHOOT MERISTEMLESS-like disrupts Cuscuta pentagona plant parasitism. Plant Cell 2012, 24, 3153–3166. [Google Scholar] [CrossRef] [Green Version]
- Press, M.C.; Smith, S.; Stewart, G.R. Carbon acquisition and assimilation in parasitic plants. Funct. Ecol. 1991, 5, 278–283. [Google Scholar] [CrossRef]
- Tennakoon, K.U.; Pate, J.S.; Fineran, B.A. Growth and partitioning of carbon and fixed nitrogen in the shrub legume Acacia littorea in the presence or absence of the root hemiparasite Olax phyllanthi. J. Exp. Bot. 1997, 48, 1047–1060. [Google Scholar] [CrossRef] [Green Version]
- Seel, W.E.; Cechin, I.; Vincent, C.A.; Press, M.C. Carbon partitioning in parasitic angiosperms and their hosts. In Carbon Partitioning Within and Between Organisms; Pollock, C.J., Farrar, J.F., Gordon, A.J., Eds.; BIOS Scientific Publishers Ltd.: Oxford, UK, 1992. [Google Scholar]
- Jeschke, W.D.; Räth, N.; Bäumel, P.; Czygan, F.C.; Proksch, P. Modelling the flow and partitioning of carbon and nitrogen in the holoparasite Cuscuta reflexa Roxb. & its host Lupinus albus L. I. Methods for estimating net flows. J. Exp. Bot. 1994, 45, 791–800. [Google Scholar]
- Hibberd, J.M.; Quick, W.P.; Press, M.C.; Scholes, J.D.; Jeschke, W.D. Solute fluxes from tobacco to the parasitic angiosperm Orobanche cernua and the influence of infection on host carbon and nitrogen relations. Plant Cell Environ. 1999, 22, 937–947. [Google Scholar] [CrossRef]
- Abbes, Z.; Kharrat, M.; Delavault, P.; Chaïbi, W.; Simier, P. Nitrogen and carbon relationships between the parasitic weed Orobanche foetida and susceptible and tolerant faba bean lines. Plant Physiol. Biochem. 2009, 47, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Cheng, L.; Guo, Y.; Turgeon, R. Phloem loading strategies and water relations in trees and herbaceous plants. Plant Physiol. 2011, 157, 1518–1527. [Google Scholar] [CrossRef] [Green Version]
- Sauer, N. Molecular physiology of higher plant sucrose transporters. FEBS Lett. 2007, 581, 2309–2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dörr, I. New results on interspecific bridges between parasites and their hosts. In Advances in Parasitic Plant Research; Moreno, M.T., Cubero, J.I., Berner, D., Joel, D., Musselman, L.J., Eds.; Junta de Andalucia: Cordoba, Spain, 1996; pp. 195–201. [Google Scholar]
- Draie, R.; Péron, T.; Pouvreau, J.-B.; Véronési, C.; Jégou, S.; Delavault, P.; Thoiron, S.; Simier, P. Invertases involved in the development of the parasitic plant Phelipanche ramosa: Characterization of the dominant soluble acid isoform, PrSAI1. Mol. Plant Pathol. 2011, 12, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Harloff, H.J.; Wegmann, D. Evidence for a mannitol cycle in Orobanche ramosa and Orobanche crenata. J. Plant Physiol. 1993, 141, 513–520. [Google Scholar] [CrossRef]
- Delavault, P.; Simier, P.; Thoiron, S.; Véronési, C.; Fer, A.; Thalouarn, P. Isolation of mannose 6- phosphate reductase cDNA, changes in enzyme activity and mannitol content in broomrape (Orobanche ramosa) parasitic on tomato roots. Physiol. Plant. 2002, 115, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Péron, T.; Candat, A.; Montiel, G.; Veronesi, C.; Macherel, D.; Delavault, P.; Simier, P. New insights into phloem unloading and expression of sucrose transporters in vegetative sinks of the parasitic plant Phelipanche ramosa L. (Pomel). Front. Plant Sci. 2016, 7, 2048. [Google Scholar]
- Smith, S.; Stewart, G.R. Effect of potassium levels on the stomatal behavior of the hemiparasite Striga hermonthica. Plant Physiol. 1990, 94, 1472–1476. [Google Scholar] [CrossRef] [Green Version]
- Lechowski, Z. Stomatal response to exogenous cytokinin treatment of the hemiparasite Melampyrum arvense L. before and after attachment to the host. Biol. Plant 1997, 39, 13–21. [Google Scholar] [CrossRef]
