Circadian Regulation Does Not Optimize Stomatal Behaviour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Measurements
2.3. Analyses
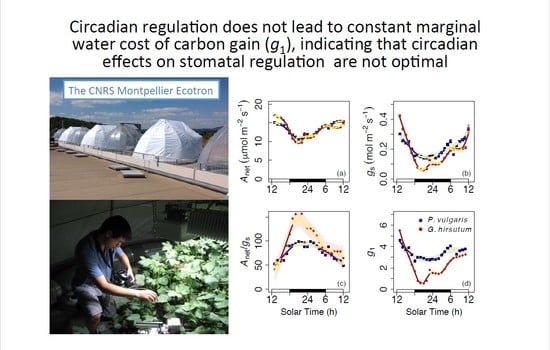
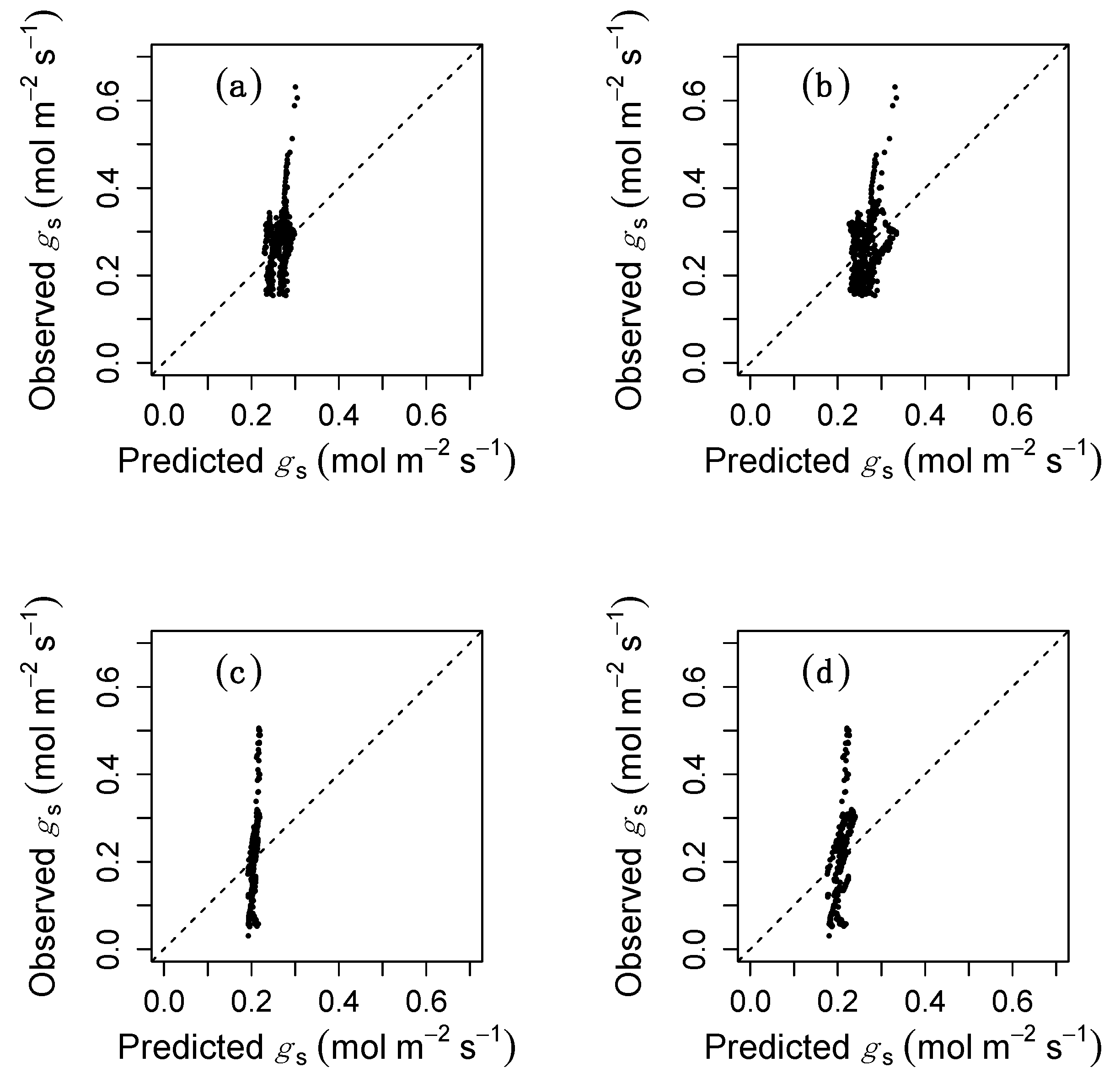
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Covington, M.F.; Maloof, J.N.; Straume, M.; Kay, S.A.; Harmer, S.L. Global transcriptome analysis reveals circadian regulation of key pathways in plant growth and development. Genome Biol. 2008, 9, R130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farré, E.M.; Weise, S.E. The interactions between the circadian clock and primary metabolism. Curr. Opin. Plant Biol. 2012, 15, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Gessler, A.; Roy, J.; Kayler, Z.; Ferrio, J.P.; Alday, J.G.; Bahn, M.; del Castillo, J.; Devidal, S.; García-Muñoz, S.; Landais, D.; et al. Night and day–circadian regulation of night-time dark respiration and light-enhanced dark respiration in plant leaves and canopies. Environ. Exp. Bot. 2017, 137, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Resco de Dios, V.; Gessler, A. Circadian regulation of photosynthesis and transpiration from genes to ecosystems. Environ. Exp. Bot. 2018, 152, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Resco de Dios, V.; Loik, M.E.; Smith, R.A.; Aspinwall, M.J.; Tissue, D.T. Genetic variation in circadian regulation of nocturnal stomatal conductance enhances plant fitness. Plant Cell Environ. 2016, 39, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Graf, A.; Schlereth, A.; Stitt, M.; Smith, A.M. Circadian control of carbohydrate availability for growth in arabidopsis plants at night. Proc. Natl. Acad. Sci. USA 2010, 107, 9458–9463. [Google Scholar] [CrossRef] [Green Version]
- de Montaigu, A.; Giakountis, A.; Rubin, M.; Toth, R.; Cremer, F.; Sokolova, V.; Porri, A.; Reymond, M.; Weinig, C.; Coupland, G. Natural diversity in daily rhythms of gene expression contributes to phenotypic variation. Proc. Natl. Acad. Sci. USA 2015, 112, 905–910. [Google Scholar] [CrossRef] [Green Version]
- Kolling, K.; Thalmann, M.; Muller, A.; Jenny, C.; Zeeman, S.C. Carbon partitioning in arabidopsis thaliana is a dynamic process controlled by the plants metabolic status and its circadian clock. Plant Cell Environ. 2015, 38, 1965–1979. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.E.; Ewers, B.E.; Williams, D.G.; Xie, Q.; Lou, P.; Xu, X.; McClung, C.R.; Weinig, C. The genetic architecture of ecophysiological and circadian traits in brassica rapa. Genetics 2011, 189, 375–390. [Google Scholar] [CrossRef] [Green Version]
