Glucosinolate Profile and Glucosinolate Biosynthesis and Breakdown Gene Expression Manifested by Black Rot Disease Infection in Cabbage
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Plant Materials
2.2. Collection of Leaf Samples for HPLC and PCR
2.3. Glucosinolates Identification, Quantification and Analysis
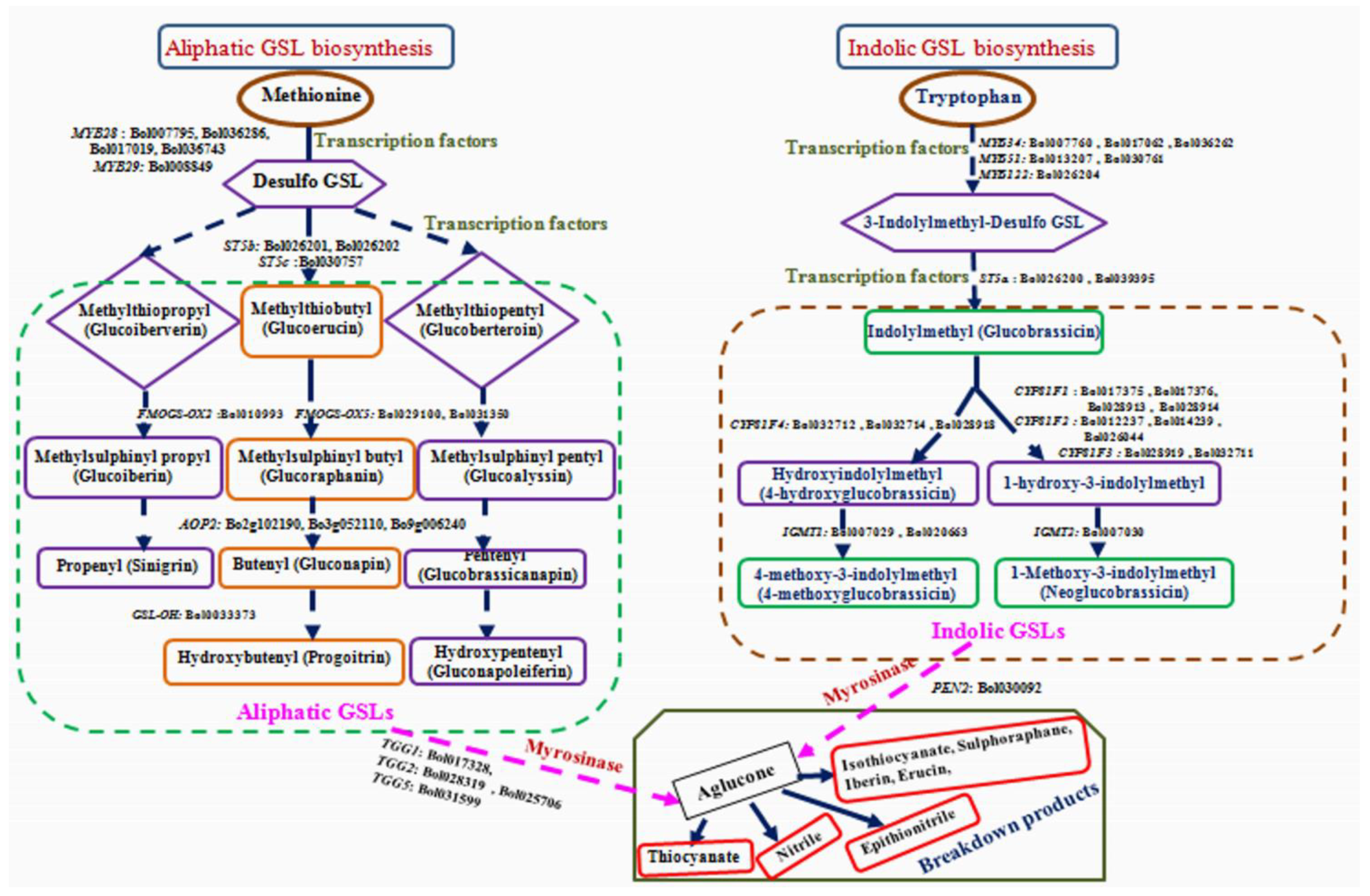
2.4. Expression Analysis of GSL Biosynthesis Pathway and Break-Down Related Genes