- Jiang, F.; Jeschke, W.D.; Hartung, W. Abscisic acid (ABA) flows from Hordeum vulgare to the hemiparasite Rhinanthus minor and the influence of infection on host and parasite abscisic acid relations. J. Exp. Bot. 2004, 55, 2323–2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lins, R.D.; Colquhoun, J.B.; Mallory-Smith, C.A. Effect of small broomrape (Orobanche minor) on red clover growth and dry matter partitioning. Weed Sci. 2007, 55, 517–520. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Flores, F.; Rubiales, D. The effect of Orobanche crenata infection severity in faba bean, field pea and grass pea productivity. Front. Plant Sci. 2016, 7, 1049. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Aparicio, M.; Flores, F.; Rubiales, D. Field response of Lathyrus cicera germplasm to crenate broomrape (Orobanche crenata). Field Crop. Res. 2009, 113, 321–327. [Google Scholar] [CrossRef]
- Jeschke, W.D.; Hilpert, A. Sink-stimulated photosynthesis and sink dependent increase in nitrate uptake: Nitrogen and carbon relations of the parasitic association Cuscuta reflexa–Ricinus communis. Plant Cell Environ. 1997, 20, 47–56. [Google Scholar] [CrossRef]
- Hibberd, J.M.; Quick, W.P.; Press, M.C.; Scholes, J.D. The influence of the parasitic angiosperm Striga gesnerioides on the growth and photosynthesis of its host, Vigna unguiculata. J. Exp. Bot. 1996, 47, 507–512. [Google Scholar] [CrossRef]
- Hibberd, J.M.; Quick, W.P.; Press, M.C.; Scholes, J.D. Can source–sink relations explain responses of tobacco to infection by the root holoparasitic angiosperm Orobanche cernua? Plant Cell Environ. 1998, 21, 333–340. [Google Scholar] [CrossRef]
- Press, M.C.; Scholes, J.D.; Watling, J.R. Parasitic plants: Physiological and ecological interactions with their hosts. In Physiological Plant Ecology: The 39th Symposium of the British Ecological Society; Press, M.C., Scholes, J.D., Barker, M.G., Eds.; University of York: New York, NY, USA, 1999; pp. 175–197. [Google Scholar]
- Press, M.C. How do the parasitic weeds Striga and Orobanche influence host carbon relations? Asp. Appl. Biol. 1995, 42, 63–70. [Google Scholar]
- Frost, D.L.; Gurney, A.L.; Press, M.C.; Scholes, J.D. Striga hermonthica reduces photosynthesis in sorghum: The importance of stomatal limitations and a potential role for ABA? Plant Cell Environ. 1997, 20, 483–492. [Google Scholar] [CrossRef]
- Allen, M.F.; Smith, W.K.; Moore, T.S.; Christensen, M. Comparative water relations and photosynthesis of mychorrhizal Bouteloua gracilis H.B.K. Lab Steud. New Phytol. 1981, 88, 683–693. [Google Scholar] [CrossRef] [Green Version]
- Manschadi, A.M.; Kroschel, J.; Sauerborn, J. Dry matter production and partitioning in the host-parasite association Vicia faba–Orobanche crenata. J. Appl. Bot. 1996, 70, 224–229. [Google Scholar]
- Barker, E.R.; Press, M.C.; Scholes, J.D.; Quick, W.P. Interactions between the parasitic angiosperm Orobanche aegyptiaca and its tomato host: Growth and biomass allocation. New Phytol. 1996, 133, 637–642. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Rispail, N.; Prats, E.; Morandi, D.; García-Garrido, J.M.; Dumas-Gaudot, E.; Duc, G.; Rubiales, D. Parasitic plant infection is partially controlled through symbiotic pathways. Weed Res. 2009, 50, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Aparicio, M.; Pérez-de-Luque, A.; Prats, E.; Rubiales, D. Variability of interactions between barrel medic (Medicago truncatula) genotypes and Orobanche species. Ann. Appl. Biol. 2008, 153, 117–126. [Google Scholar] [CrossRef]
- Joel, D.M.; Portnoy, V.H. The angiospermous root parasite Orobanche L. (Orobanchaceae) induces expression of a pathogenesis related (PR) gene in susceptible tobacco roots. Ann. Bot. 1998, 81, 779–781. [Google Scholar] [CrossRef] [Green Version]
- Westwood, J.H.; Yu, X.; Foy, C.L.; Cramer, C.L. Expression of a defense-related 3-hydroxy-3-methylglutaryl CoA reductase gene in response to parasitism by Orobanche spp. Mol. Plant Microbe Interact. 1998, 11, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Vieira Dos Santos, C.; Letousey, P.; Delavault, P.; Thalouarn, P. Defence gene expression analysis of Arabidopsis thaliana parasitized by Orobanche ramosa. Phytopathology 2003, 93, 451–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoder, J.I.; Scholes, J.D. Host plant resistance to parasitic weeds; recent progress and bottlenecks. Curr. Opin. Plant Biol. 2010, 13, 478–484. [Google Scholar] [CrossRef]