- Haydon, M.J.; Mielczarek, O.; Robertson, F.C.; Hubbard, K.E.; Webb, A.A. Photosynthetic entrainment of the arabidopsis thaliana circadian clock. Nature 2013, 502, 689–692. [Google Scholar] [CrossRef]
- Resco de Dios, V. Circadian regulation and diurnal variation in gas exchange. Plant Physiol. 2017, 175, 3–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldeira, C.F.; Jeanguenin, L.; Chaumont, F.; Tardieu, F. Circadian rhythms of hydraulic conductance and growth are enhanced by drought and improve plant performance. Nat. Commun. 2014, 5, 5365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowan, I.R.; Farquhar, G.D. Stomatal function in relation to leaf metabolism and environment. In Integration of Activity in the Higher Plant; Jennings, D.H., Ed.; Cambridge University Press: Cambridge, UK, 1977. [Google Scholar]
- Wolf, A.; Anderegg, W.R.L.; Pacala, S.W. Optimal stomatal behavior with competition for water and risk of hydraulic impairment. Proc. Natl. Acad. Sci. USA 2016, 113, E7222–E7230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holloway-Phillips, M. Improving crop water-use efficiency requires optimizing the circadian clock. Plant Physiol. 2020, 183, 29–30. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, K.E.; Webb, A.A.R. Circadian rhythms in stomata: Physiological and molecular aspects. In Rhythms in Plants; Mancuso, S., Shabala, S., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 231–255. [Google Scholar]
- Dodd, A.N.; Parkinson, K.; Webb, A.A.R. Independent circadian regulation of assimilation and stomatal conductance in the ztl-1 mutant of arabidopsis. New Phytol. 2004, 162, 63–70. [Google Scholar] [CrossRef]
- Yakir, E.; Hassidim, M.; Melamed-Book, N.; Hilman, D.; Kron, I.; Green, R.M. Cell autonomous and cell-type specific circadian rhythms in arabidopsis. Plant J. Cell Mol. Biol. 2011, 68, 520–531. [Google Scholar] [CrossRef]
- Dietze, M.C. Gaps in knowledge and data driving uncertainty in models of photosynthesis. Photosynth. Res. 2014, 119, 3–14. [Google Scholar] [CrossRef]
- Vico, G.; Manzoni, S.; Palmroth, S.; Katul, G. Effects of stomatal delays on the economics of leaf gas exchange under intermittent light regimes. New Phytol. 2011, 192, 640–652. [Google Scholar] [CrossRef]
- Cowan, I.R. Regulation of water use in relation to carbon gain in higher plants. In Physiological Plant Ecology II: Water Relations and Carbon Assimilation; Lange, O.L., Nobel, P.S., Osmond, C.B., Ziegler, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 589–613. [Google Scholar]
