First Steps in the Successful Fertilization of Rice and Arabidopsis: Pollen Longevity, Adhesion and Hydration
Abstract
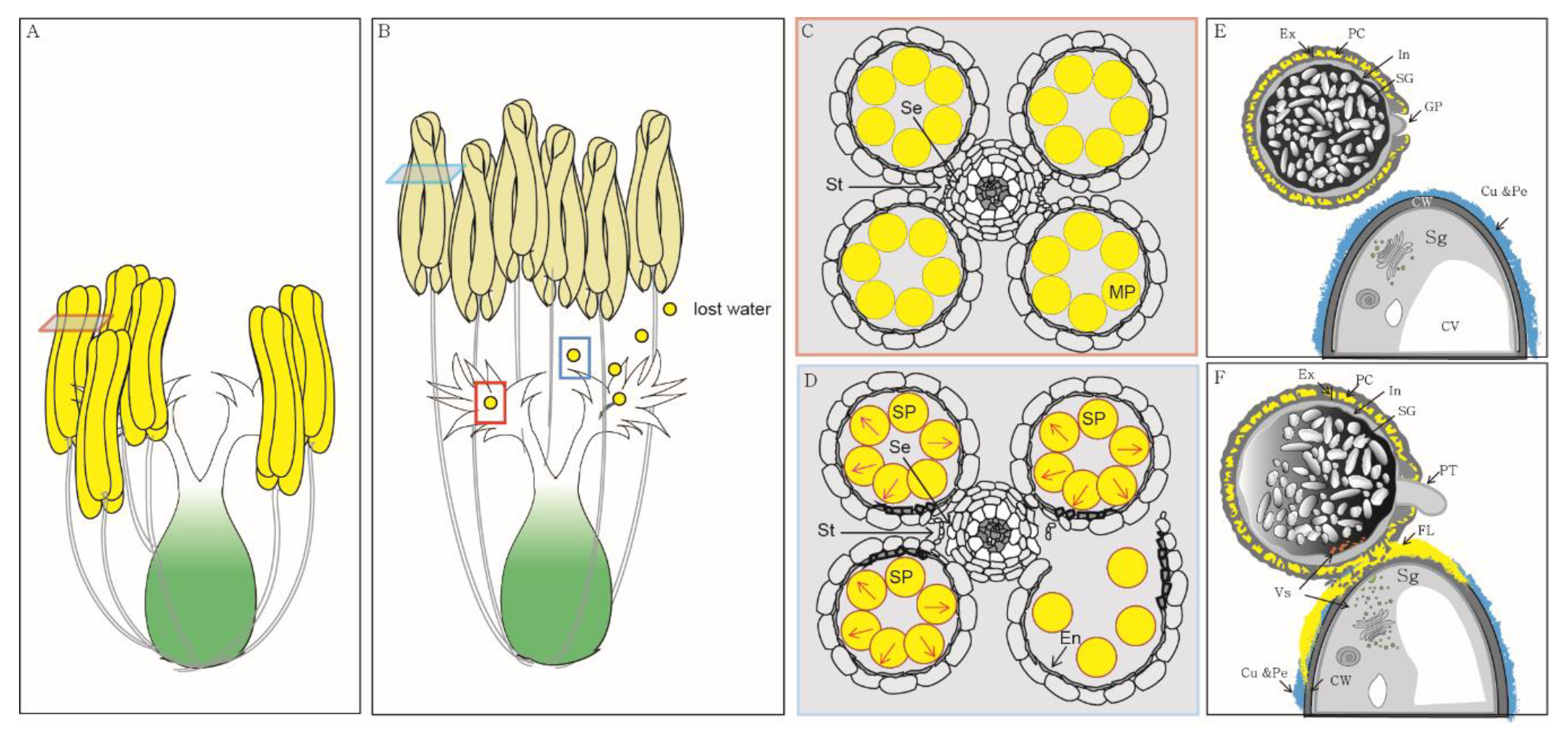
1. Introduction
2. Pollen Swelling Is a Key Event during Anther Dehiscence in Rice
3. Pollen Longevity Depends on the Structural Features of the Pollen Wall in Rice, but Not in Arabidopsis
| Stage | Species | Gene | Function | Reference |
|---|---|---|---|---|
| Pollen longevity | rice | OsGL1-4 | Involved in very-long-chain alkane biosynthesis in the pollen coat | [44] |
| HMS1 | Biosynthesis of very-long-chain fatty acids in the pollen coat and exine | [42] | ||
| OsOSC12 | Control the accumulation of fatty acids in the pollen coat | [43] | ||
| Arabidopsis | GPR1 | GTP-binding protein | [46] | |
| NMNAT | NAD biosynthesis | [45] | ||
| Pollen adhesion | Arabidopsis | LAP1 | Produces temporary callose walls between developing microspores | [47] |
| LAP3 | Contains a repetitive motif found in beta-propeller enzymes | [48] | ||
| LAP4 | Cytochrome P450 CYP703A2-involved sporopollenin synthesis | [49] | ||
| LAP5 | Chalcone synthase essential for pollen exine development | [50] | ||
| LAP6 | Chalcone synthase essential for pollen exine development | [50] | ||
| Pollen hydration | rice | MLO12 | MLO protein interacting with calmodulin in the cytosol | [51] |
| Arabidopsis | GRP17 | Oleosin-domain protein of the pollen coat | [52] | |
| EXL4 | Extracellular lipase in the pollen coat | [53] | ||
| PCP-Bs | Pollen coat protein | [54] | ||
| CER1 | Mutation causes pollen coat defect | [55] | ||
| CER3 | Mutation causes pollen coat defect | [55] | ||
| CER6 | Mutation causes pollen coat defect | [55] | ||
| KINβγ | Regulates reactive oxygen species (ROS) levels | [56] | ||
| SPIK | Transports potassium into the pollen | [57] | ||
| PME48 | Demethylesterification of homogalacturonan within the intine wall | [58] |
4. Pollen Adhesion Is Mediated by Interactions between the Pollen Wall and Stigma
5. Pollen Hydration Is a Pre-Requisite for Pollen Germination
6. Accumulated “Omics” Data are Useful Sources of the Candidate Genes for Pollen Hydration and Germination in Arabidopsis and Rice
7. Perspectives on the Research on Pollen Longevity, Adhesion and Hydration
Author Contributions
Funding
Conflicts of Interest
References
- Buitink, J.; Claessens, M.M.; Hemminga, M.A.; Hoekstra, F.A. Influence of Water Content and Temperature on Molecular Mobility and Intracellular Glasses in Seeds and Pollen. Plant Physiol. 