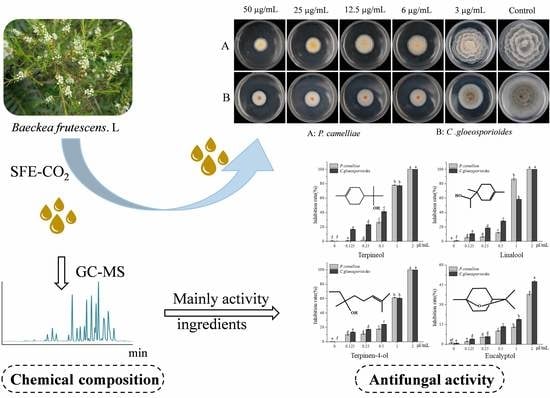
Chemical Composition of a Supercritical Fluid (Sfe-CO2) Extract from Baeckea frutescens L. Leaves and Its Bioactivity Against Two Pathogenic Fungi Isolated from the Tea Plant (Camellia sinensis (L.) O. Kuntze)
Abstract
1. Introduction
2. Results and Discussion
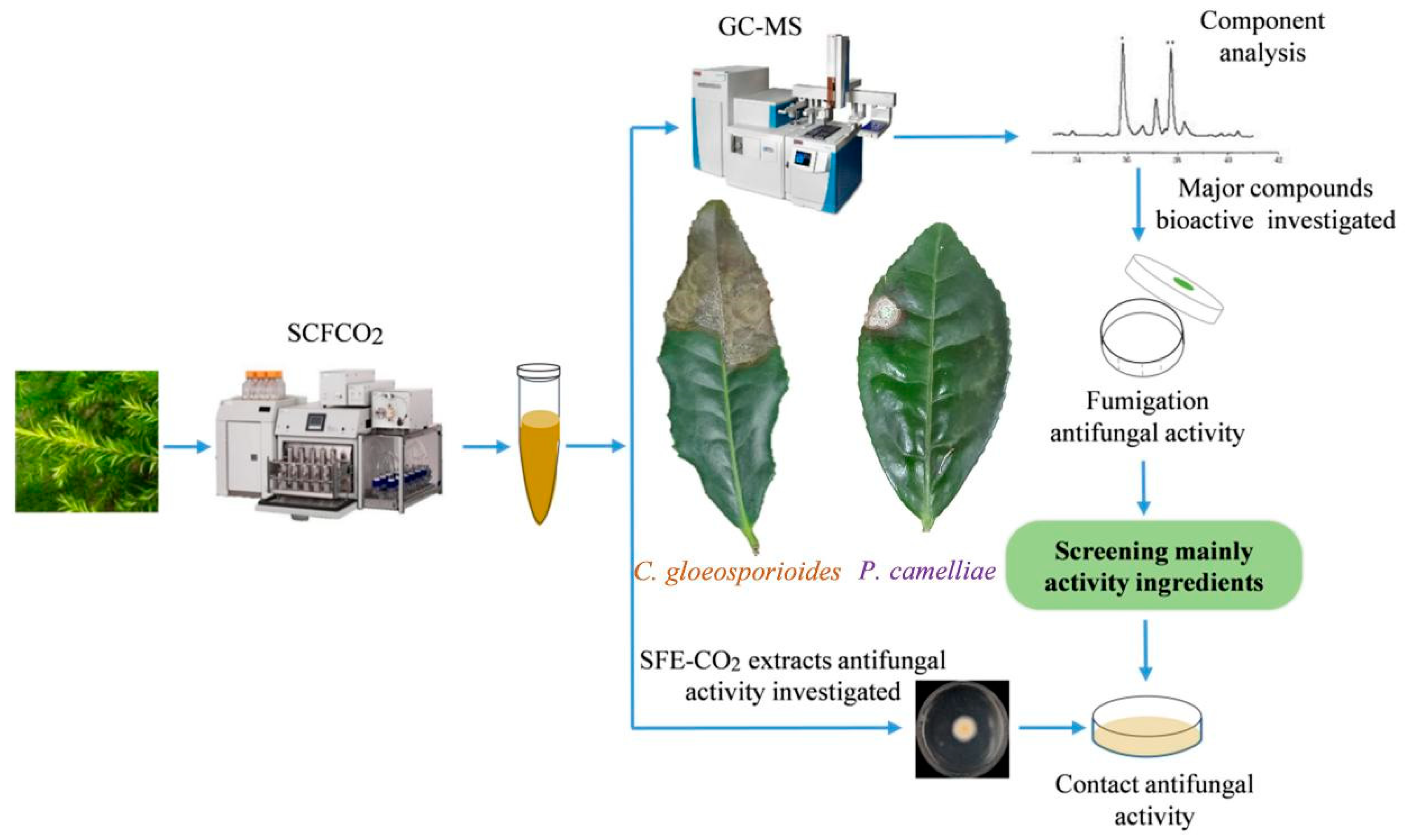
2.1. Chemical Composition of the B. Frutescens Leaf SFE-CO2 Extract
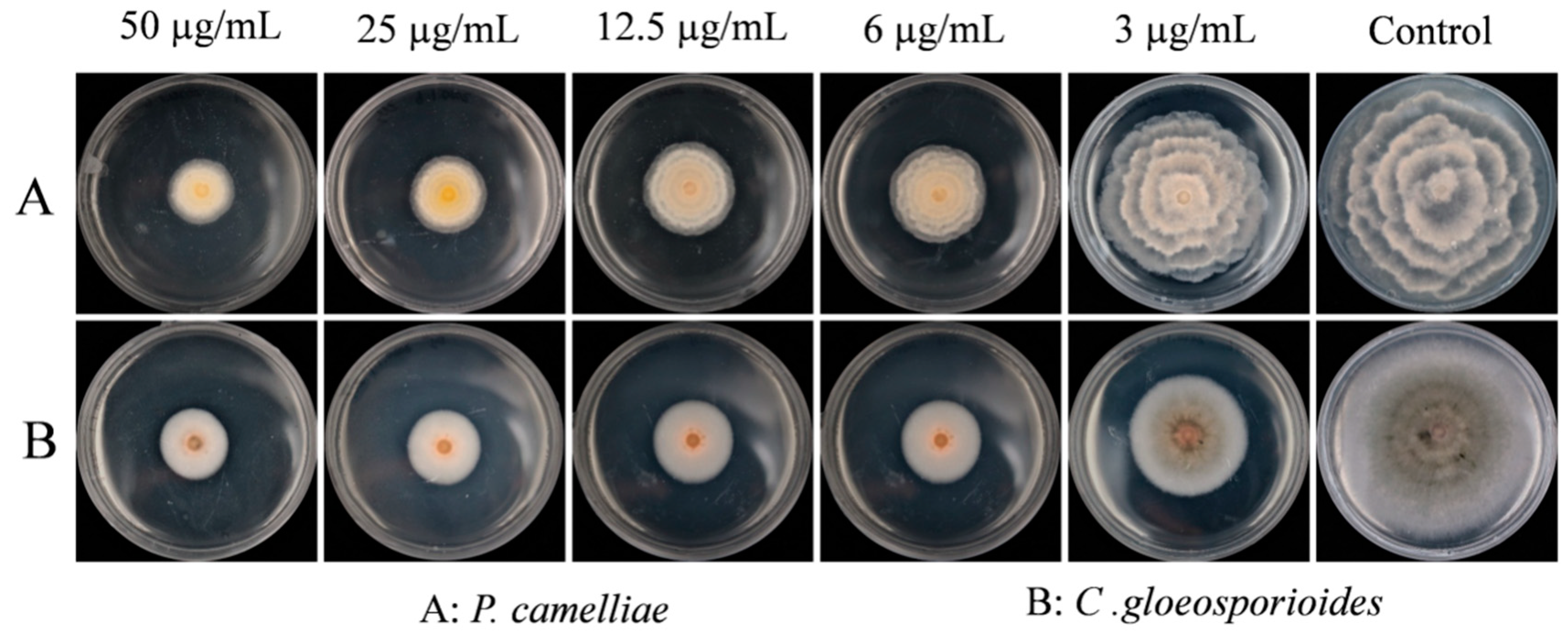
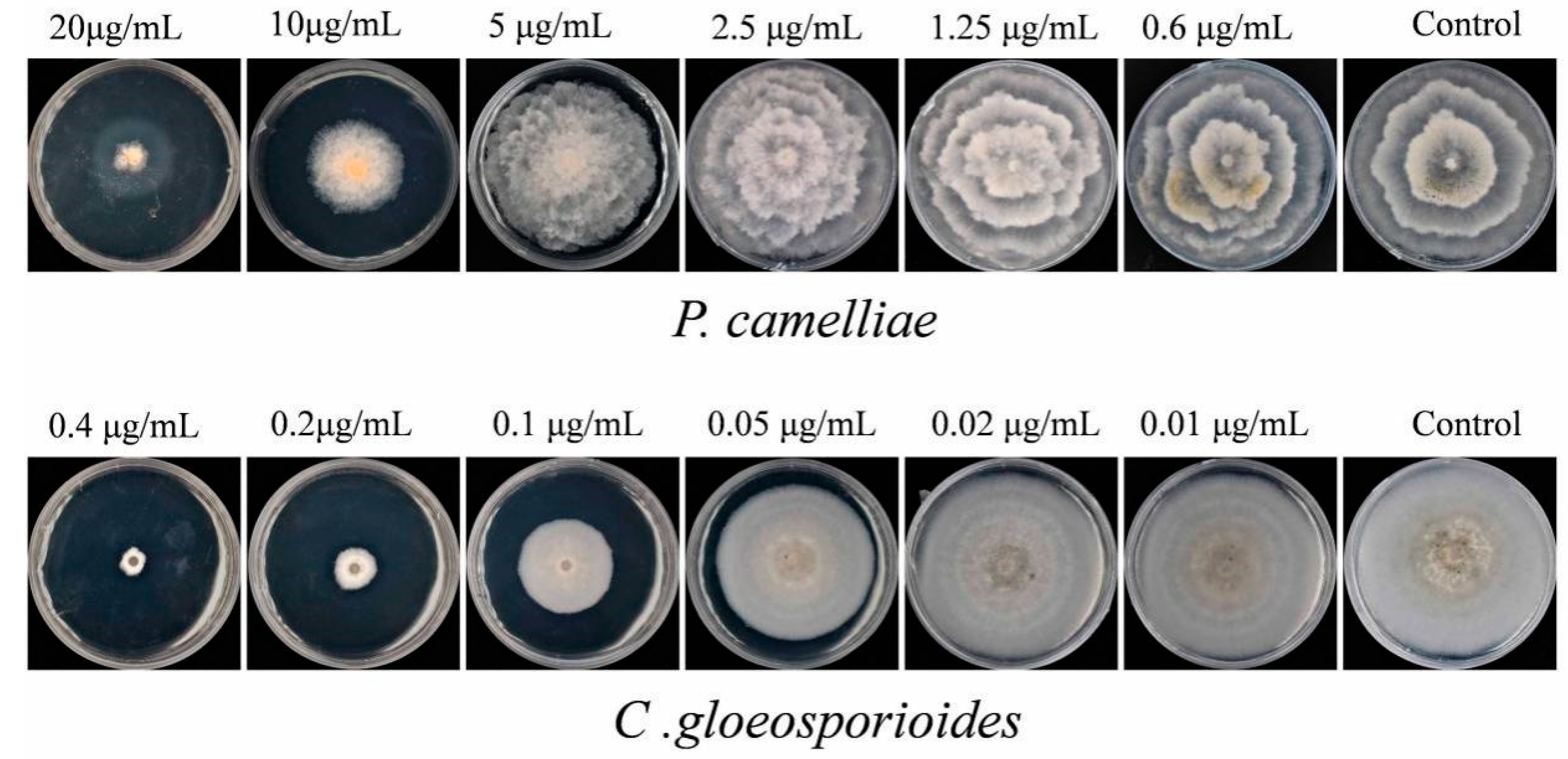
2.2. Contact Antifungal Activity of B. frutescens SFE-CO2 Extract
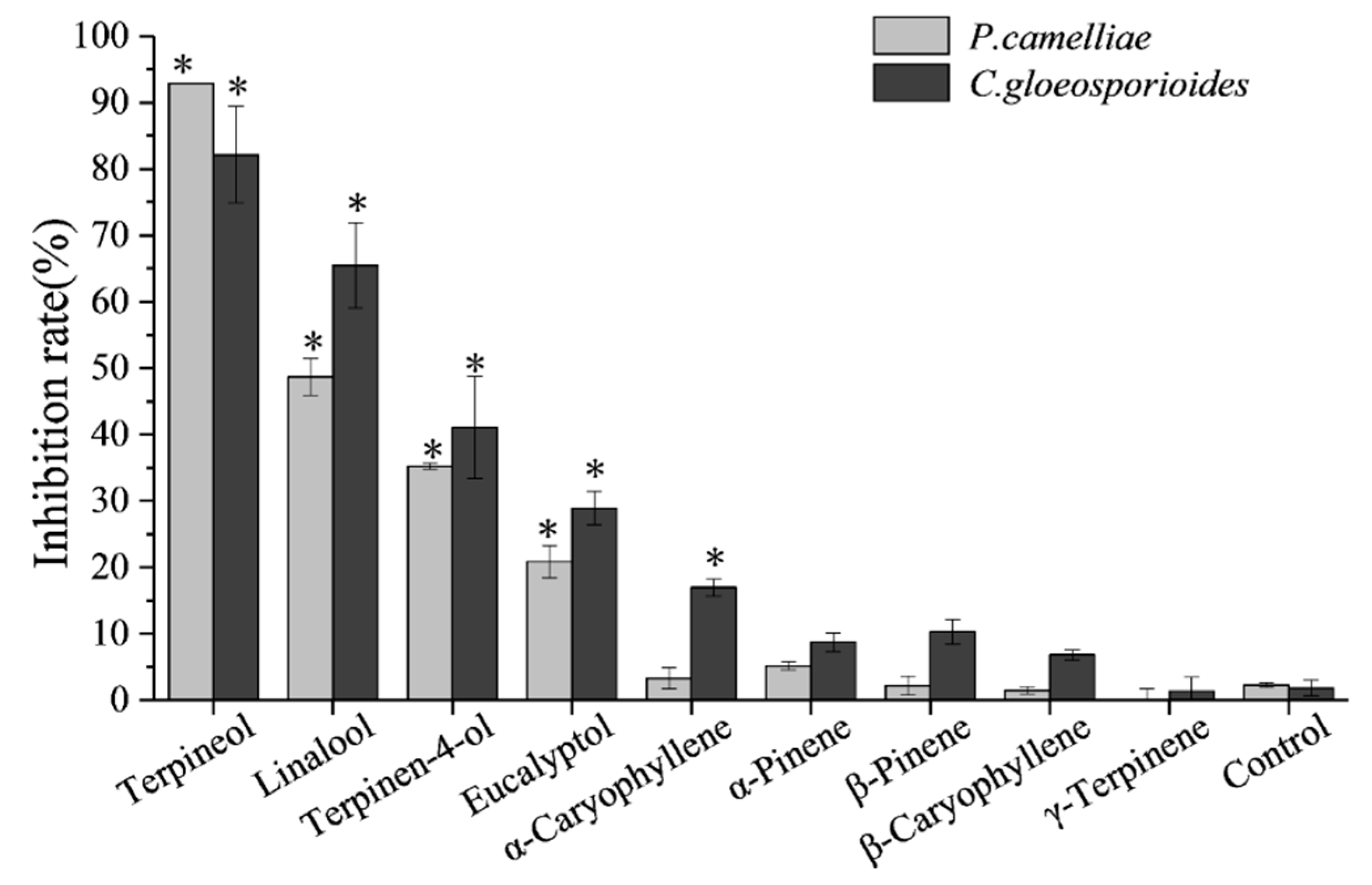
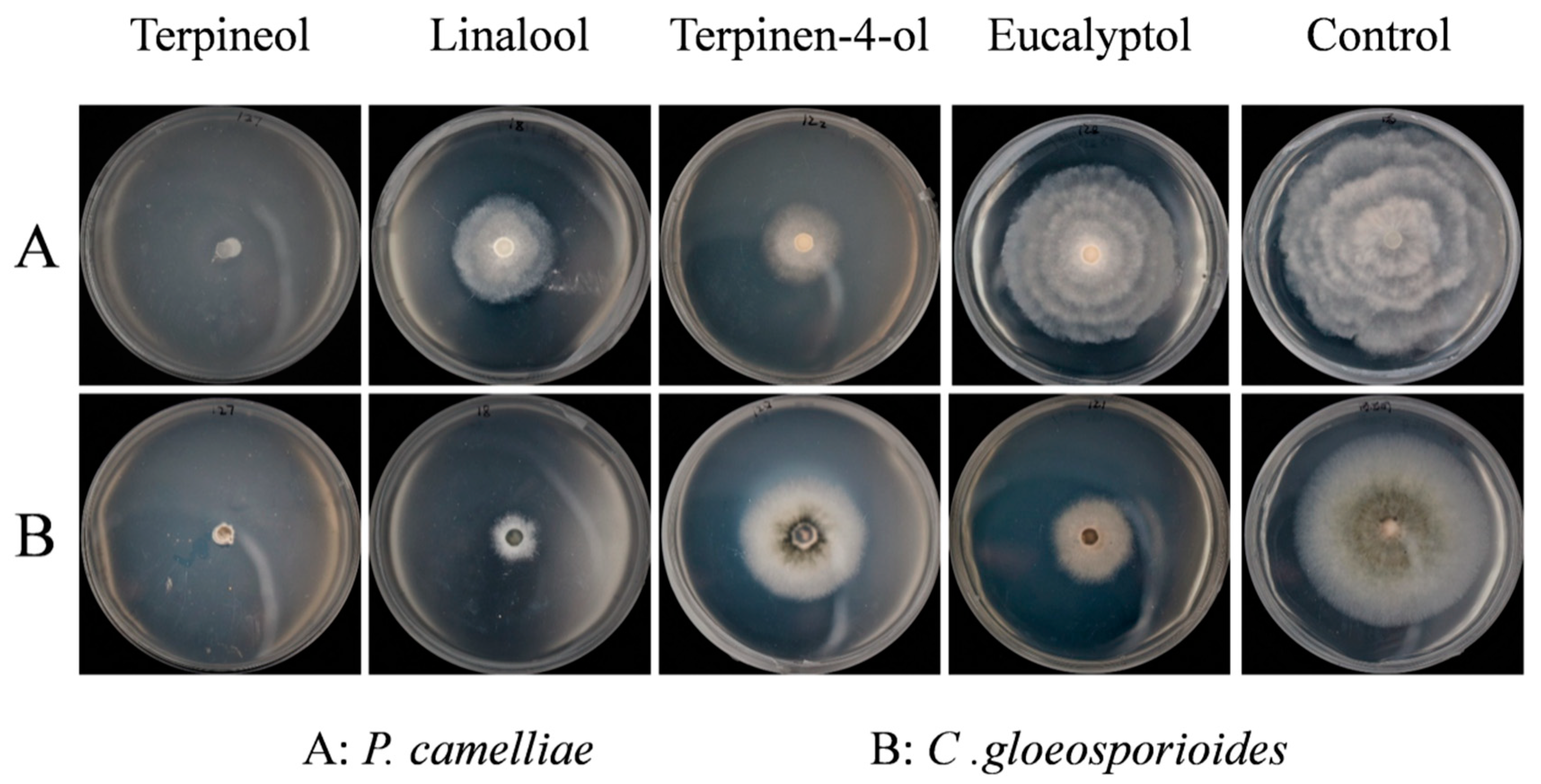
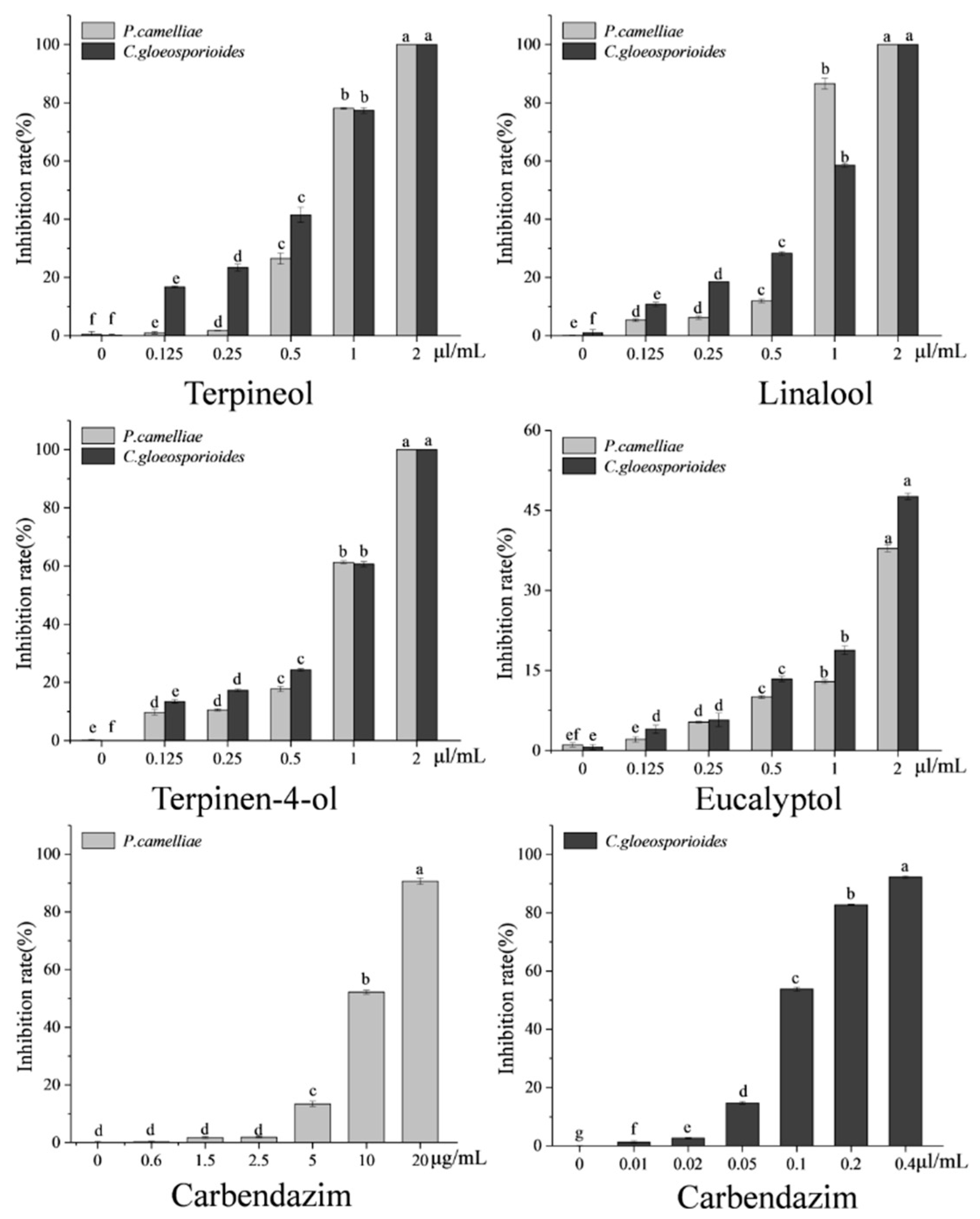
2.3. Screening for Fumigation Antifungal Activities of Major Chemical Compounds
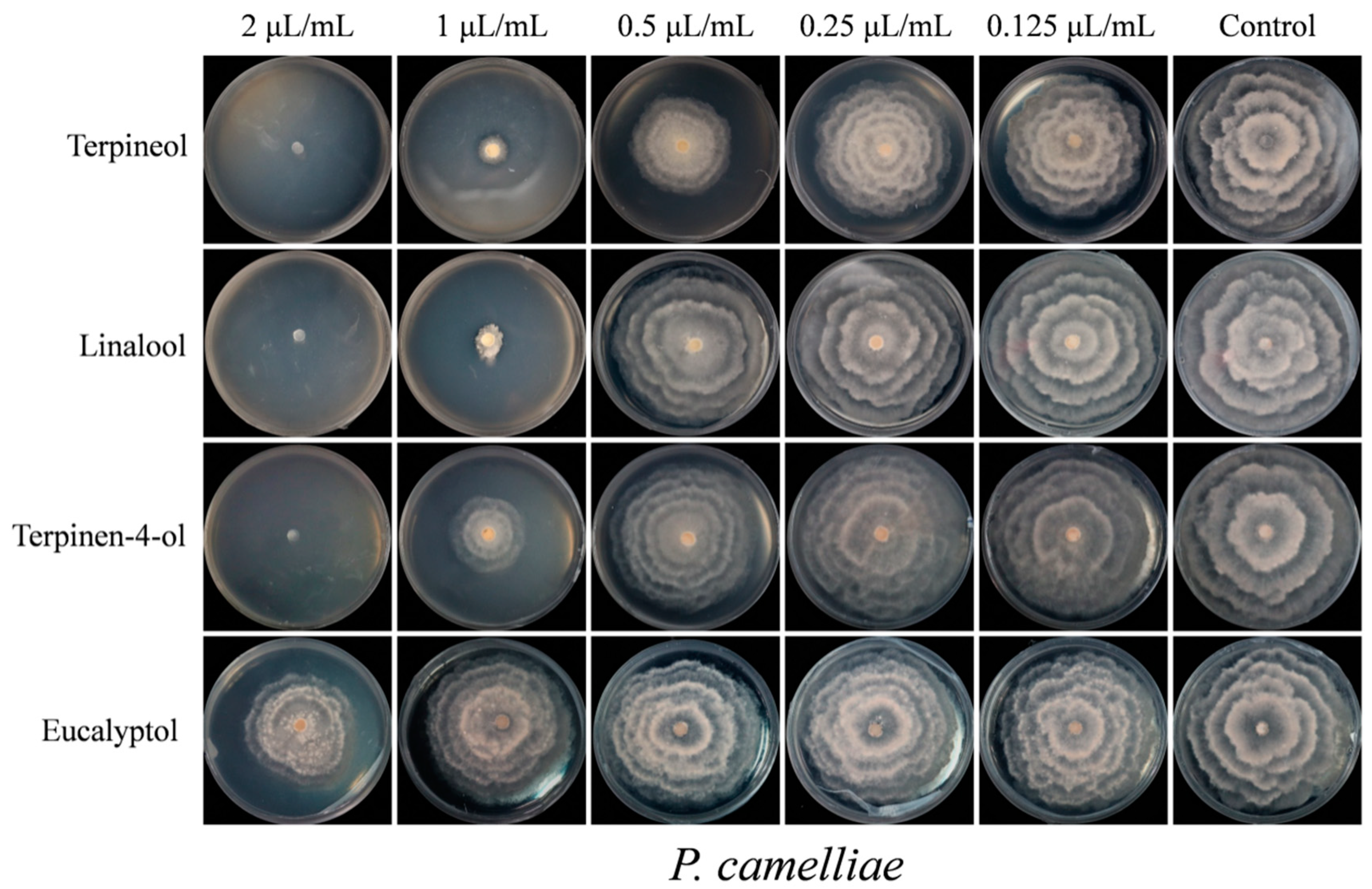
2.4. Contact Antifungal Activity of Major Activity Components
3. Materials and Methods
3.1. Plant Materials
3.2. Chemicals
3.3. Isolation and Identification of Fungal Pathogens
3.4. Supercritical Fluid (SFE-CO2) Extract of Baeckea frutescens L.
3.5. Contact Antifungal Activities Bioassay
3.6. Fumigation Antifungal Activities Bioassay
3.7. B. frutescens Extract GC-MS Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- An, N.T.G.; Huong, L.T.; Satyal, P.; Tai, T.A.; Dai, D.N.; Hung, N.H.; Ngoc, N.T.B.; Setzer, W.N. Mosquito Larvicidal Activity, Antimicrobial Activity, and Chemical Compositions of Essential Oils from Four Species of Myrtaceae from Central Vietnam. Plants 2020, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Shahruzaman, S.H.; Mustafa, M.F.; Ramli, S.; Maniam, S.; Fakurazi, S.; Maniam, S. The Cytotoxic Properties of Baeckea frutescens Branches Extracts in Eliminating Breast Cancer Cells. Evid. Based Complement. Altern. Med. 2019, 2019, 9607590. [Google Scholar] [CrossRef]
- Vu, T.T.; Kim, H.; Tran, V.K.; Le Dang, Q.; Nguyen, H.T.; Kim, H.; Kim, I.S.; Choi, G.J.; Kim, J.C. In vitro antibacterial activity of selected medicinal plants traditionally used in Vietnam against human pathogenic bacteria. BMC Complement. Altern. Med. 2016, 16, 32. [Google Scholar] [CrossRef]
