Interactive Role of Silicon and Plant–Rhizobacteria Mitigating Abiotic Stresses: A New Approach for Sustainable Agriculture and Climate Change
Abstract
1. Introduction
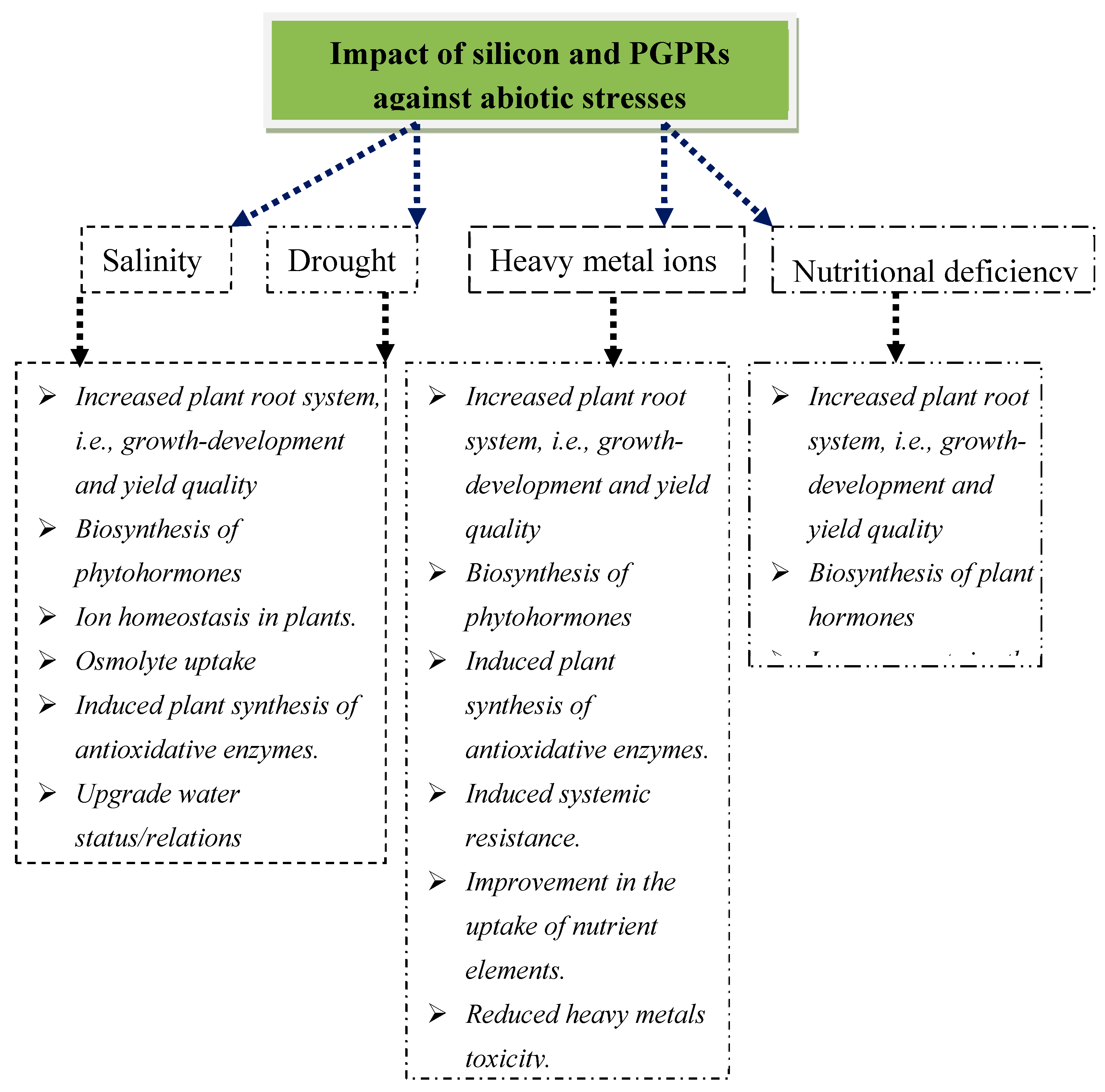
2. Role of PGPRs and Si-Mediated Mitigation Against Stress in Plants
3. Plants’ Root Development
4. Improvement of Photosynthesis and Plant Growth
5. Biosynthesis of Phytohormones
6. Uptake and Translocation of Minerals
7. Reduction of Toxic Ions
8. In Vivo Accumulation of Compatible Solutes
9. Response of Antioxidant Enzymes
10. Improvement of Plant Water Relations
11. Induced Systemic Resistance in Plants
12. PGPRs and Si Mitigating Heavy Metal Toxicity
13. PGPRs and Si Alleviating the Adverse Effects of Nutritional Deficiency
13.1. Macronutrients
13.2. Micronutrients
14. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Jeong, B.R. Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol. Environ. Saf. 2018, 147, 881–896. [Google Scholar] [CrossRef] [PubMed]
- United Nations. 2019. World Population Prospects: Highlights. Available online: https://www.un.org/development/desa/publications/world-population-prospects-2019-highlights.html (accessed on 31 March 2020).