- Timko, M.; Scholes, J. Host reaction to attack by root parasitic plants. In Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies; Springer: New York, NY, USA, 2013; pp. 115–141. [Google Scholar]
- Crescenzi, A.; Fanigliulo, A.; Fontana, A.; Fascetti, S. First Report of Orobanche nana on Celery in Italy. Plant Dis. 2015, 99, 1188. [Google Scholar] [CrossRef]
- Musselman, L.J.; Bolin, J.F. New Infestation of Branched Broomrape, Orobanche ramosa (Orobanchaceae), on Black Medic, (Medicago lupulina) (Fabaceae), in Virginia. Plant Dis. 2008, 92, 315. [Google Scholar] [CrossRef]
- Rubiales, D.; Fernández-Aparicio, M.; Rodríguez, M.J. First Report of Crenate Broomrape (Orobanche crenata) on Lentil (Lens culinaris) and Common Vetch (Vicia sativa) in Salamanca Province, Spain. Plant Dis. 2008, 92, 1368. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Emeran, A.A.; Moral, A.; Rubiales, D. First Report of Crenate Broomrape (Orobanche crenata) on White Lupine (Lupinus albus) Growing in Alkaline Soils in Spain and Egypt. Plant Dis. 2009, 93, 970. [Google Scholar] [CrossRef]
- Tsialtas, J.T.; Eleftherohorinos, I.G. First Report of Branched Broomrape (Orobanche ramosa) on Oilseed Rape (Brassica napus), Wild Mustard (Sinapis arvensis), and Wild Vetch (Vicia spp.) in Northern Greece. Plant Dis. 2011, 95, 1322. [Google Scholar] [CrossRef]
- Teimoury, M.; Karimmojeni, H.; Ehtemam, M.H.; Mehri, H.R. First Report of Orobanche aegyptiaca Parasitism on Sesame in Iran. Plant Dis. 2012, 96, 1232. [Google Scholar] [CrossRef] [PubMed]
- Gibot-Leclerc, S.; Reibel, C.; Legros, S. First Report of Branched Broomrape (Phelipanche ramosa) on Celeriac (Apium graveolens) in Eastern France. Plant Dis. 2014, 98, 1286. [Google Scholar] [CrossRef] [PubMed]
- Piwowarczyk, R. A revision of distribution and historical analysis of preferred hosts of Orobanche ramose (Orobanchaceae) in Poland. Acta Agrobot. 2012, 65, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Le Corre, V.; Reibel, C.; Gibot-Leclerc, S. Development of Microsatellite Markers in the Branched Broomrape Phelipanche ramosa L. (Pomel) and Evidence for Host-Associated Genetic Divergence. Int. J. Mol. Sci. 2014, 15, 994–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojanova, B.; Delourme, R.; Duffé, P.; Delavault, P.; Simier, P. Genetic differentiation and host preference reveal non-exclusive host races in the generalist parasitic weed Phelipanche ramosa. Weed Res. 2019, 59, 107–118. [Google Scholar] [CrossRef]
- Gibot-Leclerc, S.; Sallé, G.; Reboud, X.; Moreau, D. What are the traits of Phelipanche ramosa (L.) Pomel that contribute to the success of its biological cycle on its host Brassica napus L? Flora–Morphol. Distrib. Funct. Ecol. Plants. 2012, 207, 512–521. [Google Scholar] [CrossRef]
- Gibot-Leclerc, S.; Dessaint, F.; Reibel, C.; Le Corre, V. Phelipanche ramosa (L.) Pomel populations differ in life-history and infection response to hosts Flora-Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 247–252. [Google Scholar] [CrossRef]
- Botanga, C.J.; Timko, M.P. Genetic structure and analysis of host and nonhost interactions of Striga gesnerioides (witchweed) from Central Florida. Phytopathology 2005, 95, 1166–1173. [Google Scholar] [CrossRef] [Green Version]
- Dube, M.-P.; Belzile, F.J. Low genetic variability of Striga gesnerioides populations parasitic on cowpea might be explained by a recent origin. Weed Res. 2010, 50, 493–502. [Google Scholar] [CrossRef]
- Ramaiah, K.V. Breeding cereal grains for resistance to witchweed. In Parasitic Weeds in Agriculture; Crc-Press: Boca Raton, FL, USA, 1987; Volume 1, pp. 227–242. [Google Scholar]
- Gebisa, E. The Striga scourge in Africa: A growing pandemic. World Sci. 2007, 3–15. [Google Scholar] [CrossRef]