- Shahruzaman, S.H.; Mustafa, M.F.; Ramli, S.; Maniam, S.; Fakurazi, S.; Maniam, S. The cytotoxic effect and glucose uptake modulation of Baeckea frutescens on breast cancer cells. BMC Complement. Altern. Med. 2019, 19, 220. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.Q.; Guo, C.; Zhao, J.J.; Dong, Y.Y.; Hu, X.L.; He, Q.W.; Zhang, B.B.; Yan, M.; Wang, H. Anti-inflammatory Meroterpenoids from Baeckea frutescens. J. Nat. Prod. 2017, 80, 2204–2214. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.E.; Qi, X.J.; Liu, H.; Zeng, Y.; Ni, W.; He, L.; Wang, Z.D.; Liu, H.Y. Structurally Diverse Polymethylated Phloroglucinol Meroterpenoids from Baeckea frutescens. Nat. Prod. Bioprospect. 2018, 8, 431–439. [Google Scholar] [CrossRef]
- Wei, C.L.; Yang, H.; Wang, S.B.; Zhao, J.; Liu, C.; Gao, L.P.; Xia, E.H.; Lu, Y.; Tai, Y.L.; She, G.B.; et al. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proc. Natl. Acad. Sci. USA 2018. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, F.; Qin, L.; Zhang, N.; Cao, Q.; Schwab, W.; Li, D.X.; Song, C.K. Dynamic change in amino acids, catechins, alkaloids, and gallic acid in six types of tea processed from the same batch of fresh tea (Camellia sinensis L.) leaves. J. Food Compos. Anal. 2019, 77, 28–38. [Google Scholar] [CrossRef]
- Chen, Y.J.; Tong, H.R.; Wei, X.; Yuan, L.Y. First Report of Brown Blight Disease on Camellia sinensis Caused by Colletotrichum acutatum in China. Plant Dis. 2016, 100, 227–228. [Google Scholar] [CrossRef]
- Li, J.; Sun, K.; Ma, Q.P.; Chen, J.; Wang, L.; Yang, D.J.; Chen, X.; Li, X.H. Colletotrichum gloeosporioides–contaminated tea infusion blocks lipids reduction and induces kidney damage in mice. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Chen, Y.J.; Zeng, L.; Shu, N.; Jiang, M.Y.; Wang, H.; Huang, Y.J.; Tong, H.R. Pestalotiopsis-Like Species Causing Gray Blight Disease on Camellia sinensis in China. Plant Dis. 2018, 102, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, K.; Deng, C.; Cheng, L.L.; Deng, W.W.; Zhang, Z.Z. Report of Phoma herbarum Causing Leaf Spot Disease of Camellia sinensis in China. Plant Dis. 2018, 102, 2373. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Zeng, L.; Shu, N.; Wang, H.; Tong, H.R. First Report of Pestalotiopsis camelliae causing Grey Blight Disease on Camellia sinensis in China. Plant Dis. 2017, 101, 1034. [Google Scholar] [CrossRef]
