Communities of Fungi in Black Cherry Stumps and Effects of Herbicide
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Starfinger, U.; Kowarik, I.; Rode, M.; Schepker, H. From desirable ornamental plant to pest to accepted addition to the Flora?—The perception of an alien tree species through the centuries. Biol. Invasions 2003, 5, 323–335. [Google Scholar] [CrossRef]
- Juhász, M. Black cherry (Prunus serotina Ehrh.). In The Most Important Invasive Plants in Hungary; Botta-Dukát, Z., Balogh, L., Eds.; HAS Institute of Ecology and Botany: Vácrátót, Hungary, 2008; ISBN 9789638391421. [Google Scholar]
- Aerts, R.; Ewald, M.; Nicolas, M.; Piat, J.; Skowronek, S.; Lenoir, J.; Hattab, T.; Garzón-López, C.X.; Feilhauer, H.; Schmidtlein, S.; et al. Invasion by the Alien Tree Prunus serotina Alters Ecosystem Functions in a Temperate Deciduous Forest. Front. Plant. Sci. 2017, 8, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starfinger, U. NOBANIS—Invasive Alien Species Fact Sheet—Prunus serotina. Available online: www.nobanis.org (accessed on 6 September 2019).
- Bułaj, B.; Okpisz, K.; Rutkowski, P.; Tomczak, A. Occurrence of invasive black cherry (Prunus serotina Ehrh.) on abandoned farmland in west-central Poland. For. Lett. 2017, 110, 26–31. [Google Scholar]
- Otręba, A.; Marciszewska, K.; Janik, D. Is cut-stump and girdling an efficient method of black cherry Prunus serotina Ehrh. eradication? Folia For. Pol. 2017, 59, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Bellon, S.; Tumiłowicz, J.; Król, S. Obce Gatunki Drzew w Gospodarstwie Leśnym [Alien Tree Species in Forest Economy]; PWRiL: Warszawa, Poland, 1977. [Google Scholar]
- Łukowski, A.; Janek, W.; Baraniak, E.; Walczak, U.; Karolewski, P. Changing Host Plants Causes Structural Differences in the Parasitoid Complex of the Monophagous Moth Yponomeuta evonymella, but Does Not Improve Survival Rate. Insects 2019, 10, 197. [Google Scholar]
- Głowacka, B. (Ed.) Środki Ochrony Roślin, Środki Biobójcze Oraz Produkty do Rozkładu pni Drzew Leśnych Zalecane do Stosowania w Leśnictwie w Roku 2015 [Pesticides, Biocidal Preparations and Tree Rot and Stump Killers Recommended for Use in Forestry in 2015]; Instytut Badawczy Leśnictwa, Analizy i Raporty: Sękocin Stary, Poland; Raszyn, Poland, 2014; Volume 23, p. 74. [Google Scholar]
- Najberek, K.; Solarz, W. Gatunki obce w faunie Polski [Alien species in the fauna of Poland]. In Gatunki Obce w Faunie Polski [Alien Species in the Fauna of Poland]; Głowaciński, Z., Okarma, H., Pawłowski, J., Solarz, W., Eds.; Instytut Ochrony Przyrody PAN w Krakowie: Kraków, Poland, 2011; pp. 624–639. [Google Scholar]
- Tittenbrun, A.; Radliński, B. Practices of eliminating alien invasive species in the Roztocze National Park. In Elimination of Invasive Alien Plant Species Good and Bad Practices; Krzysztofiak, L., Krzysztofiak, A., Eds.; Stowarzyszenie “Człowiek i Przyroda”: Krzywe, Poland, 2015; pp. 49–54. (In Polish) [Google Scholar]