- Spallek, T.; Mutuku, M.; Shirasu, K. The genus Striga: A witch profile. Mol. Plant Pathol. 2013, 14, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Timko, M.; Gowda, B.S.; Ouédraogo, J.; Ousmane, B. Molecular markers for analysis of resistance to Striga gesnerioides in cowpea. In Integrating New Technologies for Striga Control; World Scientific Publishing Co Pte Ltd.: Singapore, 2007; pp. 115–128. [Google Scholar]
- Ohlson, E.W.; Timko, M.P. Race structure of cowpea witchweed (Striga gesnerioides) in West Africa and its implications for Striga resistance breeding of cowpea. Weed Sci. 2020, 68, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Timko, M.P. Gene-for-gene resistance in Striga-cowpea associations. Science 2009, 325, 1094. [Google Scholar] [CrossRef] [PubMed]
- Oren, M.; Hudgell, M.A.B.; Golconda, P.; Lun, C.M.; Smith, L.C. Evolution of the Immune System: Conservation and Diversification; Elsevier: Amsterdam, The Netherlands, 2016; pp. 295–310. [Google Scholar]
- Jubic, L.M.; Saile, S.; Furzer, O.J.; El Kasmi, F.; Dangl, J.L. Help wanted: Helper NLRs and plant immune responses. Curr. Opin. Plant Biol. 2019, 50, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Hegenauer, V.; Fürst, U.; Kaiser, B.; Smoker, M.; Zipfel, C.; Felix, G.; Stahl, M.; Albert, M. Detection of the plant parasite Cuscuta reflexa by a tomato cell surface receptor. Science 2016, 353, 478–481. [Google Scholar] [CrossRef]
- Duriez, P.; Vautrin, S.; Auriac, M.C.; Bazerque, J.; Boniface, M.C.; Callot, C.; Carrere, S.; Cauet, S.; Chabaud, M.; Gentou, F.; et al. A receptor-like kinase enhances sunflower resistance to Orobanche cumana. Nat. Plants 2019, 5, 1211–1215. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Macho, A.P.; Zipfel, C. Plant PRRs and the activation of innate immune signaling. Mol. Cell 2014, 54, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Jhu, M.-Y.; Farhi, M.; Wang, L.; Philbrook, R.N.; Belcher, M.S.; Nakayama, H.; Zumstein, K.S.; Rowland, S.D.; Ron, M.; Shih, P.M.; et al. Lignin-based resistance to Cuscuta campestris in tomato. bioRxiv 2019, 706861. [Google Scholar] [CrossRef] [Green Version]
- Molinero-Ruiz, L.; Delavault, P.; Pérez-Vich, B.; Pacureanu-Joita, M.; Bulos, M.; Altieri, E.; Domínguez, J. History of the race structure of Orobanche cumana and the breeding of sunflower for resistance to this parasitic weed: A review. Span. J. Agric. Res. 2015, 13, e10R01. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.X.; Heesacker, A.; Kishore, V.K.; Fernandez, A.; Sadik, E.S.; Cole, G.; Knapp, S.J. Genetic mapping of the Or(5) gene for resistance to Orobanche Race E in sunflower. Crop Sci. 2003, 43, 1021–1028. [Google Scholar] [CrossRef]
- Rodriguez-Ojeda, M.I.; Pineda-Martos, R.; Alonso, L.C.; Fernandez-Escobar, J.; Fernandez-Martinez, J.M.; Perez-Vich, B.; Velasco, L. A dominant avirulence gene in Orobanche cumana triggers Or5 resistance in sunflower. Weed Res. 2013, 53, 322–327. [Google Scholar] [CrossRef]
- Gauthier, M.; Véronési, C.; El-Halmouch, Y.; Leflon, M.; Jestin, C.; Labalette, F.; Simiera, P.; Delourmee, R.; Delavaulta, P. Characterisation of resistance to branched broomrape, Phelipanche ramosa, in winter oilseed rape. Crop Prot. 2012, 42, 56–63. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Van der Hoorn, R.A.L.; Terauchi, R.; Kamoun, S. Emerging concepts in effector biology of plant-associated organisms. Mol. Plant Microbe Interact. 2009, 22, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvitko, B.H.; Park, D.H.; Velásquez, A.C.; Wei, C.-F.; Russell, A.B.; Martin, G.B.; Schneider, D.J.; Collmer, A. Deletions in the Repertoire of Pseudomonas syringae pv. tomato DC3000 Type III Secretion Effector Genes Reveal Functional Overlap among Effectors. PLoS Pathog. 2009, 5, e1000388. [Google Scholar] [CrossRef] [PubMed]
- Eitas, T.K.; Dangl, J.L. NB-LRR proteins: Pairs, pieces, perception, partners, and pathways. Curr. Opin. Plant Biol. 2010, 13, 472–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.; Liu, H.; Wafula, E.K.; Honaas, L.; de Pamphilis, C.W.; Timko, M.P. SHR4z, a novel decoy effector from the haustorium of the parasitic weed Striga gesnerioides, suppresses host plant immunity. New Phytol. 2020, 226, 891–908. [Google Scholar] [CrossRef] [Green Version]