- Akbar, A.; Ali, G.S.; Pearson, B.J.; Hamid, F.S.; Sumreen, S. Screening Camelia sinensis Germplasm Against Grey Leaf Blight of Tea. J. Agric. Stud. 2017, 5, 123–135. [Google Scholar] [CrossRef]
- Wang, Y.X.; Chen, J.Y.; Xu, X.W.; Cheng, J.Y.; Zheng, L.; Huang, J.B.; Li, D.W. Identification and Characterization of Colletotrichum Species Associated with Anthracnose Disease of Camellia oleifera in China. Plant Dis. 2020, 104, 474–482. [Google Scholar] [CrossRef]
- He, L.F.; Li, X.X.; Gao, Y.Y.; Li, B.X.; Mu, W.; Liu, F. Characterization and Fungicide Sensitivity of Colletotrichum spp. from Different Hosts in Shandong, China. Plant Dis. 2019, 103, 34–43. [Google Scholar] [CrossRef]
- Jeyaraj, A.; Wang, X.W.; Wang, S.S.; Liu, S.R.; Zhang, R.; Wu, A.; Wei, C.T. Identification of Regulatory Networks of MicroRNAs and Their Targets in Response to Colletotrichum gloeosporioides in Tea Plant (Camellia sinensis L.). Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Guo, M.; Pan, Y.M.; Dai, Y.L.; Gao, Z.M. First Report of Brown Blight Disease Caused by Colletotrichum gloeosporioides on Camellia sinensis in Anhui Province, China. Plant Dis. 2014, 98, 284. [Google Scholar] [CrossRef]
- Chen, Y.J.; Qiao, W.J.; Zeng, L.; Shen, D.H.; Liu, Z.; Wang, X.S.; Tong, H.R. Characterization, Pathogenicity, and Phylogenetic Analyses of Colletotrichum Species Associated with Brown Blight Disease on Camellia sinensis in China. Plant Dis. 2017, 101, 1022–1028. [Google Scholar] [CrossRef]
- Wari, D.; Okada, R.; Takagi, M.; Yaguchi, M.; Kashima, T.; Ogawara, T. Augmentation and compatibility of Beauveria bassiana with pesticides against different growth stages of Bemisia tabaci (Gennadius); an in-vitro and field approach. Pest Manag. Sci. 2020. [Google Scholar] [CrossRef]