- Medlyn, B.E.; Duursma, R.A.; Eamus, D.; Ellsworth, D.S.; Prentice, I.C.; Barton, C.V.M.; Crous, K.Y.; De Angelis, P.; Freeman, M.; Wingate, L. Reconciling the optimal and empirical approaches to modelling stomatal conductance. Glob. Chang. Biol. 2011, 17, 2134–2144. [Google Scholar] [CrossRef] [Green Version]
- Milcu, A.; Roscher, C.; Gessler, A.; Bachmann, D.; Gockele, A.; Guderle, M.; Landais, D.; Piel, C.; Escape, C.; Devidal, S.; et al. Functional diversity of leaf nitrogen concentrations drives grassland carbon fluxes. Ecol. Lett. 2014, 17, 435–444. [Google Scholar] [CrossRef]
- Munger, L.; Bleiholder, H.; Hack, H.; Hess, M.; Stauss, R.; van Den Boom, T.; Weber, E. Phenological growth stages of the peanut plant (Arachis hypogaea L.) codification and description according to the bbch scale–with figures. J. Agron. Crop Sci. 1998, 180, 101–107. [Google Scholar]
- Feller, C.; Bleiholder, H.; Buhr, L.; Hack, H.; Hess, M.; Klose, R.; Meier, U.; Stauss, R.; Boom, T.v.d.; Weber, E. Phänologische entwicklungsstadien von gemüsepflanzen: Ii. Fruchtgemüse und hülsenfrüchte. Nachrichtenbl. Deut. Pflanzenschutzd. 1995, 47, 217–232. [Google Scholar]
- Resco de Dios, V.; Roy, J.; Ferrio, J.P.; Alday, J.G.; Landais, D.; Milcu, A.; Gessler, A. Processes driving nocturnal transpiration and implications for estimating land evapotranspiration. Sci. Rep. 2015, 5, 10975. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.J.; Simpson, G.L. Trends in bulk deposition of acidity in the uk, 1988–2007, assessed using additive models. Ecol. Indic. 2014, 37, 274–286. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Wolf, A.; Arango-Velez, A.; Choat, B.; Chmura, D.J.; Jansen, S.; Kolb, T.; Li, S.; Meinzer, F.C.; Pita, P.; et al. Woody plants optimise stomatal behaviour relative to hydraulic risk. Ecol. Lett. 2018, 21, 968–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperry, J.S.; Love, D.M. What plant hydraulics can tell us about responses to climate-change droughts. New Phytol. 2015, 207, 14–27. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [Green Version]
- Azcón-Bieto, J. Inhibition of photosynthesis by carbohydrates in wheat leaves. Plant Physiol. 1983, 73, 681–686. [Google Scholar] [CrossRef] [Green Version]
- Jones, H. Stomatal control of photosynthesis and transpiration. J. Exp. Bot. 1998, 49, 387–398. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Galmes, J.; Medrano, H.; Ribas-Carbo, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Zhang, Q.; Manzoni, S.; Katul, G.; Porporato, A.; Yang, D. The hysteretic evapotranspiration—Vapor pressure deficit relation. J. Geophys. Res. Biogeosci. 2014, 119, 2013JG002484. [Google Scholar] [CrossRef]