2.5. Total RNA Extraction, cDNA Synthesis and Quantitative Real-Time PCR Analysis
2.6. Statistical Analysis
3. Results
3.1. Phenotypic Evaluation of Cabbage Lines Against Black Rot Disease
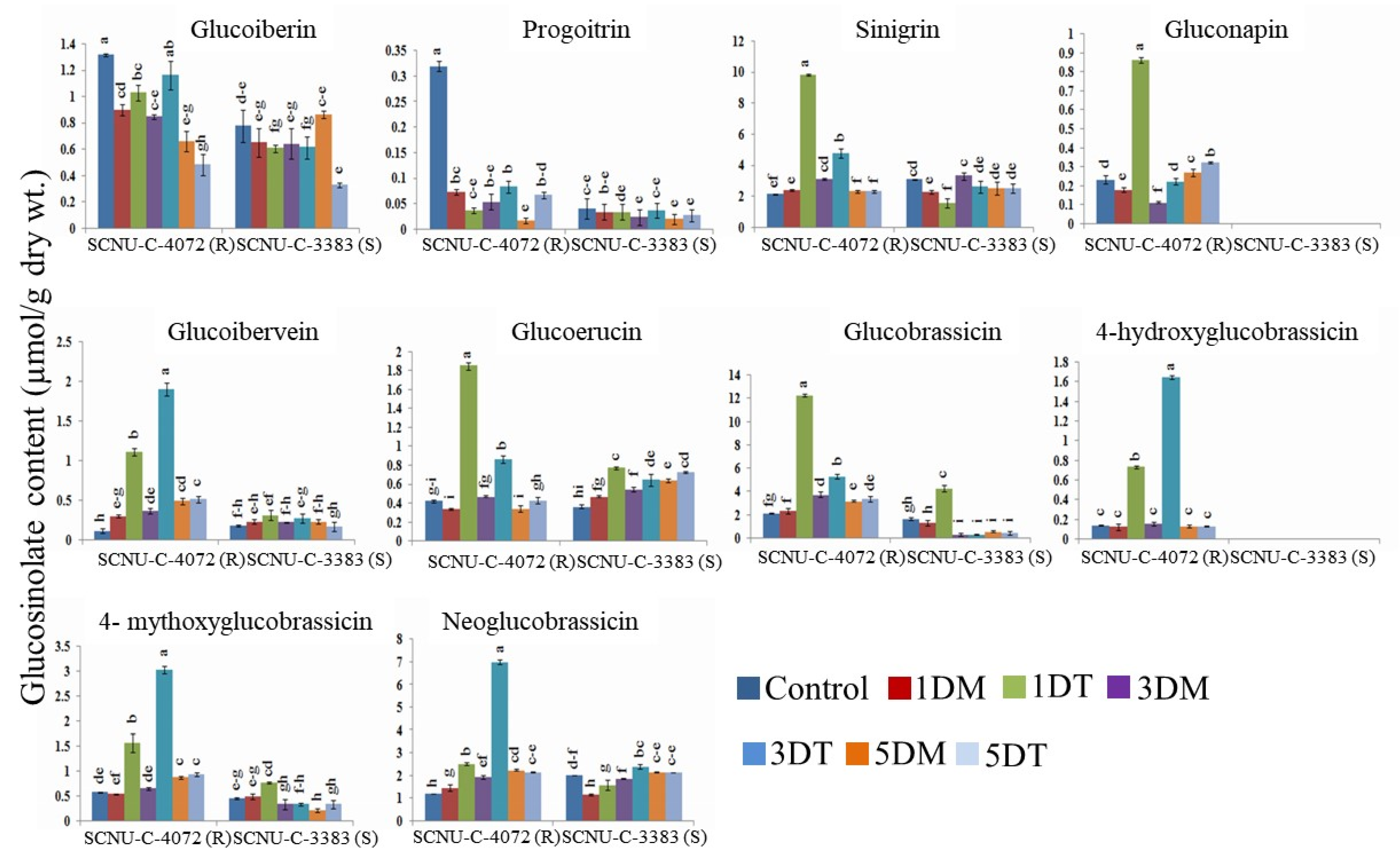
3.2. Distribution of the GSL Compounds in the Black Rot Resistant and Susceptible Lines
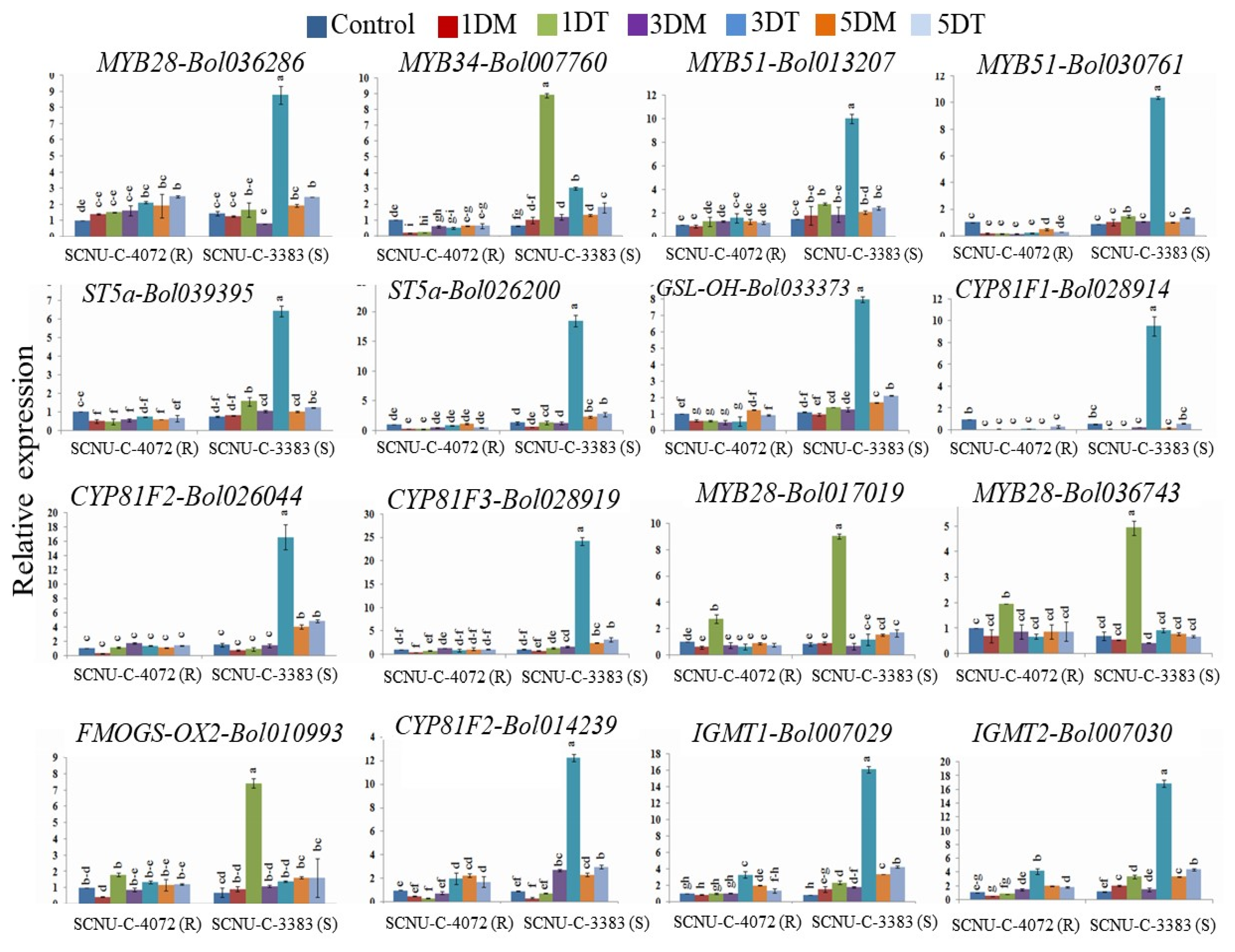