1998, 118, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Pacini, E.; Guarnieri, M.; Nepi, M. Pollen carbohydrates and water content during development, presentation, and dispersal: A short review. Protoplasma 2006, 228, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Nepi, M.; Franchi, G.G.; Padni, E. Pollen hydration status at dispersal: Cytophysiological features and strategies. Protoplasma 2001, 216, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Firon, N.; Nepi, M.; Pacini, E. Water status and associated processes mark critical stages in pollen development and functioning. Ann. Bot. 2012, 109, 1201–1214. [Google Scholar] [CrossRef]
- Pacini, E.; Dolferus, R. Pollen Developmental Arrest: Maintaining Pollen Fertility in a World With a Changing Climate. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Fu, J.-H.; Lei, L.-G.; Chen, L.; Qiu, G.-Z. Wall ultrastructure and cytochemistry and the longevity of pollen of three grass species. Aust. J. Bot. 2001, 49, 771. [Google Scholar] [CrossRef]
- Piffanelli, P.; Ross, J.H.E.; Murphy, D.J. Biogenesis and function of the lipidic structures of pollen grains. Sex. Plant Reprod. 1998, 11, 65–80. [Google Scholar] [CrossRef]
- Ariizumi, T.; Toriyama, K. Pollen exine pattern formation is dependent on three major developmental processes in Arabidopsis thaliana. Int. J. Plant Dev. Biol. 2007, 1, 106–115. [Google Scholar]
- Kim, Y.-J.; Zhang, D.; Jung, K.-H. Molecular Basis of Pollen Germination in Cereals. Trends Plant Sci. 2019, 24, 1126–1136. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Lin, X.-J.; Liang, H.-M.; Wang, F.-F.; Chen, L.-Y. The Long Journey of Pollen Tube in the Pistil. Int. J. Mol. Sci. 2018, 19, 3529. [Google Scholar] [CrossRef]
- Zheng, R.H.; De Su, S.; Xiao, H.; Tian, H.Q. Calcium: A Critical Factor in Pollen Germination and Tube Elongation. Int. J. Mol. Sci. 2019, 20, 420. [Google Scholar] [CrossRef]
- Saminathan, T.; Guo, C.-L.; Chuang, M.-H.; Lai, M.-H.; Chen, J.; Jauh, G.-Y. Rice SIZ1, a SUMO E3 ligase, controls spikelet fertility through regulation of anther dehiscence. New Phytol. 2010, 189, 869–882. [Google Scholar] [CrossRef]
- Zhu, Q.-H.; Ramm, K.; Shivakkumar, R.; Dennis, E.S.; Upadhyaya, M.N. The ANTHER INDEHISCENCE1 Gene Encoding a Single MYB Domain Protein Is Involved in Anther Development in Rice. Plant Physiol. 2004, 135, 1514–1525. [Google Scholar] [CrossRef] [PubMed]
- Bonner, L.J.; Dickinson, H.G. Anther dehiscence in Lycopersicon esculentum Mill. I. Structural aspects. New Phytol. 1989, 113, 97–115. [Google Scholar] [CrossRef]
- Keijzer, C.J. The processes of anther dehiscence and pollen dispersal. ii. the formation and the transfer mechanism of pollenkitt, cell-wall development of the loculus tissues and a function of orbicules in pollen dispersal. New Phytol. 1987, 105, 499–507. [Google Scholar] [CrossRef]