- Resco, V.; Hartwell, J.; Hall, A. Ecological implications of plants’ ability to tell the time. Ecol. Lett. 2009, 12, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Medlyn, B.E.; Duursma, R.A.; Prentice, I.C.; Wang, H.; Baig, S.; Eamus, D.; Resco de Dios, V.; Mitchell, P.; Ellsworth, D.S.; et al. Optimal stomatal behaviour around the world. Nature Clim. Chang. 2015, 5, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Kala, J.; De Kauwe, M.G.; Pitman, A.J.; Lorenz, R.; Medlyn, B.E.; Wang, Y.P.; Lin, Y.S.; Abramowitz, G. Implementation of an optimal stomatal conductance scheme in the australian community climate earth systems simulator (access1.3b). Geosci. Model Dev. 2015, 8, 3877–3889. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.M.; Smith, W.K. Low clouds and cloud immersion enhance photosynthesis in understory species of a southern appalachian spruce–fir forest (USA). Am. J. Bot. 2006, 93, 1625–1632. [Google Scholar] [CrossRef] [Green Version]
- Salmela, M.J.; McMinn, R.L.; Guadagno, C.R.; Ewers, B.E.; Weinig, C. Circadian rhythms and reproductive phenology covary in a natural plant population. J. Biol. Rhythm. 2018, 33, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Hassidim, M.; Dakhiya, Y.; Turjeman, A.; Hussien, D.; Shor, E.; Anidjar, A.; Goldberg, K.; Green, R. Circadian clock associated 1 (cca1) and the circadian control of stomatal aperture. Plant Physiol. 2017, 175, 1864–1877. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resco de Dios, V.; Anderegg, W.R.L.; Li, X.; Tissue, D.T.; Bahn, M.; Landais, D.; Milcu, A.; Yao, Y.; Nolan, R.H.; Roy, J.; et al. Circadian Regulation Does Not Optimize Stomatal Behaviour. Plants 2020, 9, 1091. https://doi.org/10.3390/plants9091091
Resco de Dios V, Anderegg WRL, Li X, Tissue DT, Bahn M, Landais D, Milcu A, Yao Y, Nolan RH, Roy J, et al. Circadian Regulation Does Not Optimize Stomatal Behaviour. Plants. 2020; 9(9):1091. https://doi.org/10.3390/plants9091091
Chicago/Turabian StyleResco de Dios, Víctor, William R.L. Anderegg, Ximeng Li, David T. Tissue, Michael Bahn, Damien Landais, Alexandru Milcu, Yinan Yao, Rachael H. Nolan, Jacques Roy, and et al. 2020. "Circadian Regulation Does Not Optimize Stomatal Behaviour" Plants 9, no. 9: 1091. https://doi.org/10.3390/plants9091091
APA StyleResco de Dios, V., Anderegg, W. R. L., Li, X., Tissue, D. T., Bahn, M., Landais, D., Milcu, A., Yao, Y., Nolan, R. H., Roy, J., & Gessler, A. (2020). Circadian Regulation Does Not Optimize Stomatal Behaviour. Plants, 9(9), 1091. https://doi.org/10.3390/plants9091091