3.3. Relative Expression of Transcription Factor- and GSL Biosynthesis-Related Genes in Black Rot Resistant Line
3.4. Relative Expression of TF-Related and GSL Biosynthesis Genes in Black Rot Susceptible Line
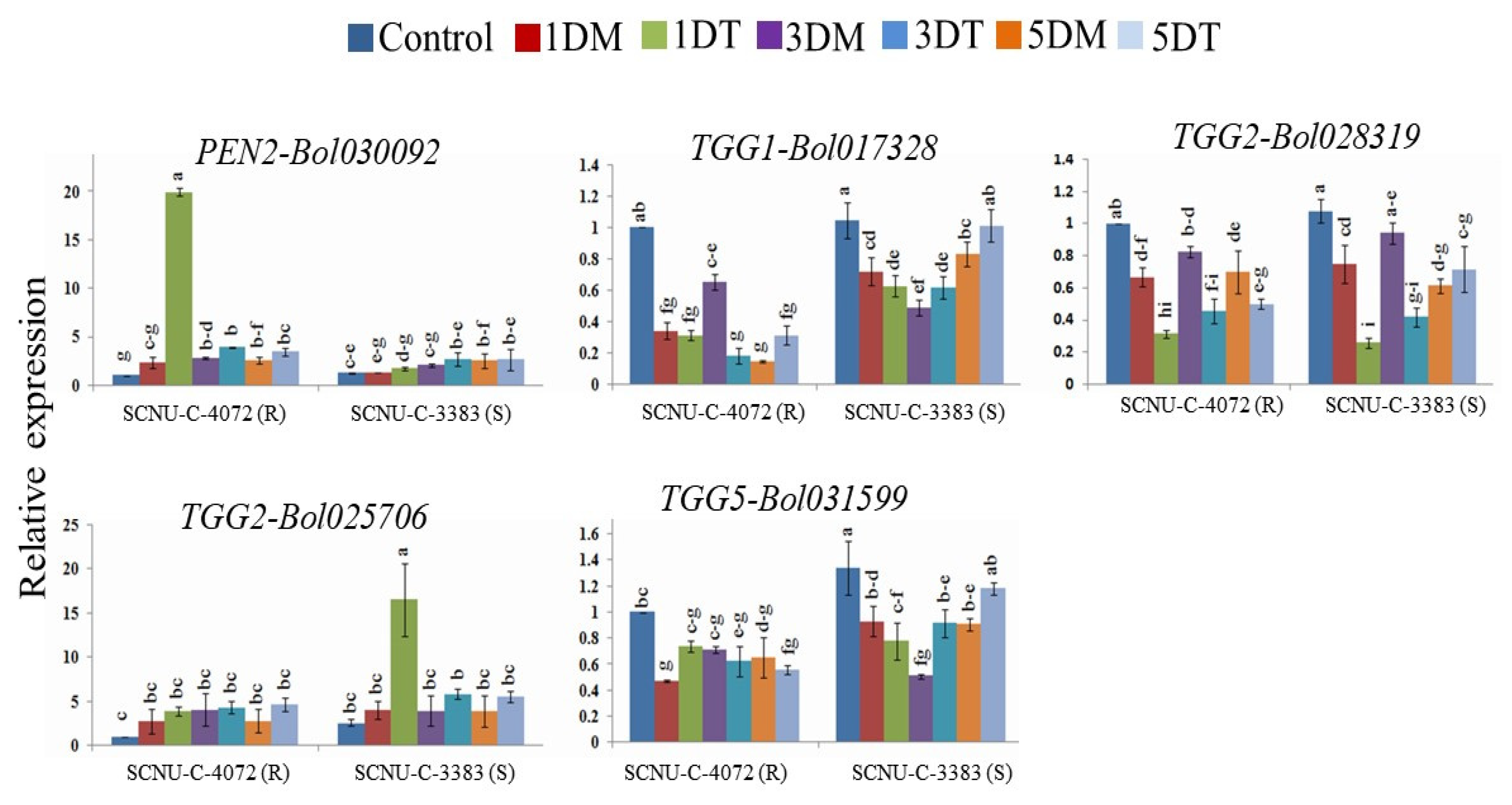
3.5. Relative Expression of GSL Breakdown Related Genes in Cabbage Lines
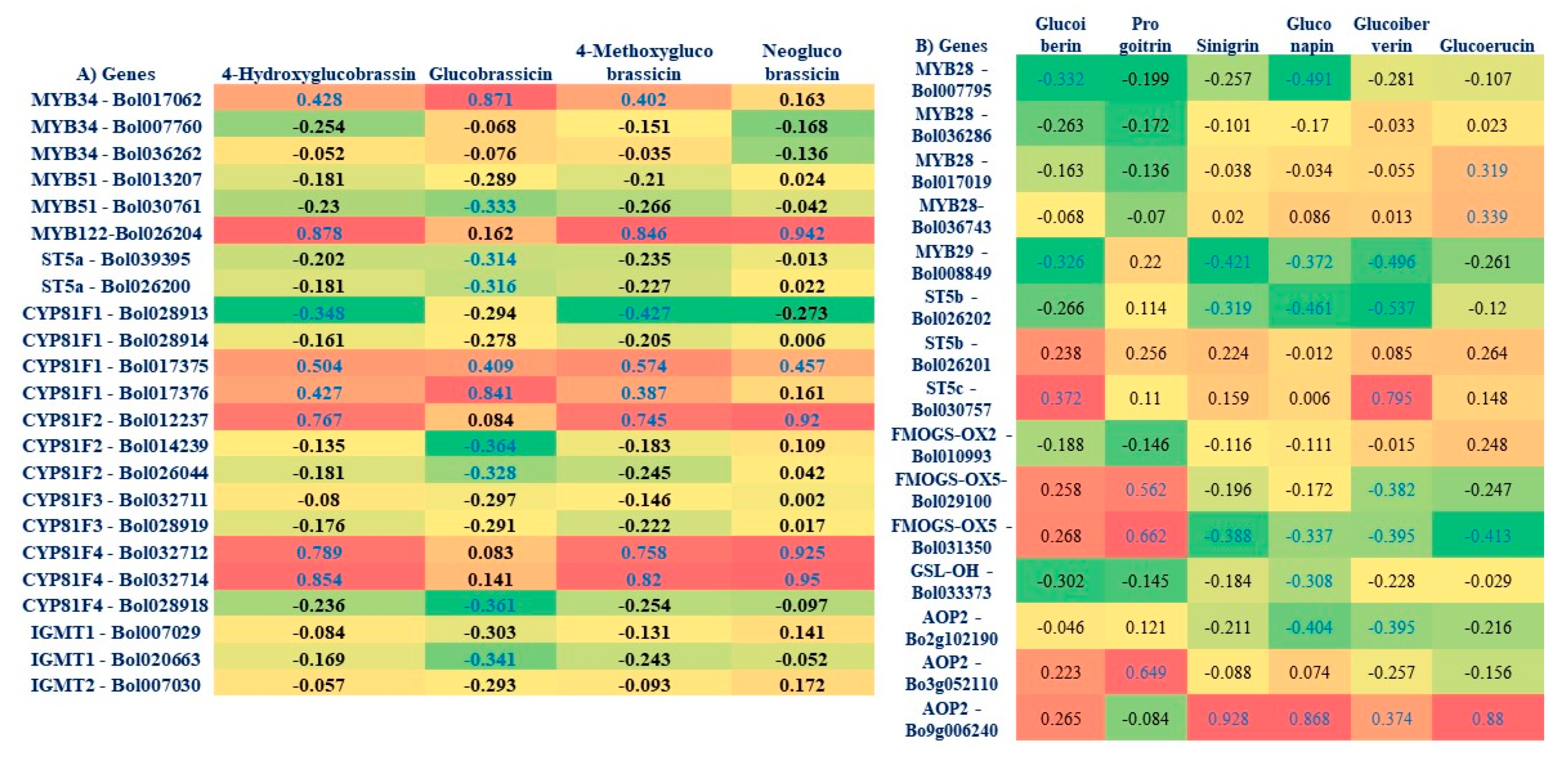
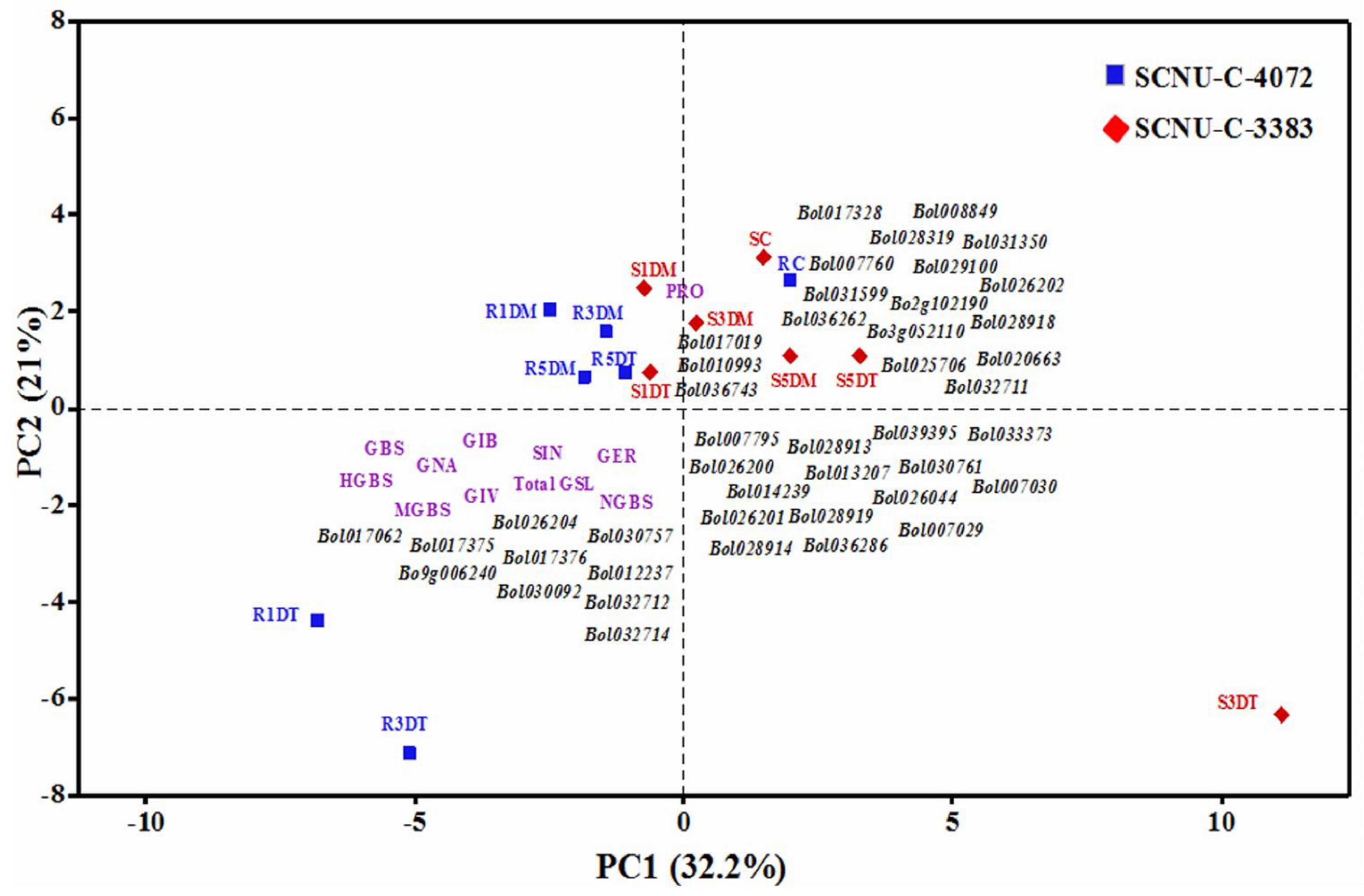
3.6. Correlation between GSL Components and Expression Level of Genes in the Cabbage Lines
4. Discussion
4.1. Black Rot Resistant Cabbage Lines Against Xcc
4.2. GSL Compounds Varied in the Leaf Tissues of Resistant and Susceptible Cabbage Lines
4.3. Black Rot Pathogen Induced GSL Biosynthesis Genes in Contrasting Cabbage Lines
4.4. Association of Aliphatic GSL Genes with Individual GSL Compound in Black Rot Resistance
4.5. Association of Indolic GSL Genes and Individual GSL Compounds in Black rot Resistance
4.6. Expression of GSL Breakdown Related Genes in Cabbage Lines after Xcc Inoculation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Statistics Division, Food and Agriculture Organization of the United Nations. Rome Italy 2015. Available online: http://ftp.fao.org/ (accessed on 30 June 2020).