- Namura-Ochalska, A.; Borowa, B. The struggle against black cherry Padus serotina (Ehrh.) Borkh. in the forest division Rózin of the Kampinos National Park. Assessment of the effectiveness of selected methods. In Elimination of Invasive Alien Plant Species Good and Bad Practices; Krzysztofiak, L., Krzysztofiak, A., Eds.; Stowarzyszenie “Człowiek i Przyroda”: Krzywe, Poland, 2015; pp. 57–74. (In Polish) [Google Scholar]
- Van den Meersschaut, D.; Lust, N. Comparison of mechanical, biological and chemical methods for controlling Black cherry (Prunus serotina) in Flanders (Belgium). Silva. Gandav. 1997, 62, 90–109. [Google Scholar] [CrossRef] [Green Version]
- Kogan, M.; Alister, C. Glyphosate Use in Forest Plantations. Chil. J. Agric. Res. 2010, 70, 652–666. [Google Scholar] [CrossRef] [Green Version]
- Skrzecz, I.; Szmidla, H. (Eds.) Środki Ochrony Roślin Oraz Środki Biobójcze do Stosowania w Leśnictwie w Roku 2020; Instytut Badawczy Leśnictwa, Analizy i Raporty nr 30: Sękocin Stary, Poland, 2020; ISBN 978-83-62830-83-1. [Google Scholar]
- Otręba, A. Wprowadzenie [Introduction]. In Metody Zwalczania Obcych Gatunków Roślin Występujących na Terenie Puszczy Kampinoskiej [Methods to Control Alien Plant Species Found in the Puszcza Kampinoska Forest]; Obidziński, A., Kołaczkowska, E., Otręba, A., Eds.; Kampinoski Park Narodowy: Izabelin, Poland, 2016. [Google Scholar]
- Kwiatkowska, M.; Jarosiewicz, P.; Bukowska, B. Glyphosate and its formulations—Toxicity, occupational and environmental exposure. Med. Pr. 2013, 64, 717. [Google Scholar] [CrossRef]
- Zaller, J.G.; Heigl, F.; Ruess, L.; Grabmaier, A. Glyphosate herbicide affects belowground interactions between earthworms and symbiotic mycorrhizal fungi in a model ecosystem. Sci. Rep. 2015, 4, 5634. [Google Scholar] [CrossRef] [Green Version]
- Morjan, W.E.; Pedigo, L.P.; Lewis, L.C. Fungicidal Effects of Glyphosate and Glyphosate Formulations on Four Species of Entomopathogenic Fungi. Environ. Entomol. 2002, 31, 1206–1212. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Mallek, A.Y.; Abdel-Kader, M.I.A.; Shonkeir, A.M.A. Effect of glyphosate on fungal population, respiration and the decay of some organic matters in Egyptian soil. Microbiol. Res. 1994, 149, 69–73. [Google Scholar] [CrossRef]
- De Jong, M.D. The BioChon story: Deployment of Chondrostereum purpureum to suppress stump sprouting in hardwoods. Mycologist 2000, 14, 58–62. [Google Scholar] [CrossRef]
- Roy, V.; Dubeau, D.; Auger, I. Biological control of intolerant hardwood competition: Silvicultural efficacy of Chondrostereum purpureum and worker productivity in conifer plantations. For. Ecol. Manag. 2010, 259, 1571–1579. [Google Scholar] [CrossRef]
- Gosselin, L.; Jobidon, R.; Bernier, L. Biological Control of Stump Sprouting of Broadleaf Species in Rights-of-Way with Chondrostereum purpureum: Incidence of the Disease on Nontarget Hosts. Biol. Control. 1999, 16, 60–67. [Google Scholar] [CrossRef]
- Harmonia+PL–Procedura Oceny Ryzyka Negatywnego Oddziaływania Inwazyjnych i Potencjalnie Inwazyjnych Gatunków Obcych w Polsce. Available online: http://projekty.gdos.gov.pl/files/artykuly/127077/Padus-serotina_czeremcha-amerykanska_PL_icon.pdf. (accessed on 10 May 2020).