- FAO. Climate Change and Food Security: A Framework Document; Food and Agricultural Organization of United Nation: Rome, Italy, 2008. [Google Scholar]
- Sahebi, M.; Hanafi, M.M.; Akmar, A.S.N.; Rafii, M.Y.; Azizi, P.; Tengoua, F.F.; Azwa, J.N.M.; Shabanimofrad, M. Importance od silicon and mechanisms of biosilica formation in plants. BioMed Res. Int. 2015, 2015, 396010. [Google Scholar] [CrossRef]
- Minhas, P.S.; Rane, J.; Pasala, R.K. Abiotic stresses in agriculture: An overview. In Abiotic Stress Management for Resilient Agriculture; Springer: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Mariani, L.; Ferrante, A. Agronomic management for enhancing plant tolerance to abiotic stresses—Drought, salinity, hypoxia, and lodging. Horticulturae 2017, 3, 52. [Google Scholar] [CrossRef]
- Gull, A.; Lone, A.A.; Ul Islam Wani, N. Biotic and abiotic stresses in plants. Abiotic Biot. Stress Plants 2019. [Google Scholar] [CrossRef]
- Berg, G. Plant microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Köberl, M.; Mostafa, A.; Ramadan, E.M.; Monschein, M.; Jensen, K.B.; Bauer, R.; Berg, G. Effects of bacterial inoculants on the indigenousmicrobiome and secondary metabolites of chamomile plants. Front Microbiol. 2014, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Cherif, H.; Marasco, R.; Rolli, E.; Ferjani, R.; Fusi, M.; Souss, A.; Mapelli, F.; Blilou, I.; Borin, S.; Boudabous, A.; et al. Oasisdesert farming selects environment-specific date palm root endo-phytic communities and cultivable bacteria that promote resistance to drought. Environ. Microbiol. Rep. 2015, 7, 668–678. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Rodriguez, R.U.; Zehnder, G.W.; Murphy, J.F.; Sikora, E.; Fernandez, C. Plant root-bacterial interactions in biological control of soil borne diseases and potential extension to systemic and foliar diseases. Aust. Plant Pathol. 1999, 28, 21–26. [Google Scholar] [CrossRef]
- Etesami, H.; Beattie, G.A. Plant-Microbe Interactions in Adaptation of Agricultural Crops to Abiotic Stress Conditions. In: Kumar V., Kumar M., Sharma S., Prasad R. (eds) Probiotics and Plant Health. Springer, Singapore. Probiotics Plant Health 2017, 163–200. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Son, J.S.; Sumayo, M.; Hwang, Y.J.; Kim, B.S.; Ghim, S.Y. Screening of plant growth promoting rhizobacteria as elicitor of systemic resistance against grey leaf spot dieses in pepper. Appl. Soil Ecol. 2014, 73, 1–8. [Google Scholar] [CrossRef]
- Lugtenberg, B.J.; Chin A-Woeng, T.F.; Bloemberg, G.V. Microbe plant interactions: Principles and mechanisms. Antonie Van Leeuwenhoek 2002, 81, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.K.; Singh, P.; Song, X.P.; Malviya, M.K.; Singh, R.K.; Chen, G.L.; Solomon, S.; Li, Y.R. Mitigating climate change for sugarcane improvement: Role of silicon in alleviating abiotic stresses. Sugar Tech. 2020. [Google Scholar] [CrossRef]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G. Biostimulant activity of silicon in horticulture. Scientia Hort. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.D. Benefits of plant silicon for crops: A review. Agron. Sustain Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Summerfield, M.A. Silcrete in Chemical Sediments and Geomorphology; Goudie, A.S., Pye, K., Eds.; Academic Press: London, UK, 1983; pp. 59–93. [Google Scholar]
- Rezanka, T.; Sigler, K. Biologically active compounds of semi metals. Stud. Nat. Prod. Chem. 2008, 35, 835–921. [Google Scholar]
- Cornelis, J.T.; Delvaux, B.; Georg, R.B.; Lucas, Y.; Ranger, J.; Opfergelt, S. Tracing the origin of dissolved silicon transferred from various soilplant systems towards rivers: A review. Biogeosciences 2011, 8, 89–112. [Google Scholar] [CrossRef]
- Clarke, J. The occurrence and significance of biogenic opal in the regolith. Earth-Sci. Rev. 2003, 60, 175–194. [Google Scholar] [CrossRef]
- Sommer, M.; Kaczorek, D.; Kuzyakov, Y.; Breuer, J. Silicon pools and fluxes in soils and landscapes—A review. J. Plant Nutr. Soil Sci. 2006, 169, 310–329. [Google Scholar] [CrossRef]
- Epstein, E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani-Ueno, N. Transport of silicon from roots to panicles in plants. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 377–385. [Google Scholar] [CrossRef]
- Verma, K.K.; Liu, X.H.; Wu, K.C.; Singh, R.K.; Song, Q.Q.; Malviya, M.K.; Song, X.P.; Singh, P.; Verma, C.L.; Li, Y.R. The impact of silicon on photosynthetic and biochemical responses of sugarcane under different soil moisture levels. Silicon 2019, 12, 1355–1367. [Google Scholar] [CrossRef]
- Verma, K.K.; Singh, R.K.; Song, Q.Q.; Singh, P.; Zhang, B.Q.; Song, X.P.; Chen, G.L.; Li, Y.R. Silicon alleviates drought stress of sugarcane plants by improving antioxidant responses. Biomed. J. Sci. Tech. Res. 2019, 17, 002957. [Google Scholar] [CrossRef]
- Verma, K.K.; Wu, K.-C.; Singh, P.; Malviya, M.K.; Singh, R.K.; Song, X.-P.; Li, Y.R. The protective role of silicon in sugarcane under water stress: Photosynthesis and antioxidant enzymes. Biomed. J. Sci. Tech. Res. 2019, 15, 002685. [Google Scholar] [CrossRef]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s role in abiotic and biotic plant stresses. Ann. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef]
- Rasool, S.; Hameed, A.; Azooz, M.; Siddiqi, T.; Ahmad, P. Salt Stress: Causes, types and responses of plants. In Ecophysiology and Responses of Plants under Salt Stress; Springer: New York, NY, USA, 2013; pp. 1–24. [Google Scholar]