- Kim, B.S. Testing for detection of Xanthomonas campestris pv. campestris in crucifer seeds and seed disinfection. Korean J. Plant Pathol. 1986, 2, 96–101. [Google Scholar]
- Vicente, J.G.; Holub, E.B. Xanthomonas campestris pv. campestris (cause of black rot of crucifers) in the genomic era is still a worldwide threat to brassica crops. Mol. Plant Pathol. 2013, 14, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.H. Black rot: A continuing threat to world crucifers. Plant Dis. 1980, 64, 736–742. [Google Scholar] [CrossRef]
- Kocks, C.; Zadoks, J.; Ruissen, M. Spatio-temporal development of black rot (X. campestris pv. campestris) in cabbage in relation to initial inoculum levels in field plots in The Netherlands. Plant Pathol. 1999, 48, 176–188. [Google Scholar] [CrossRef]
- Fargier, E.; Manceau, C. Pathogenicity assays restrict the species Xanthomonas campestris into three pathovars and reveal nine races within X. campestris pv. campestris. Plant Pathol. 2007, 56, 805–818. [Google Scholar] [CrossRef]
- Cruz, J.; Tenreiro, R.; Cruz, L. Assesment of diversity of Xanthomonas campestris pathovars affecting cruciferous plants in Portugal and disclosure of two novel X. campestris pv. campestris races. J. Plant Pathol. 2017, 99, 403–414. [Google Scholar]
- Jensen, B.D.; Vicente, J.G.; Manandhar, H.K.; Roberts, S.J. Occurrence and diversity of Xanthomonas campestris pv. campestris in vegetable Brassica fields in Nepal. Plant Dis. 2010, 94, 298–305. [Google Scholar] [CrossRef]
- Taylor, J.; Conway, J.; Roberts, S.; Astley, D.; Vicente, J.G. Sources and origin of resistance to Xanthomonas campestris pv. campestris in Brassica genomes. Phytopathology 2002, 92, 105–111. [Google Scholar] [CrossRef]
- Villeth, G.R.; Reis, F.B., Jr.; Tonietto, A.; Huergo, L.; De Souza, E.M.; Pedrosa, F.O.; Franco, O.L.; Mehta, A. Comparative proteome analysis of Xanthomonas campestris pv. campestris in the interaction with the susceptible and the resistant cultivars of Brassica oleracea. FEMS Microbiol. Lett. 2009, 298, 260–266. [Google Scholar] [CrossRef]
- Aires, A.; Dias, C.S.; Carvalho, R.; Oliveira, M.H.; Monteiro, A.A.; Simões, M.V.; Rosa, E.A.; Bennett, R.N.; Saavedra, M.J. Correlations between disease severity, glucosinolate profiles and total phenolics and Xanthomonas campestris pv. campestris inoculation of different Brassicaceae. Sci. Hortic. 2011, 129, 503–510. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Kliebenstein, D. Secondary metabolites and plant/environment interactions: A view through Arabidopsis thaliana tinged glasses. Plant Cell Environ. 2004, 27, 675–684. [Google Scholar] [CrossRef]
- Rahmanpour, S.; Backhouse, D.; Nonhebel, H. Induced tolerance of Sclerotinia sclerotiorum to isothiocyanates and toxic volatiles from Brassica species. Plant Pathol. 2009, 58, 479–486. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Bekaert, M.; Edger, P.P.; Hudson, C.M.; Pires, J.C.; Conant, G.C. Metabolic and evolutionary costs of herbivory defense: Systems biology of glucosinolate synthesis. New Phytol. 2012, 196, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Velasco, P.; Lema, M.; Francisco, M.; Soengas, P.; Cartea, M. In vivo and in vitro effects of secondary metabolites against Xanthomonas campestris pv. campestris. Molecules 2013, 18, 11131–11143. [Google Scholar] [CrossRef] [PubMed]
- Cartea, M.E.; Velasco, P. Glucosinolates in Brassica foods: Bioavailability in food and significance for human health. Phytochem. Rev. 2008, 7, 213–229. [Google Scholar] [CrossRef]
- Van Horn, L.; McCoin, M.; Kris-Etherton, P.M.; Burke, F.; Carson, J.A.S.; Champagne, C.M.; Karmally, W.; Sikand, G. The evidence for dietary prevention and treatment of cardiovascular disease. J. Am. Diet. Assoc. 2008, 108, 287–331. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Nicolas, M.E.; Bennett, R.N.; Premier, R.R.; Eagling, D.R.; Taylor, P.W.J. Developmental changes of sinigrin and glucoraphanin in three Brassica species (Brassica nigra, Brassica juncea and Brassica oleracea var. italica). Sci. Hortic. 2002, 96, 11–26. [Google Scholar] [CrossRef]
- Li, G.; Riaz, A.; Goyal, S.; Abel, S.; Quiros, C.F. Inheritance of Three Major Genes Involved in the Synthesis of Aliphatic Glucosinolates in Brassica oleracea. J. Am. Soc. Hortic. Sci. 2001, 126, 427. [Google Scholar] [CrossRef]
- Hiruma, K.; Fukunaga, S.; Bednarek, P.; Piślewska-Bednarek, M.; Watanabe, S.; Narusaka, Y.; Shirasu, K.; Takano, Y. Glutathione and tryptophan metabolism are required for Arabidopsis immunity during the hypersensitive response to hemibiotrophs. Proc. Nat. Acad. Sci. USA 2013, 110, 9589–9594. [Google Scholar] [CrossRef] [PubMed]