- Clarke, C.R.; So-Yon, P.; Tuosto, R.; Jia, X.; Yoder, A.; Van Mullekom, J.; Westwood, J. Multiple immunity-related genes control susceptibility of Arabidopsis thaliana to the parasitic weed Phelipanche Aegypt. PeerJ —J. Life Environ. Sci. PeerJ 2020, 8, e9268. [Google Scholar]
- Mur, L.A.; Kenton, P.; Atzorn, R.; Miersch, O.; Wasternack, C. The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol. 2006, 140, 249–262. [Google Scholar] [CrossRef] [Green Version]
- Kusumoto, D.; Goldwasser, Y.; Xie, X.; Yoneyama, K.; Takeuchi, Y.; Yoneyama, K. Resistance of red clover (Trifolium pratense) to the root parasitic plant Orobanche minor is activated by salicylate but not by jasmonate. Ann. Bot. 2007, 100, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Véronési, C.; Delavault, P.; Simier, P. Acibenzolar-S-methyl induces resistance in oilseed rape (Brassica napus L.) against branched broomrape (Orobanche ramosa L.). Crop Prot. 2009, 28, 104–108. [Google Scholar]
- Hiraoka, Y.; Ueda, H.; Sugimoto, Y. Molecular responses of Lotus japonicus to parasitism by the compatible species Orobanche aegyptiaca and the incompatible species Striga hermonthica. J. Exp. Bot. 2009, 60, 641–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Runyon, J.B.; Mescher, M.C.; Felton, G.W.; De Moraes, C.M. Parasitism by Cuscuta pentagona sequentially induces JA and SA defence pathways in tomato. Plant Cell Environ. 2010, 33, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Webb, M. Estimation of the seed bank of Striga spp. (Scrophulariaceae) in Malian fields and the implications for a model of biocontrol of S. hermonthica. Weed Res. 1996, 36, 85–92. [Google Scholar] [CrossRef]
- Kebreab, E.; Murdoch, A.J. Simulation of integrated control strategies for Orobanche spp. based in a life cycle model. Exp. Agric. 2001, 37, 37–51. [Google Scholar] [CrossRef]
- Gressel, J. Biotechnologies for directly generating crop resistant to parasites. In Parasitic Orobanchaceae; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 433–458. [Google Scholar]
- Fernández-Aparicio, M.; Westwood, J.H.; Rubiales, D. Agronomic, breeding and biotechnological approaches for parasitic plant management by manipulating strigolactone levels in agricultural soils. Botany 2011, 89, 813–826. [Google Scholar] [CrossRef]
- Perez-De-Luque, A.; Moreno, M.T.; Rubiales, D. Host plant resistance against broomrapes (Orobanche spp.): Defence reactions and mechanisms of resistance. Ann. Appl. Biol. 2008, 152, 131–141. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A.; Fondevilla, S.; Pérez-Vich, B.; Aly, R.; Thoiron, S.; Simier, P.; Castillejo, M.A.; Fernández, J.M.; Jorrín, J.; Rubiales, D.; et al. Understanding Orobanche and Phelipanche-host plant interactions and developing resistance. Weed Res. 2009, 49, 8–22. [Google Scholar] [CrossRef]
- Li, J.X.; Lis, K.E.; Timko, M.P. Molecular genetics of race-specific resistance of cowpea to Striga gesnerioides (Wilid.). Pest Manag. Sci. 2009, 65, 520–527. [Google Scholar] [CrossRef]
- Wickett, N.J.; Honaas, L.A.; Wafula, E.K.; Das, M.; Huang, K.; Wu, B.; Landherr, L.; Timko, M.P.; Yoder, J.; Westwood, J.H.; et al. Transcriptomes of the Parasitic Plant Family Orobanchaceae Reveal Surprising Conservation of Chlorophyll Synthesis. Curr. Biol. 2011, 21, 2098–2104. [Google Scholar] [CrossRef] [Green Version]
- Westwood, J.H.; de Pamphilis, C.W.; Das, M.; Fernández-Aparicio, M.; Honaas, L.A.; Timko, M.P.; Wickett, N.J.; Yoder, J.I. The Parasitic Plant Genome Project: New Tools for Understanding the Biology of Orobanche and Striga. Weed Sci. 2012, 60, 295–306. [Google Scholar] [CrossRef]
- Joel, D.M. The long-term approach to parasitic weeds control: Manipulation of specific developmental mechanisms of the parasite. Crop Prot. 2000, 19, 753–758. [Google Scholar] [CrossRef]
- Eizenberg, H.; Colquhoun, J.B.; Mallory-Smith, C.A. A predictive degree-days model for small broomrape (Orobanche minor) parasitism in red clover in Oregon. Weed Sci. 2005, 53, 37–40. [Google Scholar] [CrossRef]