- Bodner, G.; Nakhforoosh, A.; Kaul, H.-P. Management of crop water under drought: A review. Agron. Sustain Dev. 2015, 35, 401–442. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Compat, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Liang, Y.; Sun, W.; Zhu, Y.-G.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Poll. 2007, 147, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Miransari, M. Soil microbes and the availability of soil nutrients. Acta Physiol. Plant. 2013, 35, 3075–3084. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Exley, C. A possible mechanism of biological silicification in plants. Front. Plant Sci. 2015, 6, 853. [Google Scholar] [CrossRef]
- Pozza, E.A.; Pozza, A.A.A.; Botelho, D.M.d.S. Silicon in plant disease control. Rev. Ceres 2015, 62, 323–331. [Google Scholar] [CrossRef]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially mediated plant salt tolerance and microbiome-based solutions for Saline agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and plants: Current knowledge and technological perspectives. Front Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef]
- Etesami, H. Can interaction between silicon and plant growth promoting rhizobacteria benefits in alleviating abiotic and biotic stresses in crop plants? Agric. Ecosyst. Environ. 2018, 253, 98–112. [Google Scholar] [CrossRef]
- Mahmood, S.; Daur, I.; Al-Solaimani, S.G.; Ahmad, S.; Madkour, M.H.; Yasir, M.; Hirt, H.; Ali, S.; Ali, Z. Plant growth promoting rhizobacteria and silicon synergistically enhance salinity tolerance of mung bean. Front. Plant Sci. 2016, 7, 876. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ibrahim, M.; Farid, M.; Adrees, M.; Bharwana, S.A.; Zia-ur-Rehman, M.; Qayyum, M.F.; Abbas, F. Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: A review. Environ. Sci. Poll. Res. 2015, 22, 15416–15431. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, M.; Wani, S.P. Plant-growth-promoting rhizobacteria: Drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol. 2016, 66, 35–42. [Google Scholar] [CrossRef]
- Mahmood, S.; Daur, I.; Hussain, M.B.; Nazir, Q.; Al-Solaimani, S.G.; Ahmed, S.; Bakhashwain, A.A.; Elsafor, A.K. Silicon application and rhizobacterial inoculation regulate mung bean response to saline water irrigation. Clean Soil Air Water 2017, 45, 8. [Google Scholar] [CrossRef]
- Safoora, D.; Cyrus, G.; Bahram, B.; Mahdi, G.; Siamak, S. Effect of silicon on growth and development of strawberry under water deficit conditions. Hort. Plant J. 2018, 4, 226–232. [Google Scholar]
- Shi, Y.; Zhang, Y.; Han, W.; Feng, R.; Hu, Y.; Guo, J.; Gong, H. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front. Plant Sci. 2016, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, K.; Emam, Y.; Ashraf, M. Influence of foliar application of silicon on chlorophyll fluorescence, photosynthetic pigments, and growth in water-stressed wheat cultivars differing in drought tolerance. Turk. J. Bot. 2015, 39, 625–634. [Google Scholar] [CrossRef]
- Amin, M.; Ahmad, R.; Ali, A.; Hussain, I.; Mahmood, R.; Aslam, M.; Lee, D.J. Influence of silicon fertilization on maize performance under limited water supply. Silicon 2016, 10, 177–183. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Flam-Shepherd, R.; Huynh, W.Q.; Coskun, D.; Hamam, A.M.; Britto, D.T.; Kronzucker, H.J. Membrane fluxes, bypass flows, and sodium stress in rice: The influence of silicon. J. Exp. Bot. 2018, 69, 1679–1692. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Tanaka, K.; Fujihara, S.; Itai, A.; Den, X.; Zhang, S. Silicon mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. Plant Cell Environ. 2016, 39, 245–258. [Google Scholar] [CrossRef]
- Saleh, J.; Najafi, N.; Oustan, S.; Ghasemi-Golezani, K.; Aliasghrzad, N. Silicon affects rice growth, superoxide dismutase activity and concentrations of chlorophyll and proline under different levels and sources of soil salinity. Silicon 2018, 11, 2659–2667. [Google Scholar] [CrossRef]
- Hattori, T.; Sonobe, K.; Araki, H.; Inanaga, S.; An, P.; Morita, S. Silicon application by sorghum through the alleviation of stress-induced increase in hydraulic resistance. J. Plant Nutr. 2008, 31, 1482–1495. [Google Scholar] [CrossRef]
- Farooq, M.A.; Detterbeck, A.; Clemens, S.; Dietz, K.J. Silicon-induced reversibility of cadmium toxicity in rice. J. Exp. Bot. 2016, 67, 3573–3585. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, L.; Martins, S.C.V.; Detmann, K.C.; Silva, P.E.M.; Lavinsky, A.O.; Silva, M.M.; Detmann, E.; Araujo, W.L.; DaMatta, M. Silicon nutrition alleviates the negative impacts of arsenic on the photosynthetic apparatus of rice leaves: An analysis of the key limitations of photosynthesis. Physiol. Plant 2014, 152, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Naranjo, E.; Galle, A.; Florez-Sarasa, I.; Perdomo, J.A.; Galmes, J.; Ribas-Carbo, M.; Flexas, J. Assessment of the role of silicon in the Cu-tolerance of the C4 grass Spartina densiflora. J. Plant Physiol. 2015, 178, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Rogalla, H.; Romheld, V. Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumis sativus L. Plant Cell Environ. 2002, 25, 549–555. [Google Scholar] [CrossRef]
- Maksimovic, J.D.; Mojovic, M.; Maksimovic, V.; Romheld, V.; Nikolic, M. Silicon ameliorates manganese toxicity in cucumber by decreasing hydroxyl radical accumulation in the leaf apoplast. J. Exp. Bot. 2012, 63, 2411–2420. [Google Scholar] [CrossRef]