- Phillips, R.; Shearer, L.; Reid, D.A. Mushrooms and Other Fungi of Great Britain and Europe; A Pan Original; Pan Books: London, UK, 1981; ISBN 9780330264419. [Google Scholar]
- Proposed Regulatory Decision Document PRDD2002-01. Available online: http://publications.gc.ca/collections/Collection/H113-9-2001-7E.pdf (accessed on 10 May 2020).
- Marciszewska, K.; Szczepkowski, A.; Otręba, A.; Oktaba, L.; Kondras, M.; Zaniewski, P.; Ciurzycki, W.; Wojtan, R. The dynamics of sprouts generation and colonization by macrofungi of black cherry Prunus serotina Ehrh. eliminated mechanically in the Kampinos National Park. Folia For. Pol. 2018, 60, 34–51. [Google Scholar] [CrossRef] [Green Version]
- Lundell, T.K.; Mäkelä, M.R.; de Vries, R.P.; Hildén, K.S. Genomics, Lifestyles and Future Prospects of Wood-Decay and Litter-Decomposing Basidiomycota. In Advances in Botanical Research; Academic Press: London, UK, 2014; pp. 329–370. [Google Scholar]
- Korzeniewicz, R.; Baranowska, M.; Behnke-Borowczyk, J. The effect of size of black cherry stumps on the composition of fungal communities colonising stumps. Open Life Sci. 2019, 14, 482–493. [Google Scholar]
- Kwaśna, H.; Bateman, G.L.; Ward, E. Determining species diversity of microfungal communities in forest tree roots by pure-culture isolation and DNA sequencing. Appl. Soil Ecol. 2008, 40, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Kwaśna, H.; Mazur, A.; Łabędzki, A.; Kuźmiński, R.; Łakomy, P. Communities of fungi in decomposed wood of oak and pine. For. Res. Pap. 2016, 77, 261–275. [Google Scholar] [CrossRef] [Green Version]
- Mäkelä, M.R.; Marinović, M.; Nousiainen, P.; Liwanag, A.J.M.; Benoit, I.; Sipilä, J.; Hatakka, A.; de Vries, R.P.; Hildén, K.S. Aromatic Metabolism of Filamentous Fungi in Relation to the Presence of Aromatic Compounds in Plant Biomass. Adv. Appl. Microbiol. 2015, 91, 63–137. [Google Scholar]
- Gutowski, J.M.; Bobiec, A.; Pawlaczyk, P.; Zub, K.B. Drugie Życie Drzewa [The Second Life of Tree]; WWF: Warszawa-Hajnówka, Poland, 2004; ISBN 83-916021-6-8. [Google Scholar]
- Eaton, R.A.; Hale, M.D.C. Wood: Decay, Pests and Protection; Chapman and Hall Ltd.: London, UK, 1993; ISBN 0412531208. [Google Scholar]
- Jamil, Z.A.; Boddy, L. Wood decomposition by the ’cord-forming’ fungus Resinicium bicolor: Interactive effect of home base size and quality with its surrounding soil composition. J. Trop. Agric. Food Sci. 2004, 32, 257–269. [Google Scholar]
- Jacobson, E.S.; Tinnell, S.B. Antioxidant function of fungal melanin. J. Bacteriol. 1993, 175, 7102–7104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gessler, A.; Peuke, A.D.; Keitel, C.; Farquhar, G.D. Oxygen isotope enrichment of organic matter in Ricinus communis during the diel course and as affected by assimilate transport. New Phytol. 2007, 174, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi. Volume 1; Academic Press: London, UK, 1980; ISBN 0122204018. [Google Scholar]