- Eizenberg, H.; Colquhoun, J.B.; Mallory-Smith, C.A. Imazamox application timing for small broomrape (Orobanche minor) control in red clover. Weed Sci. 2006, 54, 923–927. [Google Scholar] [CrossRef]
- Kanampiu, F.K.; Ransom, J.K.; Friesen, D.; Gressel, J. Imazapyr and pyrithiobac movement in soil and from maize seed coats controls Striga while allowing legume intercropping. Crop Prot. 2002, 21, 611–619. [Google Scholar] [CrossRef]
- Colquhoun, J.B.; Eizenberg, H.; Mallory-Smith, C.A. Herbicide placement site affects small broomrape (Orobanche minor) control in red clover (Trifolium pratense). Weed Technol. 2006, 20, 356–360. [Google Scholar] [CrossRef]
- Nadler-Hassar, T.; Shaner, D.L.; Nissen, S.; Westra, P.; Rubin, B. Are herbicide-resistant crops the answer to controlling Cuscuta? Pest Manag. Sci. 2009, 65, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Schloss, J.V. Acetolactate synthase, mechanism of action and its herbicide binding site. Pestic. Sci. 1990, 29, 283–292. [Google Scholar] [CrossRef]
- Awad, A.E.; Worsham, A.D.; Corbin, F.T.; Eplee, R.E. Absorption, translocation and metabolism of foliarly applied 14-C dicamba in sorghum (Sorghum bicolor) and corn (Zea mays) parasitized with witchweed (Striga asiatica). In Proceedings of the 5th International Symposium of Parasitic Weeds, Nairobi, Kenya, 24–30 June 1991; Ransom, J.K., Musselman, L.J., Worsham, A.D., Parker, C., Eds.; CIMMYT: Mexico City, Mexico, 1991; pp. 535–536. [Google Scholar]
- Odhiambo, G.D.; Ransom, J.K. Effect of Dicamba on the control of Striga hermonthica in maize in western Kenya. Afr. Crop Sci. 1993, 1, 105–110. [Google Scholar]
- Joel, D.M.; Kleifeld, Y.; Losner-Goshen, D.; Herzlinger, G.; Gressel, J. Transgenic crops against parasites. Nature 1995, 374, 220–221. [Google Scholar] [CrossRef]
- Eizenberg, H.; Hershenhorn, J.; Ephrath, J.H.; Kanampiu, F. Chemical control. In Parasitic Orobanchaceae; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 415–432. [Google Scholar]
- Gressel, J.; Segel, L.; Ransom, J.K. Managing the delay of evolution of herbicide resistance in parasitic weeds. Int. J. Pest Manag. 1996, 42, 113–129. [Google Scholar] [CrossRef]
- Duke, S.O. Why have no new herbicide modes of action appeared in recent years? Pest Manag Sci. 2012, 68, 505–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Druille, M.; Omacini, M.; Golluscio, R.A.; Cabello, M.N. Arbuscular mycorrhizal fungi are directly and indirectly affected by glyphosate application. Appl. Soil Ecol. 2013, 72, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Barzman, M.; Barberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Lévesque, C.A.; Rahe, J.E. Herbicide interactions with fungal root pathogens, with special reference to glyphosate. Ann. Rev. Phytopathol. 1992, 30, 579–602. [Google Scholar] [CrossRef] [PubMed]
- Sharon, A.; Amsellem, Z.; Gressel, J. Glyphosate suppression of an elicited defense response. Plant Physiol. 1992, 98, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Sands, D.C.; Pilgeram, A.L. Methods for selecting hypervirulent biocontrol agents of weeds: Why and how? Pest Manag. Sci. 2009, 65, 581–587. [Google Scholar] [CrossRef]
- Eizenberg, H.; Plakhine, D.; Ziadne, H.; Tsechansky, L.; Graber, E.R. Non-chemical Control of Root Parasitic Weeds with Biochar. Front. Plant Sci. 2017, 8, 939. [Google Scholar] [CrossRef] [Green Version]
- Tippe, D.E.; Bastiaans, L.; van Ast, A.; Dieng, I.; Cissoko, M.; Kayeke, J.; Makokha, D.W.; Rodenburg, J. Fertilisers differentially affect facultative and obligate parasitic weeds of rice and only occasionally improve yields in infested fields. Field Crop. Res. 2020, 254, 107845. [Google Scholar] [CrossRef]
- Scavo, A.; Mauromicale, G. Integrated Weed Management in Herbaceous Field Crops. Agronomy 2020, 10, 466. [Google Scholar] [CrossRef] [Green Version]
- Eplee, R.E.; Norris, R.S. Chemical control of Striga. In Parasitic weeds in agriculture: Vol. I. Striga; Musselman, L.J., Ed.; CRC Press: Boca Raton, FI, USA, 1987; pp. 173–182. [Google Scholar]