- Wang, Y.X.; Stass, A.; Horst, W.J. Apoplastic binding of aluminum is involved in silicon-induced amelioration of aluminum toxicity in maize. Plant Physiol. 2004, 136, 3762–3770. [Google Scholar] [CrossRef]
- Chen, D.Q.; Cao, B.B.; Qi, L.Y.; Yin, L.N.; Wang, S.W.; Deng, X.P. Silicon-moderated K-deficiency-induced leaf chlorosis by decreasing putrescine accumulation in sorghum. Ann. Bot. 2016, 118, 305–315. [Google Scholar] [CrossRef]
- Kaya, C.; Tuna, L.; Higgs, D. Effect of silicon on plant growth and mineral nutrition of maize grown under water-stress conditions. J. Plant Nutr. 2006, 29, 1469–1480. [Google Scholar] [CrossRef]
- Dardanelli, M.S.; Fernández de Córdoba, F.J.; Rosario Espuny, M.; Rodríguez Carvajal, M.A.; Soria Díaz, M.E.; Gil Serrano, A.M.; Okon, Y.; Megías, M. Effect of Azospirillum brasilense coinoculated with Rhizobium on Phaseolus vulgaris flavonoids and Nod factor production under salt stress. Soil Biol. Biochem. 2008, 40, 2713–2721. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Arshad, M. Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity. Can. J. Microbiol. 2007, 53, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, D.; Samiyappan, R. ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogaea) plants. J. App. Microbiol. 2007, 102, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Barassi, C.A.; Ayrault, G.; Creus, C.M.; Sueldo, R.J.; Sobrero, M.T. Seed inoculation with Azospirillum mitigates NaCl effects on lettuce. Sci. Hortic. 2006, 109, 8–14. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef]
- Ashraf, M.; Hasnain, S.; Berge, O.; Mahmood, T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils 2004, 40, 157–162. [Google Scholar] [CrossRef]
- Hamdia, A.B.E.; Shaddad, M.A.K.; Doaa, M.M. Mechanisms of salt tolerance and interactive effects of Azospirillum brasilense inoculation on maize cultivars grown under salt stress conditions. Plant Growth Regul. 2004, 44, 165–174. [Google Scholar] [CrossRef]
- Hamaoui, B.; Abbadi, J.M.; Burdman, S.; Rashid, A.; Sarig, S.; Okon, Y. Effects of inoculation with Azospirillum brasilense on chickpeas (Cicer arietinum) and faba beans (Vicia faba) under different growth conditions. Agronomie 2001, 2, 553–560. [Google Scholar] [CrossRef][Green Version]
- El-Akhal, M.R.; Rincon, A.; Coba de la Pena, T.; Lucas, M.M.; El Mourabit, N.; Barrijal, S.; Pueyo, J.J. Effects of salt stress and rhizobial inoculation on growth and nitrogen fixation of three peanut cultivars. Plant Biol. 2013, 15, 415–421. [Google Scholar] [CrossRef]
- Yao, L.; Wu, Z.; Zheng, Y. Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton. Eur. J. Soil Biol. 2010, 46, 49–54. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 2004, 166, 525–530. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Khalid, M. Efficacy of Rhizobium and Pseudomonas strains to improve physiology, ionic balance and quality of mung bean under salt-affected conditions on farmer’s fields. Plant Physiol. Biochem. 2013, 63, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Creus, C.M.; Sueldo, R.J.; Barassi, C.A. Water relations and yield in Azospirillum-inoculated wheat exposed to drought in the field. Can. J. Bot. 2004, 82, 273–281. [Google Scholar] [CrossRef]
- Casanovas, E.M.; Barassi, C.A.; Sueldo, R.J. Azospirillum inoculation mitigates water stress effects in maize seedlings. Cereal Res. Commun. 2002, 30, 343–350. [Google Scholar] [CrossRef]
- German, M.A.; Burdman, S.; Okon, Y.; Kigel, J. Effects of Azospirillum brasilense on root morphology of common bean (Phaseolus vulgaris L.) under different water regimes. Biol. Fertil. Soils 2000, 32, 259–264. [Google Scholar] [CrossRef]
- Belimov, A.A.; Dodd, I.C.; Hontzeas, N.; Theobald, J.C.; Safronova, V.I.; Davies, W.J. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol. 2009, 181, 413–423. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Prinsen, E.; Veselov, S.U.; Martinenko, E.V.; Melentiev, A.I.; Kudoyarova, G.R. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil 2007, 292, 305–315. [Google Scholar] [CrossRef]
- Sziderics, A.H.; Rasche, F.; Trognitz, F.; Sessitsch, A.; Wilhelm, E. Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.). Can. J. Microbiol. 2007, 53, 1195–1202. [Google Scholar] [CrossRef]
- Pereyra, M.A.; Zalazar, C.A.; Barassi, C.A. Root phospholipids in Azospirillum-inoculated wheat seedlings exposed to water stress. Plant Physiol. Biochem. 2006, 44, 873–879. [Google Scholar] [CrossRef]
- Creus, C.M.; Sueldo, R.J.; Barassi, C.A. Water relations in Azospirillum-inoculated wheat seedlings under osmotic stress. Can. J. Bot. Revue Canadienne De Botanique 1998, 76, 238–244. [Google Scholar] [CrossRef]
- Grichko, V.P.; Glick, B.R. Amelioration of flooding stress byACC deaminase-containing plant growth-promoting bacteria. Plant Physiol. Biochem. 2001, 39, 11–17. [Google Scholar] [CrossRef]
- Bensalim, S.; Nowak, J.; Asiedu, S.K. A plant growth promoting rhizobacterium and temperature effects on performance of 18 clones of potato. Am. J Potato Res. 1998, 75, 145–152. [Google Scholar] [CrossRef]
- Zhang, F.; Dashti, N.; Hynes, R.K.; Smith, D.L. Plant growth promoting rhizobacteria and soybean [Glycine max (L.) Merr] growth and physiology at suboptimal root zone temperatures. Ann. Bot. 1997, 79, 243–249. [Google Scholar] [CrossRef]
- Egamberdiyeva, D. The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. App. Soil Ecol. 2007, 36, 184–189. [Google Scholar] [CrossRef]