- Madloo, P.; Lema, M.; Francisco, M.; Soengas, P. Changes in glucosinolates content in Brassica oleracea modulate disease severity caused by Xanthomonas campestris pv. campestris. In Proceedings of the VII International Symposium on Brassicas 1202, Berlin, Germany, 17–20 September 2017; pp. 75–80. [Google Scholar]
- Bednarek, P.; Piślewska-Bednarek, M.; Svatoš, A.; Schneider, B.; Doubský, J.; Mansurova, M.; Humphry, M.; Consonni, C.; Panstruga, R.; Sanchez-Vallet, A.; et al. A Glucosinolate Metabolism Pathway in Living Plant Cells Mediates Broad-Spectrum Antifungal Defense. Science 2009, 323, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Brader, G.; Mikkelsen, M.D.; Halkier, B.A.; Tapio Palva, E. Altering glucosinolate profiles modulates disease resistance in plants. Plant J. 2006, 46, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Giamoustaris, A.; Mithen, R. The effect of modifying the glucosinolate content of leaves of oilseed rape (Brassica napus ssp. oleifera) on its interaction with specialist and generalist pests. Ann. Appl. Biol. 1995, 126, 347–363. [Google Scholar] [CrossRef]
- Lazzeri, L.; Manici, L.M. Allelopathic Effect of Glucosinolate-containing Plant Green Manure on Pythium sp. and Total Fungal Population in Soil. HortScience 2001, 36, 1283. [Google Scholar] [CrossRef]
- Motisi, N.; Montfort, F.; Doré, T.; Romillac, N.; Lucas, P. Duration of control of two soilborne pathogens following incorporation of above- and below-ground residues of Brassica juncea into soil. Plant Pathol. 2009, 58, 470–478. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Kurashige, N.S. A role for isothiocyanates in plant resistance against the specialist herbivore Pieris rapae. J. Chem. Ecol. 2003, 29, 1403–1415. [Google Scholar] [CrossRef]
- Vicente, J.G.; Conway, J.; Roberts, S.; Taylor, J. Identification and origin of Xanthomonas campestris pv. campestris races and related pathovars. Phytopathology 2001, 91, 492–499. [Google Scholar] [CrossRef]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. Transl. Res. 1954, 44, 301–307. [Google Scholar]
- Pandey, K.K.; Pandey, P.K.; Singh, B. Artificial screening for black rot resistance based on different disease parameter in early cauliflower. Mycobiology 2003, 31, 173–178. [Google Scholar] [CrossRef]
- Robin, A.; Yi, G.-E.; Laila, R.; Yang, K.; Park, J.-I.; Kim, H.; Nou, I.-S. Expression profiling of glucosinolate biosynthetic genes in Brassica oleracea L. var. capitata inbred lines reveals their association with glucosinolate content. Molecules 2016, 21, 787. [Google Scholar] [CrossRef] [PubMed]
- Abuyusuf, M.; Robin, A.; Kim, H.-T.; Islam, M.; Park, J.-I.; Nou, I.-S. Altered glucosinolate profiles and expression of glucosinolate biosynthesis genes in ringspot-resistant and susceptible cabbage lines. Int. J. Mol. Sci. 2018, 19, 2833. [Google Scholar] [CrossRef]
- Yi, G.-E.; Robin, A.; Yang, K.; Park, J.-I.; Kang, J.-G.; Yang, T.-J.; Nou, I.-S. Identification and expression analysis of glucosinolate biosynthetic genes and estimation of glucosinolate contents in edible organs of Brassica oleracea subspecies. Molecules 2015, 20, 13089–13111. [Google Scholar] [CrossRef] [PubMed]
- Abuyusuf, M.; Robin, A.; Lee, J.-H.; Jung, H.-J.; Kim, H.-T.; Park, J.-I.; Nou, I.-S. Glucosinolate Profiling and Expression Analysis of Glucosinolate Biosynthesis Genes Differentiate White Mold Resistant and Susceptible Cabbage Lines. Int. J. Mol. Sci. 2018, 19, 4037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Liang, J.; Wu, J.; Cheng, F.; Wang, X. Three genes encoding AOP2, a protein involved in aliphatic glucosinolate biosynthesis, are differentially expressed in Brassica rapa. J. Exp. Bot. 2015, 66, 6205–6218. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Yang, X.; Tong, C.; Edwards, D.; Parkin, I.A.; Zhao, M.; Ma, J.; Yu, J.; Huang, S. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 2014, 5, 3930. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, Z.; Zhang, Y.; Li, Y.; Zhou, S.; Chen, G. A putative functional MYB transcription factor induced by low temperature regulates anthocyanin biosynthesis in purple kale (Brassica Oleracea var. acephala f. tricolor). Plant Cell Rep. 2012, 31, 281–289. [Google Scholar] [CrossRef]
- Nawaz, I.; Iqbal, M.; Hakvoort, H.W.; Bliek, M.; de Boer, B.; Schat, H. Expression levels and promoter activities of candidate salt tolerance genes in halophytic and glycophytic Brassicaceae. Environ. Exp. Bot. 2014, 99, 59–66. [Google Scholar] [CrossRef]