- Bai, Y.B.; Gao, Y.Q.; Nie, X.D.; Tuong, T.M.; Li, D.; Gao, J.M. Antifungal Activity of Griseofulvin Derivatives against Phytopathogenic Fungi in Vitro and in Vivo and Three-Dimensional Quantitative Structure-Activity Relationship Analysis. J. Agric. Food Chem. 2019, 67, 6125–6132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mei, X.; Du, M.; Chen, K.; Jiang, M.; Wang, K.; Zalan, Z.; Kan, J. Potential modes of action of Pseudomonas fluorescens ZX during biocontrol of blue mold decay on postharvest citrus. J. Sci. Food Agric. 2020, 100, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Shuping, D.S.S.; Eloff, J.N. The use of plants to protect plants and food against fungal pathogens: A review. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Tyskiewicz, K.; Tyskiewicz, R.; Konkol, M.; Roj, E.; Jaroszuk-Scisel, J.; Skalicka-Wozniak, K. Antifungal Properties of Fucus vesiculosus L. Supercritical Fluid Extract Against Fusarium culmorum and Fusarium oxysporum. Molecules 2019, 24, 3518. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Z.; Liu, Y.; Wang, Q. Leveraging botanical resources for crop protection: The isolation, bioactivity and structure-activity relationships of lycoris alkaloids. Pest Manag. Sci. 2018, 74, 2783–2792. [Google Scholar] [CrossRef]
- Li, Y.; Wei, W.; Zhang, J.; Li, G.; Gao, K. Structures and antipathogenic fungi activities of flavonoids from pathogen-infected Astragalus adsurgens. Nat. Prod. Res. 2019, 33, 822–826. [Google Scholar] [CrossRef]
- Han, X.B.; Zhao, J.; Cao, J.M.; Zhang, C.S. Essential oil of Chrysanthemum indicum L.: Potential biocontrol agent against plant pathogen Phytophthora nicotianae. Environ. Sci. Pollut. Res. Int. 2019, 26, 7013–7023. [Google Scholar] [CrossRef]
- Capuzzo, A.; Maffei, M.E.; Occhipinti, A. Supercritical fluid extraction of plant flavors and fragrances. Molecules 2013, 18, 7194–7238. [Google Scholar] [CrossRef]
- Messyasz, B.; Michalak, I.; Leska, B.; Schroeder, G.; Gorka, B.; Korzeniowska, K.; Lipok, J.; Wieczorek, P.; Roj, E.; Wilk, R.; et al. Valuable natural products from marine and freshwater macroalgae obtained from supercritical fluid extracts. J. Appl. Phycol. 2018, 30, 591–603. [Google Scholar] [CrossRef]
- Tyskiewicz, K.; Konkol, M.; Roj, E. The Application of Supercritical Fluid Extraction in Phenolic Compounds Isolation from Natural Plant Materials. Molecules 2018, 23, 2625. [Google Scholar] [CrossRef]