- Asch, F.; Padham, J.L. Root associated bacteria suppress symptoms of iron toxicity in lowland rice. In The Global Food & Product Chain—Dynamics, Innovations, Conflicts, Strategies; Tielkes, E., Hülsebusch, C., Häuser, I., Deininger, A., Becker, K., Eds.; MDD GmbH: Stuttgart, Germany, 2005; p. 276. [Google Scholar]
- Terré, S.; Asch, F.; Padham, J.; Sikora, R.A.; Becker, M. Influence of root zone bacteria on root iron plaque formation in rice subjected to iron toxicity. In Proceedings of the Conference on Utilisation of Diversity in Land Use Systems: Sustainable and Organic Approaches to Meet Human Needs, Tropentag, Witzenhausen, Germany, 8 October 2007. [Google Scholar]
- Lee, S.K.; Sohn, E.Y.; Hamayun, M.; Yoon, J.Y.; Lee, I.J. Effect of silicon on growth and salinity stress of soybean plant grown under hydroponic system. Agrofor. Syst. 2010, 80, 333–340. [Google Scholar] [CrossRef]
- Kim, Y.H.; Khan, A.L.; Waqas, M.; Shim, J.K.; Kim, D.H.; Lee, K.Y.; Lee, I.J. Silicon application to rice root zone influenced the phytohormonal and antioxidant responses under salinity stress. J. Plant Growth Regul. 2014, 33, 137–149. [Google Scholar] [CrossRef]
- Hameed, A.; Sheikh, M.A.; Jamil, A.; Basra, S.M.A. Seed priming with sodium silicate enhances seed germination and seedling growth in wheat (Triticum aestivum L.) under water deficit stress induced by polyethylene glycol. Pak. J. Life Soc. Sci. 2013, 11, 19–24. [Google Scholar]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Gong, H.; Chen, K. The regulatory role of silicon on water relations, photosynthetic gas exchange, and carboxylation activities of wheat leaves in field drought conditions. Acta Physiol. Plant. 2012, 34, 1589–1594. [Google Scholar] [CrossRef]
- Liu, P.; Yin, L.; Wang, S.; Zhang, M.; Deng, X.; Zhang, S.; Tanaka, K. Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum Bicolor L. Environ. Exp. Bot. 2015, 111, 42–51. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Sharma, K.P.; Gaur, R.K. Biotechnological perspectives of microbes in agro-ecosystems. Biotechnol. Lett. 2011, 33, 1905–1910. [Google Scholar] [CrossRef] [PubMed]
- García-Fraile, P.; Menéndez, E.; Rivas, R. Role of bacterial biofertilizers in agriculture and forestry. AIMS Bioeng. 2015, 2, 183–205. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Boyce, A.N. Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Cho, Y.-G. Plant hormones in salt stress tolerance. J. Plant Biol. 2015, 58, 147–155. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Khan, M.I.R. A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Tsukanova, K.A.; Chebotar, V.; Meyer, J.J.M.; Bibikova, T.N. Effect of plant growth-promoting Rhizobacteria on plant hormone homeostasis. S. Afr. J. Bot. 2017, 113, 91–102. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A.; Hosseini, H.M. Indole-3-acetic acid (IAA) production trait, a useful screening to select endophytic and rhizosphere competent bacteria for rice growth promoting agents. MethodsX 2015, 2, 72–78. [Google Scholar] [CrossRef]
- Wang, Y.; Mopper, S.; Hasenstein, K.H. Effects of salinity on endogenous ABA, IAA, JA, and SA in Iris hexagona. J. Chem. Ecol. 2001, 27, 327–342. [Google Scholar] [CrossRef]
- Hamayun, M.; Sohn, E.-Y.; Khan, S.A.; Shinwari, Z.K.; Khan, A.L.; Lee, I.-J. Silicon alleviates the adverse effects of salinity and drought stress on growth and endogenous plant growth hormones of soybean (Glycine max L.). Pak. J. Bot. 2010, 42, 1713–1722. [Google Scholar]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Amsterdam, The Netherlands, 2009; pp. 153–188. [Google Scholar]
- Liang, Y.; Zhang, W.; Chen, Q.; Ding, R. Effects of silicon on H+-ATPase and H+- PPase activity, fatty acid composition and fluidity of tonoplast vesicles from roots of salt-stressed barley (Hordeum vulgare L.). Environ. Exp. Bot. 2005, 53, 29–37. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Z.; Ke, Y.; Xiao, C.; Peng, Z.; Wu, L.; Li, Y.; Jian, H.; Cen, J.; Zhang, Y. Effects of silicate fertilizer on nutrition of leaves, yield and sugar of sugarcanes. Trop. Subtrop. Soil Sci. 1997, 6, 242–246. [Google Scholar]
- Soundararajan, P.; Manivannan, A.; Jeong, B.R. Regulatory mechanisms by silicon to overcome the salinity-induced imbalance of essential nutrient elements. In Silicon in Plants: Advances and Future Prospects; CRC Press: Boca Raton, FL, USA, 2016; pp. 47–66. [Google Scholar]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Coskun, D.; Schulze, L.M.; Wong, J.R.; Britto, D.T. Sodium as nutrient and toxicant. Plant Soil 2013, 369, 1–23. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The role of silicon in higher plants under salinity and drought stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef]
- Geddie, J.L.; Sutherland, I.W. Uptake of metals by bacterial polysaccharides. J. Appl. Bacteriol. 1993, 74, 467–472. [Google Scholar] [CrossRef]
- Xie, Z.; Song, R.; Shao, H.; Song, F.; Xu, H.; Lu, Y. Silicon improves maize photosynthesis in saline-alkaline soils. Sci. World J. 2015, 2015, 245072. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Gzik, A. Accumulation of proline and pattern of α-amino acids in sugar beet plants in response to osmotic, water and salt stress. Environ. Exp. Bot. 1996, 36, 29–38. [Google Scholar] [CrossRef]
- Pei, Z.; Ming, D.; Liu, D.; Wan, G.; Geng, X.; Gong, H.; Zhou, W. Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J. Plant Growth Regul. 2010, 29, 106–115. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Protection of plasma membrane of onion epidermal cells by glycinebetaine and proline against NaCl stress. Plant Physiol. Biochem. 1998, 36, 767–772. [Google Scholar] [CrossRef]