- Zwanenburg, B.; Mwakaboko, A.S.; Reizelman, A.; Anilkumar, G.; Sethumadhavan, D. Structure and function of natural and synthetic signalling molecules in parasitic weed germination. Pest Manag. Sci. 2009, 65, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aparicio, M.; Bernard, A.; Falchetto, L.; Marget, P.; Chauvel, B.; Steinberg, C.; Morris, C.E.; Gibot-Leclerc, S.; Boari, A.; Vurro, M.; et al. Investigation of Amino Acids As Herbicides for Control of Orobanche minor Parasitism in Red Clover. Front. Plant Sci. 2017, 8, 842. [Google Scholar] [CrossRef] [PubMed]
- Westwood, J.H.; Charudattan, R.; Duke, S.O.; Fennimore, S.A.; Marrone, P.; Slaughter, D.C.; Swanton, C.; Zollinger, R. Weed Management in 2050: Perspectives on the Future of Weed Science. Weed Sci. 2018. [Google Scholar] [CrossRef] [Green Version]
- Watson, A.K. Biocontrol. In Parasitic Orobanchaceae; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 469–497. [Google Scholar]
- Cohen, B.; Amsellem, Z.; Maor, R.; Sharon, A.; Gressel, J. Transgenically enhanced expression of indole-3-acetic acid (IAA) confers hypervirulence to plant pathogens. Phytopathology 2002, 92, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Meir, S.; Amsellem, Z.; Al-Ahmad, H.; Safran, E.; Gressel, J. Transforming a NEP1 toxin gene into two Fusarium spp. to enhance mycoherbicide activity on Orobanche–Failure and success. Pest Manag. Sci. 2009, 65, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Nzioki, H.S.; Oyosi, F.; Morris, C.E.; Kaya, E.; Pilgeram, A.L.; Baker, C.S.; Sands, D. Striga Biocontrol on a Toothpick: A Readily Deployable and Inexpensive Method for Smallholder Farmers. Front. Plant Sci. 2016, 7, 1121. [Google Scholar] [CrossRef] [Green Version]
- Cimmino, A.; Fernández-Aparicio, M.; Andolfi, A.; Basso, S.; Rubiales, D.; Evidente, A. Effect of fungal and plant metabolites on broomrapes (Orobanche and Phelipanche spp.) seed germination and radicle growth. J. Agric. Food Chem. 2014, 62, 10485–10492. [Google Scholar] [CrossRef]
- Pouvreau, J.; Gaudin, Z.; Auger, B.; Lechat, M.M.; Gauthier, M.; Delavault, P.; Simier, P. A high-throughput seed germination assay for root parasitic plants. Plant Methods 2013, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Macías, F.A.; Mejías, F.J.R.; Molinillo, J.M.G. Recent advances in allelopathy for weed control: From knowledge to appplications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef]
- Aly, R. Conventional and biotechnological approaches for control of parasitic weeds. Vitr. Cell. Dev. Biol. Plant 2007, 43, 304–317. [Google Scholar] [CrossRef]
- Cvejić, S.; Radanović, A.; Dedić, B.; Jocković, M.; Jocić, S.; Miladinović, D. Genetic and Genomic Tools in Sunflower Breeding for Broomrape Resistance. Genes 2020, 11, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letousey, P.; De Zélicourt, A.; Vieira Dos Santos, C.; Thoiron, S.; Monteau, F.; Simier, P.; Thalouarn, P.; Delavault, P. Molecular analysis of resistance mechanisms to Orobanche cumana in sunflower. Plant Pathol. 2007, 56, 536–546. [Google Scholar] [CrossRef]
- De Zélicourt, A.; Letousey, P.; Thoiron, S.; Campion, C.; Simoneau, P.; Elmorjani, K.; Marion, D.; Simier, P.; Delavault, P. Ha-DEF1, a sunflower defensin, induces cell death in Orobanche parasitic plants. Planta 2007, 226, 591–600. [Google Scholar] [CrossRef] [PubMed]
- McCarville, M.T.; O′Neal, M.E.; Potter, B.D.; Tilmon, K.J.; Cullen, E.M.; McCornack, B.P.; Tooker, J.F.; Prischmann-Voldseth, D.A. One Gene Versus Two: A Regional Study on the Efficacy of Single Gene Versus Pyramided Resistance for Soybean Aphid Management. J. Econ. Entomol. 2014, 107, 1680–1687. [Google Scholar] [CrossRef]
- Varenhorst, A.J.; Pritchard, S.R.; O′Neal, M.E.; Hodgson, E.W.; Singh, A.K. Determining the Effectiveness of Three-Gene Pyramids Against Aphis glycines (Hemiptera: Aphididae) Biotypes. J. Econ. Entomol. 2017, 110, 2428–2435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.C.; Wen, Z.X.; DiFonzo, C.; Song, Q.J.; Wang, D.C. Pyramiding different aphid-resistance genes in elite soybean germplasm to combat dynamic aphid populations. Mol. Breed. 2018, 38, 29. [Google Scholar] [CrossRef]