- Malaman, F.S.; Moraes, L.A.B.; West, C.; Ferreira, N.J.; Oliveira, A.L. Supercritical fluid extracts from the Brazilian cherry (Eugenia uniflora L.): Relationship between the extracted compounds and the characteristic flavour intensity of the fruit. Food Chem. 2011, 124, 85–92. [Google Scholar] [CrossRef]
- Tyskiewicz, K.; Konkol, M.; Roj, E. Supercritical Carbon Dioxide (scCO2) Extraction of Phenolic Compounds from Lavender (Lavandula angustifolia) Flowers: A Box-Behnken Experimental Optimization. Molecules 2019, 24, 3354. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Mi, X.Z.; Wu, Z.R.; Zhang, L.X.; Wei, C.L. Characterization and Pathogenicity of Pestalotiopsis-Like Species Associated With Gray Blight Disease on Camellia sinensis in Anhui Province, China. Plant Dis. 2019, 103, 2786–2797. [Google Scholar] [CrossRef]
- Soylu, E.M.; Soylu, S.; Kurt, S. Antimicrobial activities of the essential oils of various plants against tomato late blight disease agent Phytophthora infestans. Mycopathologia 2006, 161, 119–128. [Google Scholar] [CrossRef]
- Dai, D.N.; Thang, T.D.; Olayiwola, T.; Ogunwande, I.A. Chemical Composition of Essential Oil of Baeckea frutescens L. Int. Res. J. Pure Appl. Chem. 2015, 8, 26–32. [Google Scholar] [CrossRef]
- Padovan, A.; Keszei, A.; Külheim, C.; Foley, W.J. The evolution of foliar terpene diversity in Myrtaceae. Phytochem. Rev. 2014, 13, 695–716. [Google Scholar] [CrossRef]
- Tam, N.T.; Thuam, D.T.; Bighelli, A.; Castola, V.; Muselli, A.; Richomme, P.; Casanova, J. Baeckea frutescens leaf oil from Vietnam: Composition and chemical variability. Flavour Fragr. J. 2004, 19, 217–220. [Google Scholar] [CrossRef]
- Ouzzar, M.L.; Louaer, W.; Zemane, A.; Meniai, A.H. Comparison of the performances of hydrodistillation and supercritical CO2 extraction processes for essential oil extraction from Rosemary (Rosmarinus officinalis L.). Chem. Eng. Trans. 2015, 43, 1129–1134. [Google Scholar]
- Bhavya, M.L.; Chandu, A.G.S.; Devi, S.S.; Quirin, K.W.; Pasha, A.; Vijayendra, S.V.N. In-vitro evaluation of antimicrobial and insect repellent potential of supercritical-carbon dioxide (SCF-CO(2)) extracts of selected botanicals against stored product pests and foodborne pathogens. J. Food Sci. Technol. 2020, 57, 1071–1079. [Google Scholar] [CrossRef]