- Breitenbach, J.; Kränzlin, F. Fungi of Switzerland: Ascomycetes; Fungi of Switzerland: A Contribution to the Knowledge of the Fungal Flora of Switzerland. Verl. Mykol. 1984, 1, 310. [Google Scholar]
- Dong-Yeo, K.; Ju-Kyeong, E.; Hyeok, P.; Ahn-Heum, E. A Note on a Dark Septate Endophyte Phialocephala piceae Isolated from Needle Leaves of Thuja koraiensis in Korea. Korean J. Mycol. 2016, 44, 338–341. [Google Scholar]
- Tanney, J.B.; Douglas, B.; Seifert, K.A. Sexual and asexual states of some endophytic Phialocephala species of Picea. Mycologia 2016, 108, 255–280. [Google Scholar] [CrossRef] [Green Version]
- Whitton, S.R.; McKenzie, E.H.C.; Hyde, K.D. Dictyochaeta and Dictyochaetopsis species from the Pandanaceae. Fungal Divers. 2000, 4, 133–158. [Google Scholar]
- Perry, B.A. A Taxonomic Investigation of Mycena in California. Ph.D. Thesis, San Francisco State University, San Francisco, CA, USA, 2002. [Google Scholar]
- Bodziarczyk, J.; Chachuła, P. Charakterystyka przyrodnicza rezerwatu “Cisy w Serednicy” w Górach Słonnych (Bieszczady Zachodnie) [Characteristics of nature value of the Cisy w Serednicy nature reserve in the Słonne Mts. (Western Bieszczady Mts.)]. Rocz. Bieszcz. 2008, 16, 179–190. [Google Scholar]
- Cruz, A.C.R.; Leão-Ferreira, S.M.; Barbosa, F.R.; Gusmão, L.F.P. Conidial fungi from semi-arid Caatinga biome of Brazil. New and interesting Zanclospora species. Mycosphere 2008, 106, 15–27. [Google Scholar]
- Han, J.-G.; Sung, G.-H.; Shin, H.-D. Proliferodiscus inspersus var. magniascus and Rodwayella citrinula, Two Unrecorded Taxa of Hyaloscyphaceae (Tribe Arachnopezizeae) in Korea. Mycobiology 2014, 42, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Crous, P.W.; Carnegie, A.J.; Wingfield, M.J.; Sharma, R.; Mughini, G.; Noordeloos, M.E.; Santini, A.; Shouche, Y.S.; Bezerra, J.D.P.; Dima, B.; et al. Fungal Planet description sheets: 868–950. Pers. Mol. Phylogeny Evol. Fungi 2019, 42, 291–473. [Google Scholar] [CrossRef]
- Haelewaters, D.; Dirks, A.C.; Kappler, L.A.; Mitchell, J.K.; Quijada, L.; Vandegrift, R.; Buyck, B.; Pfister, D.H. A Preliminary Checklist of Fungi at the Boston Harbor Islands. Northeast. Nat. 2018, 25, 45–76. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.-M.; Wang, M.; Cai, L. Phylogenetic assessment and taxonomic revision of Mariannaea. Mycol. Prog. 2017, 16, 271–283. [Google Scholar] [CrossRef]
- Hernandez, V. Role of Non-Decay Fungi on the Weathering of Wood. Ph.D. Thesis, The University of British Columbia, Vancouver, BC, Canada, 2012. [Google Scholar]
- Gremmen, J. A new, crystalline, antibiotic substance produced by Mollisia species (Discomycetes). Antonie Van Leeuwenhoek 1956, 22, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, J.M.; Sabat, G.; Cullen, D. The Foliar Endophyte Phialocephala scopiformis DAOMC 229536 Proteome When Grown on Wood Used as the Sole Carbon Source. Microbiol. Resour. Announc. 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crous, P.W.; Lombard, L.; Giraldo, A.; Smith, A.J.; Groenewald, J.Z.; Schumacher, R.K.; Wingfield, M.J.; Christensen, M.; Gardiennet, A.; Nakashima, C.; et al. Fungal Systematics and Evolution: FUSE 1. Sydowia 2015, 67, 81–118. [Google Scholar]