- Wang, H.; Mao, Y.; Yang, J.; He, Y. TCP24 modulates secondary cell wall thickening and anther endothecium development. Front. Plant Sci. 2015, 6, 436. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Omasa, K.; Horie, T. Mechanism of Anther Dehiscence in Rice (Oryza sativa L.). Ann. Bot. 1999, 84, 501–506. [Google Scholar] [CrossRef]
- Xu, X.; Wang, B.; Feng, Y.-F.; Xue, J.-S.; Qian, X.-X.; Liu, S.-Q.; Zhou, J.; Yu, Y.-H.; Yang, N.-Y.; Xu, P.; et al. AUXIN RESPONSE FACTOR17 Directly Regulates MYB108 for Anther Dehiscence. Plant Physiol. 2019, 181, 645–655. [Google Scholar] [CrossRef]
- Yang, C.; Xu, Z.; Song, J.; Conner, K.; Barrena, G.V.; Wilson, Z.A. Arabidopsis MYB26/MALE STERILE35 Regulates Secondary Thickening in the Endothecium and Is Essential for Anther Dehiscence. Plant Cell 2007, 19, 534–548. [Google Scholar] [CrossRef]
- Mitsuda, N.; Seki, M.; Shinozaki, K.; Ohme-Takagi, M. The NAC Transcription Factors NST1 and NST2 of Arabidopsis Regulate Secondary Wall Thickenings and Are Required for Anther Dehiscence. Plant Cell 2005, 17, 2993–3006. [Google Scholar] [CrossRef]
- Song, S.; Chen, Y.; Liu, L.; See, Y.H.B.; Mao, C.; Gan, Y.; Yu, H. Author Correction: OsFTIP7 determines auxin-mediated anther dehiscence in rice. Nat. Plants 2018, 4, 1124. [Google Scholar] [CrossRef] [PubMed]
- Cecchetti, V.; Altamura, M.M.; Brunetti, P.; Petrocelli, V.; Falasca, G.; Ljung, K.; Costantino, P.; Cardarelli, M. Auxin controls Arabidopsis anther dehiscence by regulating endothecium lignification and jasmonic acid biosynthesis. Plant J. 2013, 74, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Cecchetti, V.; Celebrin, D.; Napoli, N.; Ghelli, R.; Brunetti, P.; Costantino, P.; Cardarelli, M. An auxin maximum in the middle layer controls stamen development and pollen maturation in Arabidopsis. New Phytol. 2016, 213, 1194–1207. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Avci, U.; Tan, L.; Zhu, X.; Glushka, J.; Pattathil, S.; Eberhard, S.; Sholes, T.; Rothstein, G.E.; Lukowitz, W.; et al. Loss of Arabidopsis GAUT12/IRX8 causes anther indehiscence and leads to reduced G lignin associated with altered matrix polysaccharide deposition. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Osakabe, Y.; Maruyama, K.; Ito, T.; Osakabe, K.; Sato, T.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Receptor-like protein kinase 2 (RPK 2) is a novel factor controlling anther development in Arabidopsis thaliana. Plant J. 2007, 50, 751–766. [Google Scholar] [CrossRef]
- Jung, K.W.; Oh, S.-I.; Kim, Y.Y.; Yoo, K.S.; Cui, M.H.; Shin, J.S. Arabidopsis histidine-containing phosphotransfer factor 4 (AHP4) negatively regulates secondary wall thickening of the anther endothecium during flowering. Mol. Cells 2008, 25, 294–300. [Google Scholar]