- Kohler, J.; Hernández, J.A.; Caravaca, F.; Roldán, A. Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ. Exp. Bot. 2009, 65, 245–252. [Google Scholar] [CrossRef]
- Pereira, T.S.; da Silva Lobato, A.K.; Tan, D.K.Y.; da Costa, D.V.; Uchôa, E.B.; do Nascimento Ferreira, R.; dos Santos Pereira, E.; Ávila, F.W.; Marques, D.J.; Guedes, E.M.S. Positive interference of silicon on water relations, nitrogen metabolism, and osmotic adjustment in two pepper (Capsicum annuum) cultivars under water deficit. Aust. J. Crop. Sci. 2013, 7, 1064. [Google Scholar]
- Kim, Y.-H.; Khan, A.L.; Waqas, M.; Lee, I.-J. Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: A review. Front. Plant Sci. 2017, 8, 510. [Google Scholar] [CrossRef]
- Demidchik, V. Reactive oxygen species and oxidative stress in plants. In Plant Stress Physiology; Shabala, S., Ed.; CAB International: Wallingford, UK; Oxford, UK, 2012; pp. 24–58. [Google Scholar]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon decreases transpiration rate and conductance from stomata of maize plants. J. Plant. Nutr. 2006, 29, 1637–1647. [Google Scholar] [CrossRef]
- Zhu, Y.-X.; Xu, X.-B.; Hu, Y.-H.; Han, W.-H.; Yin, J.-L.; Li, H.-L.; Gong, H.-J. Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep. 2015, 34, 1629–1646. [Google Scholar] [CrossRef]
- Gong, H.J.; Chen, K.M.; Zhao, Z.G.; Chen, G.C.; Zhou, W.J. Effects of silicon on defense of wheat against oxidative stress under drought at different developmental stages. Biol. Plant. 2008, 52, 592–596. [Google Scholar] [CrossRef]
- Hattori, T.; Inanaga, S.; Araki, H.; An, P.; Morita, S.; Luxova, M.; Lux, A. Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol. Plant. 2005, 123, 459–466. [Google Scholar] [CrossRef]
- Rubio-Asensio, J.S.; López-Berenguer, C.; García-de la Garma, J.; Burger, M.; Bloom, A.J. Root strategies for nitrate assimilation. In Root Engineering; Springer: New York, NY, USA, 2014; pp. 251–267. [Google Scholar]
- Sutka, M.; Li, G.; Boudet, J.; Boursiac, Y.; Doumas, P.; Maurel, C. Natural variation of root hydraulics in Arabidopsis grown in normal and salt-stressed conditions. Plant Physiol. 2011, 155, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Boursiac, Y.; Chen, S.; Luu, D.-T.; Sorieul, M.; van den Dries, N.; Maurel, C. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 2005, 139, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Vardharajula, S.; Zulfikar Ali, S.; Grover, M.; Reddy, G.; Bandi, V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, J.S.; Singh, D.P. Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 2011, 21, 214–222. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Bakker, P.; Pieterse, C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Lugtenberg, B. Use of plant growth-promoting rhizobacteria to alleviate salinity stress in plants. Use Microb. Allev. Soil Stress 2014, 1, 73–96. [Google Scholar]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springer Plus 2013, 2, 587. [Google Scholar] [CrossRef]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Kloepper, J.W.; Paré, P.W. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef]
- Keller, C.; Rizwan, M.; Davidian, J.-C.; Pokrovsky, O.; Bovet, N.; Chaurand, P.; Meunier, J.-D. Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 μM Cu. Planta 2015, 241, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Anwaar, S.A.; Ali, S.; Ali, S.; Ishaque, W.; Farid, M.; Farooq, M.A.; Najeeb, U.; Abbas, F.; Sharif, M. Silicon (Si) alleviates cotton (Gossypium hirsutum L.) from zinc (Zn) toxicity stress by limiting Zn uptake and oxidative damage. Environ. Sci. Poll. Res. 2015, 22, 3441–3450. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K.; Rai, A.K. Silicon-mediated alleviation of Cr (VI) toxicity in wheat seedlings as evidenced by chlorophyll florescence, laser induced breakdown spectroscopy and anatomical changes. Ecotoxicol. Environ. Saf. 2015, 113, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Maier, P.; Fecht, M.; Horst, W.J. Leaf apoplastic silicon enhances manganese tolerance of cowpea (Vigna unguiculata). J. Plant Physiol. 2002, 159, 167–173. [Google Scholar] [CrossRef]
- Sessitsch, A.; Kuffner, M.; Kidd, P.; Vangronsveld, J.; Wenzel, W.W.; Fallmann, K.; Puschenreiter, M. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol. Biochem. 2013, 60, 182–194. [Google Scholar] [CrossRef]
- Chmielowska-Bak, J.; Gzyl, J.; Rucinska-Sobkowiak, R.; Arasimowicz-Jelonek, M.; Deckert, J. The new insights into cadmium sensing. Front. Plant Sci. 2014, 5, 245. [Google Scholar]
- Hernandez-Apaolaza, L. Can silicon partially alleviate micronutrient deficiency in plants? A review. Planta 2014, 240, 447–458. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 5, 963401. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A. Co-inoculation with endophytic and rhizosphere bacteria allows reduced application rates of N-fertilizer for rice plant. Rhizosphere 2016, 2, 5–12. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A. Rhizosphere and endorhiza of oilseed rape (Brassica napus L.) plant harbor bacteria with multifaceted beneficial effects. Biol. Control 2016, 94, 11–24. [Google Scholar] [CrossRef]
- Eneji, A.E.; Inanaga, S.; Muranaka, S.; Li, J.; Hattori, T.; An, P.; Tsuji, W. Growth and nutrient use in four grasses under drought stress as mediated by silicon fertilizers. J. Plant Nutr. 2008, 31, 355–365. [Google Scholar] [CrossRef]