- Siepmann, R. Ein Beitrag zur Regeneration und zur Infektion von Douglasienwurzeln (Pseudotsuga menziesii [Mirb.] Franco) durch Pilze aus dem Boden. For. Pathol. 1981, 11, 162–169. [Google Scholar] [CrossRef]
- Brady, K.C.; O’Kiely, P.; Forristal, P.D.; Fuller, H. Schizophyllum commune on big-bale grass silage in Ireland. Mycologist 2005, 19, 30–35. [Google Scholar] [CrossRef]
- Nilsson, T. Studies on wood degradation and cellulolytic activity of microfungi. Stud. For. Suec. 1973, 104, 1–40. [Google Scholar]
- Nilsson, T. The degradation of cellulose and the production of cellulase, xylanase, mannanase and amylase by wood-attacking microfungi. Stud. For. Suec. 1974, 114, 1–61. [Google Scholar]
- Poszytek, K. Mikrobiologiczna utylizacja celulozy [Microbiological utilisation of cellulose]. Postępy Mikrobiol. 2016, 55, 132–146. [Google Scholar]
- Szwajkowska-Michałek, L.; Kwaśna, H.; Łakomy, P.; Perkowski, J. Inhibition of Armillaria and Heterobasidion growth by Penicillium adametzii isolated from Pinus sylvestris forest soil. For. Pathol. 2012, 42, 454–466. [Google Scholar] [CrossRef]
- Krzysko-Łupicka, T.; Grata, K. Ekologiczne skutki działania herbicydu fosforoorganicznego na diazotrofy glebowe w okresie jesiennym [Ecological effects of an organophosphorus herbicide on soil diazotrophs in the autumn period]. Proc. ECOpole 2007, 1, 1169–1173. [Google Scholar]
- Rueppel, M.L.; Brightwell, B.B.; Schaefer, J.; Marvel, J.T. Metabolism and degradation of glyphosate in soil and water. J. Agric. Food Chem. 1977, 25, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.S.F.; Monteiro, R.T.R.; Abarkeli, R.B. Effect of glyphosate on the microbial activity of two Brazilian soils. Chemosphere 2003, 52, 799–804. [Google Scholar] [CrossRef] [Green Version]
- Hunter, M.L., Jr. Wildlife, Forests, and Forestry. Principles of Managing Forests for Biological Diversity; Prentice Hall: Englewood Cliffs, NJ, USA, 1990; ISBN 0139594795. [Google Scholar]
- Schmidt, P.-A.; Bálint, M.; Greshake, B.; Bandow, C.; Römbke, J.; Schmitt, I. Illumina metabarcoding of a soil fungal community. Soil Biol. Biochem. 2013, 65, 128–132. [Google Scholar] [CrossRef]
- Vilgalys, R.; Gonzalez, D. Organization of ribosomal DNA in the basidiomycete Thanatephorus praticola. Curr. Genet. 1990, 18, 277–280. [Google Scholar] [CrossRef]
- Behnke-Borowczyk, J.; Kwaśna, H.; Kulawinek, B. Fungi associated with Cyclaneusma needle cast in Scots pine in the west of Poland. For. Pathol. 2019, 49, e12487. [Google Scholar] [CrossRef]
- GenBank. Available online: https://www.ncbi.nlm.nih.gov/genbank (accessed on 6 September 2019).