- Lee, J.; Yang, K.; Lee, M.; Kim, S.; Kim, J.; Lim, S.; Kang, G.-H.; Min, S.R.; Kim, S.-J.; Park, S.U. Differentiated cuticular wax content and expression patterns of cuticular wax biosynthetic genes in bloomed and bloomless broccoli (Brassica oleracea var. italica). Proc. Biochem. 2015, 50, 456–462. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Saha, P.; Kalia, P.; Sharma, M.; Singh, D. New source of black rot disease resistance in Brassica oleracea and genetic analysis of resistance. Euphytica 2016, 207, 35–48. [Google Scholar] [CrossRef]
- Abdel-Farid, I.B.; Jahangir, M.; Mustafa, N.R.; Van Dam, N.M.; Van den Hondel, C.A.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Glucosinolate profiling of Brassica rapa cultivars after infection by Leptosphaeria maculans and Fusarium oxysporum. Biochem. Syst. Ecol. 2010, 38, 612–620. [Google Scholar] [CrossRef]
- Doughty, K.; Porter, A.; Morton, A.; Kiddle, G.; Bock, C.; Wallsgrove, R. Variation in the glucosinolate content of oilseed rape (Brassica napus L.) leaves: II. Response to infection by Alternaria brassicae (Berk.) Sacc. Ann. Appl. Biol. 1991, 118, 469–477. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Bennett, R.; Kiddle, G.; Wallsgrove, R.; Huang, Y.; He, Y. Breeding, inheritance, and biochemical studies on Brassica napus cv. Zhougyou 821: Tolerance to Sclerotinia sclerotiorum (stem rot). In Proceedings of Proceedings of the 10th International Rapeseed Congress’, Canberra, Australia, 5–10 July 1999. [Google Scholar]
- Madloo, P.; Lema, M.; Francisco, M.; Soengas, P. Role of Major Glucosinolates in the Defense of Kale Against Sclerotinia sclerotiorum and Xanthomonas campestris pv. campestris. Phytopathology 2019, 109, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Burow, M.; Atwell, S.; Francisco, M.; Kerwin, R.E.; Halkier, B.A.; Kliebenstein, D.J. The glucosinolate biosynthetic gene AOP2 mediates feed-back regulation of jasmonic acid signaling in Arabidopsis. Mol. Plant 2015, 8, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, T.; Lema, M.; Soengas, P.; Cartea, M.; Velasco, P. In vitro activity of glucosinolates and their degradation products against brassica-pathogenic bacteria and fungi. Appl. Environ. Microbiol. 2015, 81, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Frerigmann, H.; Piślewska-Bednarek, M.; Sánchez-Vallet, A.; Molina, A.; Glawischnig, E.; Gigolashvili, T.; Bednarek, P. Regulation of pathogen-triggered tryptophan metabolism in Arabidopsis thaliana by MYB transcription factors and indole glucosinolate conversion products. Mol. Plant 2016, 9, 682–695. [Google Scholar] [CrossRef]
- Wretblad, S.; Dixelius, C. B-genome derived resistance to Leptosphaeria maculans in near isogenic Brassica napus lines is independent of glucosinolate profile. Physiol. Plant. 2000, 110, 461–468. [Google Scholar] [CrossRef]
- Mithen, R.; Lewis, B.; Fenwick, G. In vitro activity of glucosinolates and their products against Leptosphaeria maculans. Trans. Br. Mycol. Soc. 1986, 87, 433–440. [Google Scholar] [CrossRef]
- Clay, N.K.; Adio, A.M.; Denoux, C.; Jander, G.; Ausubel, F.M. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 2009, 323, 95–101. [Google Scholar] [CrossRef]
- Schlaeppi, K.; Bodenhausen, N.; Buchala, A.; Mauch, F.; Reymond, P. The glutathione-deficient mutant pad2-1 accumulates lower amounts of glucosinolates and is more susceptible to the insect herbivore Spodoptera littoralis. Plant J. 2008, 55, 774–786. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubel, M.H.; Abuyusuf, M.; Nath, U.K.; Robin, A.H.K.; Jung, H.J.; Kim, H.T.; Park, J.I.; Nou, I.S. Glucosinolate Profile and Glucosinolate Biosynthesis and Breakdown Gene Expression Manifested by Black Rot Disease Infection in Cabbage. Plants 2020, 9, 1121. https://doi.org/10.3390/plants9091121
Rubel MH, Abuyusuf M, Nath UK, Robin AHK, Jung HJ, Kim HT, Park JI, Nou IS. Glucosinolate Profile and Glucosinolate Biosynthesis and Breakdown Gene Expression Manifested by Black Rot Disease Infection in Cabbage. Plants. 2020; 9(9):1121. https://doi.org/10.3390/plants9091121
Chicago/Turabian StyleRubel, Mehede Hassan, Md. Abuyusuf, Ujjal Kumar Nath, Arif Hasan Khan Robin, Hee Jeong Jung, Hoy Taek Kim, Jong In Park, and Ill Sup Nou. 2020. "Glucosinolate Profile and Glucosinolate Biosynthesis and Breakdown Gene Expression Manifested by Black Rot Disease Infection in Cabbage" Plants 9, no. 9: 1121. https://doi.org/10.3390/plants9091121
APA StyleRubel, M. H., Abuyusuf, M., Nath, U. K., Robin, A. H. K., Jung, H. J., Kim, H. T., Park, J. I., & Nou, I. S. (2020). Glucosinolate Profile and Glucosinolate Biosynthesis and Breakdown Gene Expression Manifested by Black Rot Disease Infection in Cabbage. Plants, 9(9), 1121. https://doi.org/10.3390/plants9091121