- Brenchley, W.E.; Maskell, E.J. The inter-relation between silicon and other elements in plant nutrition. Ann. Appl. Biol. 1927, 14, 45–82. [Google Scholar] [CrossRef]
- Fisher, R.A. A preliminary note on the effect of sodium silicate in increasing the yield of barley. J. Agric. Sci. 1929, 19, 132–139. [Google Scholar] [CrossRef]
- Ma, J.F.; Miyake, Y.; Takahashi, E. Silicon as a beneficial element for crop plants. In Silicon in Agriculture; Datnoff, L.E., Snyder, G.H., Korndorfer, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 8, pp. 17–39. [Google Scholar]
- Meena, V.S.; Maurya, B.R.; Verma, J.P. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol. Res. 2014, 169, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Mali, M.; Aery, N.C. Influence of silicon on growth, relative water contents and uptake of silicon, calcium and potassium in wheat grown in nutrient solution. J. Plant Nutr. 2008, 31, 1867–1876. [Google Scholar] [CrossRef]
- Pavlovic, J.; Samardzic, J.; Maksimović, V.; Timotijevic, G.; Stevic, N.; Laursen, K.H.; Hansen, T.H.; Husted, S.; Schjoerring, J.K.; Liang, Y. Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root apoplast. New Phytol. 2013, 198, 1096–1107. [Google Scholar] [CrossRef]
- Bityutskii, N.; Pavlovic, J.; Yakkonen, K.; Maksimovic, V.; Nikolic, M. Contrasting effect of silicon on iron, zinc and manganese status and accumulation of metal-mobilizing compounds in micronutrient-deficient cucumber. Plant Physiol. Biochem. 2014, 74, 205–211. [Google Scholar] [CrossRef]
- Maksimovic, J.D.; Bogdanovic, J.; Maksimovic, V.; Nikolic, M. Siliconmodulates the metabolism and utilization of phenolic compounds incucumber (Cucumis sativus L.) grown at excess manganese. J. Plant Nutr. Soil Sci. 2007, 170, 739–744. [Google Scholar] [CrossRef]
- Pich, A.; Scholz, G.; Stephan, U.W. Iron-dependent changes of heavy metals, nicotianamine, and citrate in different plant organs and in the xylem exudate of two tomato genotypes. Nicotianamine as possible copper translocator. Plant Soil 1994, 165, 189–196. [Google Scholar] [CrossRef]
- Wallace, A. Participation of silicon in cation–anion balance as a possible mechanism for aluminum and iron tolerance in some Gramineae. J. Plant Nutr. 1993, 16, 547–553. [Google Scholar] [CrossRef]
- Gonzalo, M.J.; Lucena, J.J.; Hernández-Apaolaza, L. Effect of silicon addition on soybean (Glycine max) and cucumber (Cucumis sativus) plants grown under iron deficiency. Plant Physiol. Biochem. 2013, 70, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Frantz, J.M.; Khandekar, S.; Leisner, S. Silicon differentially influences copper toxicity response in silicon-accumulator and non-accumulator species. J. Am. Soc. Hortic. Sci. 2011, 136, 329–338. [Google Scholar] [CrossRef]
- Ramos-Solano, B.; García, J.A.L.; Garcia-Villaraco, A.; Algar, E.; Garcia-Cristobal, J.; Mañero, F.J.G. Siderophore and chitinase producing isolates from the rhizosphere of Nicotiana glauca Graham enhance growth and induce systemic resistance in Solanum lycopersicum L. Plant Soil 2010, 334, 189–197. [Google Scholar] [CrossRef]
- Osorio Vega, N.W. A review on beneficial effects of rhizosphere bacteria on soil nutrient availability and plant nutrient uptake. Rev. Fac. Nac. Agron. Medellin 2007, 60, 3621–3643. [Google Scholar]
- Dutta, S.; Podile, A.R. Plant growth promoting rhizobacteria (PGPR): The bugs to debug the root zone. Crit. Rev. Microbiol. 2010, 36, 232–244. [Google Scholar] [CrossRef]

| Growth Condition | Plant/ Cultivars | Source of Si | Variables | Effect of Si with Stress | Reference |
|---|---|---|---|---|---|
| Drought | Saccharum officinarum | Calcium-metasilicate | Net CO2 assimilation | + | [17,29,30,31] |
| Stomatal conductance | + | ||||
| Transpiration rate | + | ||||
| Photosynthesis pigments | + | ||||
| RWC (%) | + | ||||
| CAT | + | ||||
| POD | + | ||||
| SOD | + | ||||
| Plant hormones (ABA, IAA, and GA3) | + | ||||
| Strawberry (Fragaria x ananassa ‘Camarosa’) | Potassium silicate | Leaf area-number | no effect | [49] | |
| Petiole length | + | ||||
| Electrolyte leakage (%) | no effect | ||||
| Chlorophyll index | + | ||||
| Fv/Fm | + | ||||
| PN | + | ||||
| E | + | ||||
| WUE | + | ||||
| Proline content | + | ||||
| Shoot/ root FM/DM | + | ||||
| Solanum lycopersicum | Potassium silicate | EC (%) | + | [50] | |
| Root hydraulic conductance | + | ||||
| Photosynthesis | + | ||||
| Transpiration | + | ||||
| MDA | + | ||||
| H2O2 | + | ||||
| CAT activity | + | ||||
| SOD activity | + | ||||
| Ascorbic acid | + | ||||
| Reduced GSH activity | + | ||||
| Triticum aestivum and Zea mays | Sodium and calcium silicate | Growth and yield traits | + | [51,52] | |
| Chlorophyll fluorescence | + | ||||
| Leaf gas exchange | + | ||||
| Salinity | Cucumis sativus | Calcium silicate | Total DM | + | [53] |
| Root EC (%) | + | ||||
| Protein content | + | ||||
| MDA | + | ||||
| H2O2 content | + | ||||
| CAT activity | - | ||||
| APx activity | + | ||||
| GPx activity | + | ||||
| SOD activity | + | ||||
| GR activity | + | ||||
| DHAR activity | + | ||||
| Oryza sativa | Sodium silicate | Dry mass | + | [54] | |
| Na+ content | + | ||||
| Na+ influx and efflux | - | ||||
| Transpiration rate | + | ||||
| Apoplastic bypass flow | + | ||||
| Vigna radiata | Potassium silicate and PGPR strains (E. cloacae and B. drentensis) | Growth traits | + | [45] | |