- Dai, S.-Y.; Hsu, W.-H.; Yang, C.-H. The Gene ANTHER DEHISCENCE REPRESSOR (ADR) Controls Male Fertility by Suppressing the ROS Accumulation and Anther Cell Wall Thickening in Arabidopsis. Sci. Rep. 2019, 9, 5112. [Google Scholar] [CrossRef]
- Jung, K.W.; Kim, Y.Y.; Yoo, K.S.; Ok, S.H.; Cui, M.H.; Jeong, B.-C.; Yoo, S.D.; Jeung, J.U.; Shin, J.S. A Cystathionine-β-Synthase Domain-Containing Protein, CBSX2, Regulates Endothecial Secondary Cell Wall Thickening in Anther Development. Plant Cell Physiol. 2012, 54, 195–208. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Jung, K.W.; Jeung, J.U.; Shin, J.S. A novel F-box protein represses endothecial secondary wall thickening for anther dehiscence in Arabidopsis thaliana. J. Plant Physiol. 2012, 169, 212–216. [Google Scholar] [CrossRef]
- Xiao, Y.-G.; Chen, Y.; Charnikhova, T.; Mulder, P.P.J.; Heijmans, J.; Hoogenboom, A.; Agalou, A.; Michel, C.; Morel, J.-B.; Dreni, L.; et al. OsJAR1 is required for JA-regulated floret opening and anther dehiscence in rice. Plant Mol. Biol. 2014, 86, 19–33. [Google Scholar] [CrossRef]
- Song, S.; Qi, T.; Huang, H.; Ren, Q.; Wu, D.; Chang, C.; Peng, W.; Liu, Y.; Peng, J.; Xie, D. The Jasmonate-ZIM Domain Proteins Interact with the R2R3-MYB Transcription Factors MYB21 and MYB24 to Affect Jasmonate-Regulated Stamen Development in Arabidopsis. Plant Cell 2011, 23, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Omasa, K.; Horie, T. Rapid Swelling of Pollen Grains in Response to Floret Opening Unfolds Anther Locules in Rice (Oryza sativa L.). Plant Prod. Sci. 1999, 2, 196–199. [Google Scholar] [CrossRef]
- Matsui, T.; Omasa, K.; Horie, T. Rapid Swelling of Pollen Grains in the Dehiscing Anther of Two-rowed Barley. (Hordeum distichum L. emend. L?). Ann. Bot. 2000, 85, 345–350. [Google Scholar] [CrossRef][Green Version]
- Koike, S.; Satake, T. Sterility caused by cooling treatment at the flowering stage in rice plants. II. The abnormal digestion of starch in pollen grain and metabolic changes in anthers following cooling treatment. Jpn. J. Crop. Sci. 1987, 56, 666–672. [Google Scholar] [CrossRef][Green Version]
- Satake, T.; Yoshida, S. High Temperature-Induced Sterility in Indica Rices at Flowering. Jpn. J. Crop. Sci. 1978, 47, 6–17. [Google Scholar] [CrossRef]
- Sato, K.; Inaba, K.; Tozawa, M. High Temperature Injury of ripening in rice plant: I. The effects of high temperature Treatments as different stages of panicle development on the ripening. Jpn. J. Crop. Sci. 1973, 42, 207–213. [Google Scholar] [CrossRef][Green Version]
- Rehman, S.; Yun, S.J. Developmental regulation of K accumulation in pollen, anthers, and papillae: Are anther dehiscence, papillae hydration, and pollen swelling leading to pollination and fertilization in barley (Hordeum vulgare L.) regulated by changes in K concentration? J. Exp. Bot. 2006, 57, 1315–1321. [Google Scholar] [CrossRef]