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Ostell, J.; Pruitt, K.D.; Sayers, E.W. GenBank. Nucleic Acids Res. 2018, 46, D41–D47. [Google Scholar] [CrossRef] [Green Version]
- Morgulis, A.; Coulouris, G.; Raytselis, Y.; Madden, T.L.; Agarwala, R.; Schäffer, A.A. Database indexing for production MegaBLAST searches. Bioinformatics 2008, 24, 1757–1764. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A Greedy Algorithm for Aligning DNA Sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Springer: Dordrecht, The Netherlands, 1988; ISBN 978-94-015-7360-3. [Google Scholar]

| Taxon | Frequency in the Fungal Community (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| May Samples | July–August Samples | |||||||
| <5 cm Diam. | >5 cm Diam. | <5 cm Diam. | >5 cm Diam. | |||||
| Treated | Untreated | Treated | Untreated | Treated | Untreated | Treated | Untreated | |
| Blastocladiomycota | 0.046 | 0 | 0 | 0 | 0 | 0.097 | 0 | 0 |
| Rozellomycota | 0 | 0.164 | 0 | 0.017 | 0.019 | 0 | 0.049 | 0.672 |
| Glomeromycota | 0 | 0.25 | 0 | 0.02 | 0.02 | 0 | 0.05 | 0.67 |
| Zygomycota | 0.023 | 0.62 | 0.34 | 0.49 | 0.02 | 0.02 | 0.01 | 0.09 |