| Shoot & root fresh/ dry mass | + | ||||
| Green pigments | + | ||||
| Gas exchange | + | ||||
| Salt tolerance index | + | ||||
| Crop output | + | ||||
| Sorghum bicolor | Sodium silicate | Total dry mass | + | [55] | |
| Photosynthetic pigments | + | ||||
| Root Na+ and K+ content | - | ||||
| Total polyamine content | + | ||||
| Total ACC content | + | ||||
| Oryza sativa | Silicic acid | Total no. of tillers | - | [56] | |
| Leaf area | - | ||||
| Dry mass | - | ||||
| Chlorophyll content | - | ||||
| Proline content | + | ||||
| SOD activity | + | ||||
| Osmotic | Sorghum bicolor | Sodium silicate | Photosynthetic responses | + | [57] |
| Root hydraulic resistance | + | ||||
| Plant dry mass | + | ||||
| Solanum lycopersicum | Potassium silicate | Leaf gas exchange | + | [50] | |
| LWC | + | ||||
| Root EC | + | ||||
| MDA, H2O2 | + | ||||
| Antioxidant enzymes | + | ||||
| Ascorbic acid content | + | ||||
| GSH activity | + | ||||
| Cd Toxicity | Oryza sativa | Sodium silicate | Total DM | + | [58] |
| H2O2 content (leaf and root) | + | ||||
| Ascorbate content (leaf & root) | + | ||||
| GSH content (leaf and root) | + | ||||
| NPT content (leaf and root) | + | ||||
| As Toxicity | Oryza sativa | Sodium silicate | Photosynthesis | + | [59] |
| gs | + | ||||
| gm | + | ||||
| Fv/Fm | no effect | ||||
| Vcmax and Jmax | ,, | ||||
| Cu Toxicity | Spartina densiflora | Potassium silicate | Shoot FM | ,, | [60] |
| Root FM and RGR | + | ||||
| Leaf gas exchange | + | ||||
| Chlorophyll content | + | ||||
| Rubisco content | + | ||||
| Mn Toxicity | Cucumis sativa | Sodium silicate | Shoot fresh and dry mass | + | [61,62] |
| Leaf Mn content | - | ||||
| H2O2 and GPx activity | + | ||||
| Al Toxicity | Zea mays | Potassium silicate | Root size | + | [63] |
| Root citrate and malate exudation | - | ||||
| Root phenol exudation | - | ||||
| K+ Deficiency | Sorghum bicolor | Sodium silicate | Total dry wt. | + | [64,65] |
| CO2 assimilation | + | ||||
| Protein content | + | ||||
| Green pigments | + | ||||
| Leaf polyamine and arginine content | + | ||||
| Antioxidant enzymes | + |
| Stress Condition | Plant/ Cultivar | Bacterial Inoculate | Reference |
|---|---|---|---|
| Salinity | Phaseolus vulgaria | Azospirillum brasilense | [66] |
| ,, | Vicia faba | Enterobacter cloacae, Bacillus drentensis | [45] |
| ,, | Zea mays | Pseudomonas syringae, P. fluorescens, Enterobacter aerogenes | [67] |
| ,, | Arachis hypogaea | P. fluorescens | [68] |
| ,, | Lactuca sativa | Azospirillum | [69] |
| ,, | Lycopersicon esculentum | Achromobacter piechaudii | [70] |
| ,, | Triticum aestivum | Aeromonas hydrophila/caviae, Bacillus insolitus, Bacillus spp. | [71] |
| ,, | Zea mays | Azospirillum | [72] |
| ,, | Cicer arietinum, Vicia faba | A. brasilense | [73] |
| ,, | Zea mays | Bacillus | [74] |
| ,, | Vicia faba, Gossypium hirsutum | Pseudomonas | [75,76] |
| Low water | Lycopersicon esculentum, Capsicum annuum | Achromobacter piechaudii | [77] |
| ,, | Triticum aestivum | Azospirillum | [78] |
| ,, | Zea mays | Azospirillum brasilense | [79] |
| ,, | Phaseolus vulgaris | Azospirillum brasilense | [80] |
| Drying soil | Pisum sativum | Variovorax paradoxus | [81] |
| Drying soil | Lactuca sativa | Bacillus | [82] |
| Osmotic stress (PEG −45%) | Capsicum annuum | Arthrobacter spp. Bacillus spp. | [83] |
| Osmotic stress (PEG −20%) in dark | Triticum aestivum | Azospirillum | [84] |
| Osmotic stress (PEG −20%) | Triticum aestivum | Azospirillum brasilense | [85] |
| Waterlogging | Lycopersicon esculentum | Enterobacter cloacae, Pseudomonas putida | [86] |
| High temperature | Vitis vinifera | Burkholderia phytofirmans | |
| High temperature | Solanum tuberosum | Burkholderia phytofirmans | [87] |
| High temperature | Glycine max | Aeromonas hydrophila, Serratia liquefaciens, Serratia proteamaculans | [88] |
| Nutrient imbalance | Zea mays | Bacillus polymyxa, Mycobacterium phlei, Pseudomonas alcaligenes | [89] |
| Iron toxicity | Oryza sativa | Bacillus subtilis, B. megaterium, Bacillus spp. | [90,91] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, K.K.; Song, X.-P.; Li, D.-M.; Singh, M.; Rajput, V.D.; Malviya, M.K.; Minkina, T.; Singh, R.K.; Singh, P.; Li, Y.-R. Interactive Role of Silicon and Plant–Rhizobacteria Mitigating Abiotic Stresses: A New Approach for Sustainable Agriculture and Climate Change. Plants 2020, 9, 1055. https://doi.org/10.3390/plants9091055
Verma KK, Song X-P, Li D-M, Singh M, Rajput VD, Malviya MK, Minkina T, Singh RK, Singh P, Li Y-R. Interactive Role of Silicon and Plant–Rhizobacteria Mitigating Abiotic Stresses: A New Approach for Sustainable Agriculture and Climate Change. Plants. 2020; 9(9):1055. https://doi.org/10.3390/plants9091055
Chicago/Turabian StyleVerma, Krishan K., Xiu-Peng Song, Dong-Mei Li, Munna Singh, Vishnu D. Rajput, Mukesh Kumar Malviya, Tatiana Minkina, Rajesh Kumar Singh, Pratiksha Singh, and Yang-Rui Li. 2020. "Interactive Role of Silicon and Plant–Rhizobacteria Mitigating Abiotic Stresses: A New Approach for Sustainable Agriculture and Climate Change" Plants 9, no. 9: 1055. https://doi.org/10.3390/plants9091055
APA StyleVerma, K. K., Song, X.-P., Li, D.-M., Singh, M., Rajput, V. D., Malviya, M. K., Minkina, T., Singh, R. K., Singh, P., & Li, Y.-R. (2020). Interactive Role of Silicon and Plant–Rhizobacteria Mitigating Abiotic Stresses: A New Approach for Sustainable Agriculture and Climate Change. Plants, 9(9), 1055. https://doi.org/10.3390/plants9091055