- Guza, C.J. Weed Control with Glyphosate and Glufosinate in Herbicide-Resistant Sugarbeets (Beta vulgaris L.). Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2000. [Google Scholar]
- Nadler-Hassar, T.; Rubin, B. Natural tolerance of Cuscuta campestris to herbicides inhibiting amino acid biosynthesis. Weed Res. 2003, 43, 341–347. [Google Scholar] [CrossRef]
- De Blok, M.; Botterman, J.; Vanderwiele, M.; Dockx, J.; Thoen, C.; Gossele, V.; Rao Movva, N.; Thompson, C.; Van Montagu, M.; Leemans, J. Engineering herbicide resistance in plants by expression of a detoxifying enzyme. EMBO J. 1987, 6, 2513–2518. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Qu, F.; Li, Z.; Doohan, D. Inter-species protein trafficking endows dodder (Cuscuta pentagona) with a host-specific herbicide-tolerant trait. New Phytol. 2013, 198, 1017–1022. [Google Scholar] [CrossRef]
- Slavov, S.; Valkov, V.; Batchvarova, R.; Atanassova, S.; Alexandrova, M.; Atanassov, A. Chlorsulfuron resistant transgenic tobacco as a tool for broomrape control. Transgenic Res. 2005, 14, 273–278. [Google Scholar] [CrossRef]
- Aviv, D.; Amsellem, Z.; Gressel, J. Transformation of carrots with mutant acetolactate synthase for Orobanche (broomrape) control. Pest Manag. Sci. 2002, 58, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Nandula, V.K.; Foy, C.L.; Orcutt, D.M. Glyphosate for Orobanche aegyptiaca control in Vicia sativa and Brassica napus. Weed Sci. 1999, 47, 486–491. [Google Scholar] [CrossRef]
- Senseman, S.A. Herbicide Handbook; Weed Science Society of America: Champaign, IL, USA, 2007; pp. 155–157. [Google Scholar]
- Hamamouch, N.; Westwood, J.H.; Banner, I.; Cramer, C.L.; Gepstein, S.; Aly, R. A peptide from insects protects transgenic tobacco from a parasitic weed. Transgenic Res. 2005, 14, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Tomilov, A.A.; Tomilova, N.B.; Wroblewski, T.; Michelmore, R.; Yoder, J.I. Trans-specific gene silencing between host and parasitic plants. Plant J. 2008, 56, 389–397. [Google Scholar] [CrossRef]
- De Framond, A.R.P.; Mcmillan, J.; Ejeta, G. Effects on Striga parastitism of transgenic maize armed with RNAi constructs targeting essential S. asiatica genes. In Integrating New Technologies for Striga Control-Towards Ending the Witch-Hunt; Ejeta, G., Gressel, J., Eds.; World Scientific: Singapore, 2007; pp. 185–196. [Google Scholar]
- Dubey, N.K.; Eizenberg, H.; Leibman, D.; Wolf, D.; Edelstein, M.; Abu-Nassar, J.; Marzouk, S.; Gal-On, A.; Aly, R. Enhanced host-parasite resistance based on down-regulation of Phelipanche aegyptiaca target genes is likely by mobile small RNA. Front. Plant Sci. 2017, 8, 1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butt, H.; Jamil, M.; Wang, J.Y.; Al-Babili, S.; Mahfouz, M. Engineering plant architecture via CRISPR/Cas9-mediated alteration of strigolactone biosynthesis. BMC Plant Biol. 2018, 18, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bari, V.K.; Nassar, J.A.; Kheredin, S.M.; Gal-On, A.; Ron, M.; Britt, A.; Steele, D.; Yoder, J.; Aly, R. CRISPR/Cas9-mediated mutagenesis of CAROTENOID CLEAVAGE DIOXYGENASE 8 in tomato provides resistance against the parasitic weed Phelipanche aegyptiaca. Sci. Rep. 2019, 9, e11438. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Aparicio, M.; Delavault, P.; Timko, M.P. Management of Infection by Parasitic Weeds: A Review. Plants 2020, 9, 1184. https://doi.org/10.3390/plants9091184
Fernández-Aparicio M, Delavault P, Timko MP. Management of Infection by Parasitic Weeds: A Review. Plants. 2020; 9(9):1184. https://doi.org/10.3390/plants9091184
Chicago/Turabian StyleFernández-Aparicio, Mónica, Philippe Delavault, and Michael P. Timko. 2020. "Management of Infection by Parasitic Weeds: A Review" Plants 9, no. 9: 1184. https://doi.org/10.3390/plants9091184
APA StyleFernández-Aparicio, M., Delavault, P., & Timko, M. P. (2020). Management of Infection by Parasitic Weeds: A Review. Plants, 9(9), 1184. https://doi.org/10.3390/plants9091184