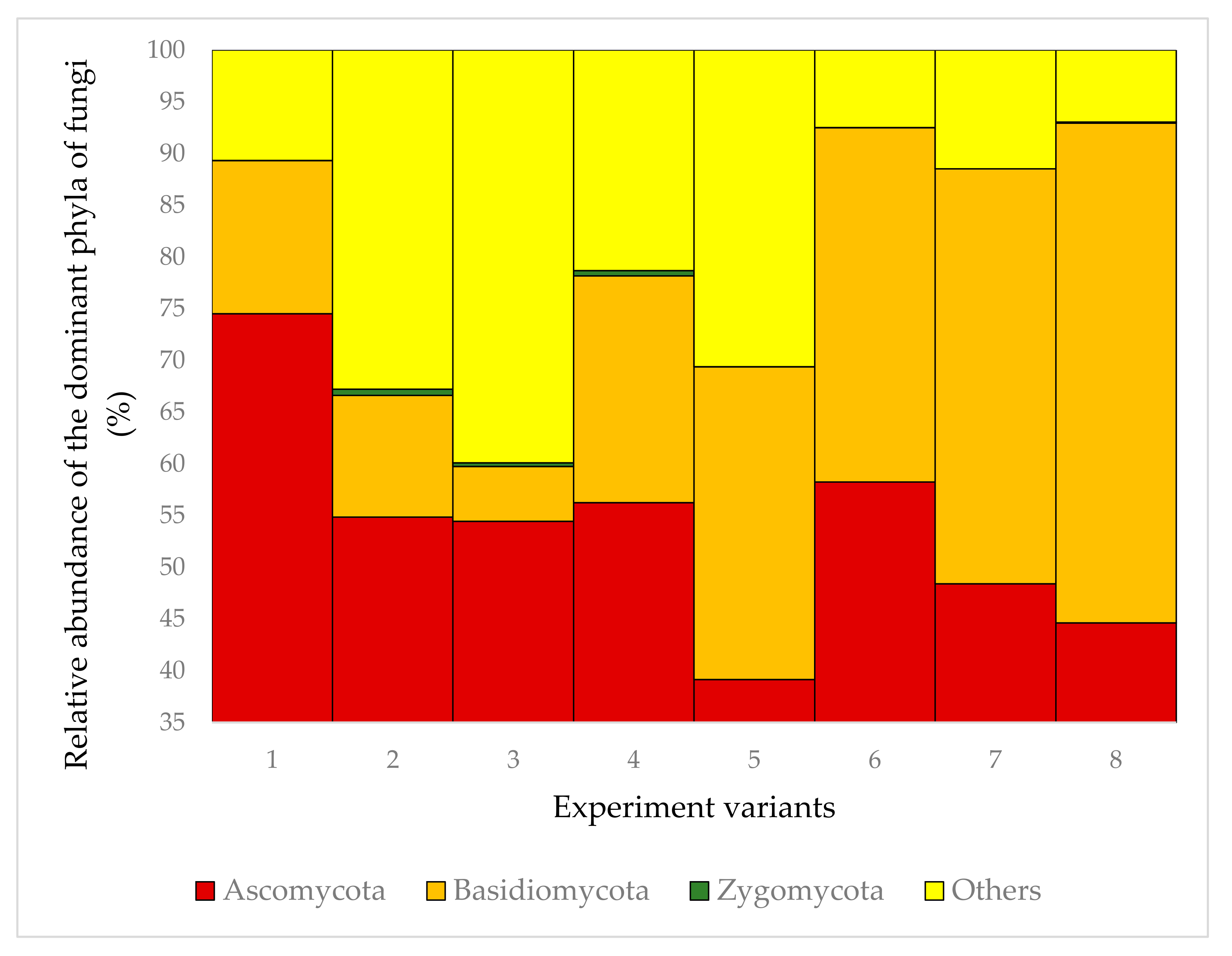
| Ascomycota | 74.51 | 54.85 | 54.45 | 56.25 | 39.15 | 58.25 | 48.4 | 44.62 |
| Basidiomycota | 14.8 | 11.76 | 5.30 | 21.93 | 30.22 | 34.26 | 40.11 | 48.32 |
| Other taxa | 3.96 | 0.58 | 13.38 | 2.53 | 4.77 | 2.57 | 3.61 | 2.65 |
| Non-cultured fungi | 3.65 | 12.75 | 21.0 | 5.81 | 21.75 | 3.16 | 4.86 | 2.45 |
| Organisms with no NCBI reference sequence | 3.01 | 19.2 | 5.49 | 12.98 | 4.07 | 1.65 | 2.96 | 1.24 |
| Number of Operational Taxonomic Units | 20,198 | 38,770 | ||||||
| 6756 | 13,442 | 14,585 | 24,185 | |||||
| 4324 a | 2432 a | 7673 b | 5769 b | 5282 c | 9303 c | 8110 d | 16,075 d | |
| Number of fungal Operational Taxonomic Units | 17,073 | 36,758 | ||||||
| 5974 | 11,099 | 13,726 | 23,032 | |||||
| 4023 a | 1951 a | 6225b | 4874 b | 4815 c | 8911 c | 7577 d | 15,455 d | |
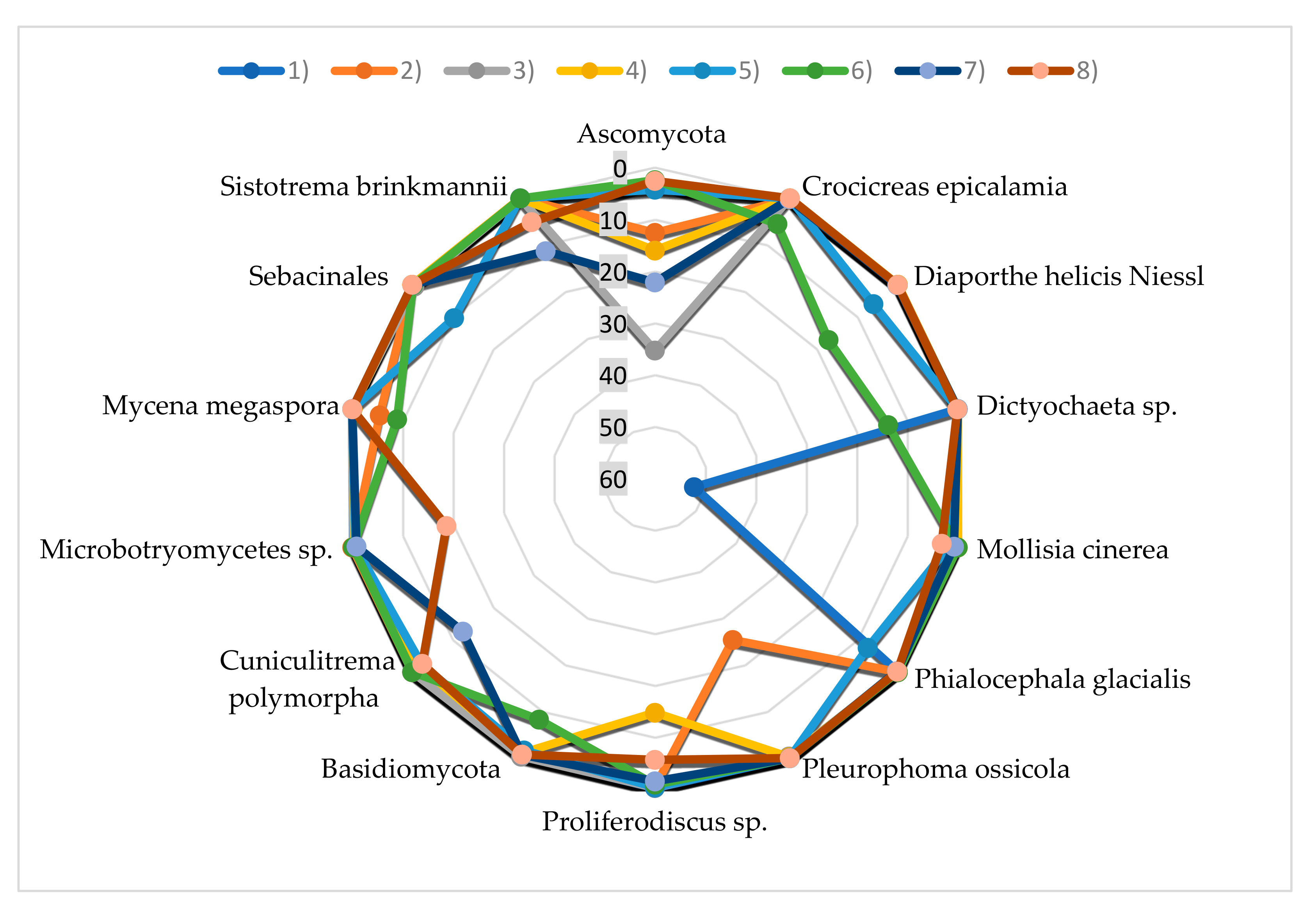
| Number of taxa | 285 a | 120 a | 242 b | 304 b | 267 | 287 | 319 | 306 |
| Number of fungal taxa | 249 a | 83 a | 217 b | 281 b | 229 | 254 | 277 | 274 |
| May | July–August | ||||||
|---|---|---|---|---|---|---|---|
| 0.8892 | |||||||
| <5 cm diam. | >5 cm diam. | ||||||
| 0.8419 | 0.7904 | ||||||