- Koyu, H.; Kazan, A.; Nalbantsoy, A.; Yalcin, H.T.; Yesil-Celiktas, O. Cytotoxic, antimicrobial and nitric oxide inhibitory activities of supercritical carbon dioxide extracted Prunus persica leaves. Mol. Biol. Rep. 2020, 47, 569–581. [Google Scholar] [CrossRef]
- Kim, E.; Park, I.K. Fumigant antifungal activity of Myrtaceae essential oils and constituents from Leptospermum petersonii against three Aspergillus species. Molecules 2012, 17, 10459–10469. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, T.; Guo, Z.; Zhang, L.; Mao, L.; Zhang, Y.; Jiang, H. Fumigation and contact activities of 18 plant essential oils on Villosiclava virens, the pathogenic fungus of rice false smut. Sci. Rep. 2019, 9, 7330. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.K.; Yang, H.J.; Jung, H.; Yoon, D.J.; Sang, M.K.; Jeun, Y.C. Application of Volatile Antifungal Plant Essential Oils for Controlling Pepper Fruit Anthracnose by Colletotrichum gloeosporioides. Plant Pathol. J. 2015, 31, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Mondello, F.; De Bernardis, F.; Girolamo, A.; Cassone, A.; Salvatore, G. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect. Dis. 2006, 6. [Google Scholar] [CrossRef] [PubMed]
- Zengin, H.; Baysal, A.H. Antibacterial and Antioxidant Activity of Essential Oil Terpenes against Pathogenic and Spoilage-Forming Bacteria and Cell Structure-Activity Relationships Evaluated by SEM Microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef]
- Jing, G.-X.; Tao, N.-G.; Jia, L.; Zhou, H.-E. Influence of α-terpineol on the growth and morphogenesis of Penicillium digitatum. Bot. Stud. 2015, 56, 35. [Google Scholar] [CrossRef]
- Sun, W.M.; Ma, Y.N.; Yin, Y.J.; Chen, C.J.; Xu, F.R.; Dong, X.; Cheng, Y.X. Effects of Essential Oils from Zingiberaceae Plants on Root-Rot Disease of Panax notoginseng. Molecules 2018, 23, 1021. [Google Scholar] [CrossRef]