| <5 cm diam. | >5 cm diam. | <5 cm diam. | >5 cm diam. | ||||
| Treated | Untreated | Treated | Untreated | Treated | Untreated | Treated | Untreated |
| 0.22 | 0.44 | 0.46 | 0.53 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korzeniewicz, R.; Baranowska, M.; Kwaśna, H.; Niedbała, G.; Behnke-Borowczyk, J. Communities of Fungi in Black Cherry Stumps and Effects of Herbicide. Plants 2020, 9, 1126. https://doi.org/10.3390/plants9091126
Korzeniewicz R, Baranowska M, Kwaśna H, Niedbała G, Behnke-Borowczyk J. Communities of Fungi in Black Cherry Stumps and Effects of Herbicide. Plants. 2020; 9(9):1126. https://doi.org/10.3390/plants9091126
Chicago/Turabian StyleKorzeniewicz, Robert, Marlena Baranowska, Hanna Kwaśna, Gniewko Niedbała, and Jolanta Behnke-Borowczyk. 2020. "Communities of Fungi in Black Cherry Stumps and Effects of Herbicide" Plants 9, no. 9: 1126. https://doi.org/10.3390/plants9091126
APA StyleKorzeniewicz, R., Baranowska, M., Kwaśna, H., Niedbała, G., & Behnke-Borowczyk, J. (2020). Communities of Fungi in Black Cherry Stumps and Effects of Herbicide. Plants, 9(9), 1126. https://doi.org/10.3390/plants9091126