- Park, S.; Park, A.R.; Im, S.; Han, Y.J.; Lee, S.; Back, K.; Kim, J.I.; Kim, Y.S. Developmentally Regulated Sesquiterpene Production Confers Resistance to Colletotrichum gloeosporioides in Ripe Pepper Fruits. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Jia, X.M.; Xin, L.; Zhai, H. Antifungal Effects and Potential Mechanism of Essential Oils on Collelotrichum gloeosporioides In Vitro and In Vivo. Molecules 2019, 24, 3386. [Google Scholar] [CrossRef]

| Peak NO | Compound Name | RI (C) 1 | RI (L) 2 | Relative Area (%) 3 | Identification Methods |
|---|---|---|---|---|---|
| 1 | α-Pinene | 940 | 944 | 2.11 | RI, MS, STD 4 |
| 2 | β-Pinene | 987 | 979 | 5.21 | RI, MS, STD |
| 3 | o- Cymene | 1044 | 1042 | 1.18 | RI, MS |
| 4 | Eucalyptol | 1049 | 1047 | 5.46 | RI, MS, STD |
| 5 | γ-Terpinene | 1074 | 1063 | 2.49 | RI, MS, STD |
| 6 | α-Terpinolene | 1104 | 1097 | 0.38 | RI, MS |
| 7 | Linalool | 1115 | 1104 | 1.47 | RI, MS, STD |
| 8 | Terpinen-4-ol | 1194 | 1182 | 0.83 | RI, MS, STD |
| 9 | Terpineol | 1209 | 1200 | 4.54 | RI, MS, STD |
| 10 | Epicubebol | 1433 | 1494 | 0.19 | RI, MS |
| 11 | β-Caryophyllene | 1448 | 1439 | 28.05 | RI, MS, STD |
| 12 | α-Caryophyllene | 1485 | 1452 | 24.02 | RI, MS, STD |
| 13 | α-Muurolene | 1520 | 1500 | 1.60 | RI, MS |
| 14 | δ-Guaiene | 1523 | 1502 | 0.57 | RI, MS |
| 15 | δ-Cadinene | 1536 | 1524 | 6.29 | RI, MS |
| 16 | trans-Calamenene | 1542 | 1527 | 1.28 | RI, MS |
| 17 | 6-epi-Shyobunol | 1548 | 1548 | 0.29 | RI, MS |
| 18 | Cadine-1,4-diene | 1552 | 1546 | 0.64 | RI, MS |
| 19 | Isoshyobunone | 1605 | 1562 | 1.63 | RI, MS |
| 20 | Caryophyllene oxide | 1610 | 1581 | 0.70 | RI, MS |
| 21 | Unkown | 1618 | - | 0.77 | RI, MS |
| 22 | Humulene epoxide II | 1624 | 1616 | 0.86 | RI, MS |
| 23 | Unkown | 1633 | - | 0.38 | RI, MS |
| 24 | α-Acorenol | 1643 | 1630 | 0.73 | RI, MS |
| 25 | Cubenol | 1648 | 1637 | 2.81 | RI, MS |
| 26 | Longifolenaldehyde | 1656 | 1631 | 2.40 | RI, MS |
| 27 | β-Acorenol | 1661 | 1649 | 2.11 | RI, MS |
| Total | 98.99 |
| Content (μg/mL) | Inhibitory Rate (%) 1 | |
|---|---|---|
| P. camelliae | C. gloeosporioides | |
| 50 | 66.63 ± 2.05 a,2 | 64.59 ± 1.59 a |
| 25 | 65.77 ± 1.35 a | 63.94 ± 0.39 a |
| 12.5 | 63.09 ± 2.84 a | 61.69 ± 0.46 a |
| 6 | 54.11 ± 1.18 b | 53.05 ± 0.87 b |
| 3 | 32.48 ± 4.37 c | 40.29 ± 1.65 c |
| Control | 0.00 ± 1.08 d | 0.00 ± 0.16 d |
| MIC50 | 5.11 μg/mL | 4.79 μg/mL |
| Compound | P. camelliae | C. gloeosporioides | ||||||
|---|---|---|---|---|---|---|---|---|
| MIC50 (μL/mL) | 95% CI 1 (μL/mL) | Chi Square (χ2) | R2 | MIC50 (μL/mL) | 95% CI (μL/mL) | Chi Square (χ2) | R2 | |
| Terpineol | 0.69 | 0.66–0.72 | 1.40 | 0.999 | 0.62 | 0.49–0.75 | 9.88 | 0.988 |
| Linalool | 0.73 | 0.63–0.82 | 10.61 | 0.994 | 0.93 | 0.74–1.13 | 12.32 | 0.990 |
| Terpinen-4-ol | 0.86 | 0.69–1.09 | 25.82 | 0.986 | 0.94 | 0.66–1.21 | 23.90 | 0.987 |
| Eucalyptol | 2.79 | 2.62–2.97 | 6.50 | 0.980 | 2.18 | 2.05–2.3 | 5.07 | 0.981 |
| Carbendazim | 9.70 | 9.41–10.01 | 0.55 | 0.999 | 0.095 | 0.091–0.100 | 1.22 | 0.999 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Zhang, M.; Qin, L.; Wang, D.; Yu, F.; Liang, W.; Song, C.; Granato, D. Chemical Composition of a Supercritical Fluid (Sfe-CO2) Extract from Baeckea frutescens L. Leaves and Its Bioactivity Against Two Pathogenic Fungi Isolated from the Tea Plant (Camellia sinensis (L.) O. Kuntze). Plants 2020, 9, 1119. https://doi.org/10.3390/plants9091119
Jiang H, Zhang M, Qin L, Wang D, Yu F, Liang W, Song C, Granato D. Chemical Composition of a Supercritical Fluid (Sfe-CO2) Extract from Baeckea frutescens L. Leaves and Its Bioactivity Against Two Pathogenic Fungi Isolated from the Tea Plant (Camellia sinensis (L.) O. Kuntze). Plants. 2020; 9(9):1119. https://doi.org/10.3390/plants9091119
Chicago/Turabian StyleJiang, Hao, Mengting Zhang, Li Qin, Dongxu Wang, Feng Yu, Wenhui Liang, Chuankui Song, and Daniel Granato. 2020. "Chemical Composition of a Supercritical Fluid (Sfe-CO2) Extract from Baeckea frutescens L. Leaves and Its Bioactivity Against Two Pathogenic Fungi Isolated from the Tea Plant (Camellia sinensis (L.) O. Kuntze)" Plants 9, no. 9: 1119. https://doi.org/10.3390/plants9091119
APA StyleJiang, H., Zhang, M., Qin, L., Wang, D., Yu, F., Liang, W., Song, C., & Granato, D. (2020). Chemical Composition of a Supercritical Fluid (Sfe-CO2) Extract from Baeckea frutescens L. Leaves and Its Bioactivity Against Two Pathogenic Fungi Isolated from the Tea Plant (Camellia sinensis (L.) O. Kuntze). Plants, 9(9), 1119. https://doi.org/10.3390/plants9091119