- Abeysekera, S.; Abeysiriwardana, D.; Dehideniyz, E. Characteristics associated with outcrossing rate of cytoplasmic male sterile CMS lines in rice under local conditions. Ann. Sri Lanka Dep. Agric. 2003, 5, 1–6. [Google Scholar]
- Virmani, S.S. Heterosis and Hybrid Rice Breeding; Springer Science and Business Media LLC: Berlin, Germany, 1994; Volume 22. [Google Scholar]
- Koga, Y.; Akihama, T.; Fujimaki, H.; Yokoo, M. Studies on the Longevity of Pollen Grains of Rice, Oriza sativa L. Cytologia 1971, 36, 104–110. [Google Scholar] [CrossRef]
- Pickert, M. In vitro germination and storage of trinucleate Arabidopsis thaliana (L.) pollen grains. Arabidopsis Inf. Serv. 1988, 26, 39–42. [Google Scholar]
- Chen, H.; Zhang, Z.; Ni, E.; Lin, J.; Peng, G.; Huang, J.; Zhu, L.; Deng, L.; Yang, F.; Luo, Q.; et al. HMS1 interacts with HMS1I to regulate very-long-chain fatty acid biosynthesis and the humidity-sensitive genic male sterility in rice (Oryza sativa). New Phytol. 2019, 225, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Xu, X.; Zhou, Y.; Wang, X.; Zhang, Y.; Liu, D.; Zhao, B.; Duan, L.; Qi, X. Deficiency of a triterpene pathway results in humidity-sensitive genic male sterility in rice. Nat. Commun. 2018, 9, 604. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, L.; Wang, T. Deficiency of very long chain alkanes biosynthesis causes humidity-sensitive male sterility via affecting pollen adhesion and hydration in rice. Plant Cell Environ. 2019, 42, 3340–3354. [Google Scholar] [CrossRef] [PubMed]
- Hashida, S.-N.; Takahashi, H.; Takahara, K.; Kawai-Yamada, M.; Kitazaki, K.; Shoji, K.; Goto, F.; Yoshihara, T.; Uchimiya, H. NAD+ Accumulation during Pollen Maturation in Arabidopsis Regulating Onset of Germination. Mol. Plant 2013, 6, 216–225. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Q.; Zhao, K.; Luo, Q.; Bao, S.; Liu, H.; Men, S. The Arabidopsis GPR1 Gene Negatively Affects Pollen Germination, Pollen Tube Growth, and Gametophyte Senescence. Int. J. Mol. Sci. 2017, 18, 1303. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.-I.; Zinkl, G.M.; Swanson, R.J.; Maruyama, D.; Preuss, D. Callose (β-1,3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol. 2005, 5, 22. [Google Scholar] [CrossRef]
- Dobritsa, A.A.; Nishikawa, S.-I.; Preuss, D.; Urbanczyk-Wochniak, E.; Sumner, L.; Hammond, A.; Carlson, A.L.; Swanson, R.J. LAP3, a novel plant protein required for pollen development, is essential for proper exine formation. Sex. Plant Reprod. 2009, 22, 167–177. [Google Scholar] [CrossRef]
- Dobritsa, A.A.; Shrestha, J.; Morant, M.; Pinot, F.; Matsuno, M.; Swanson, R.; Møller, B.L.; Preuss, D. CYP704B1 Is a Long-Chain Fatty Acid -Hydroxylase Essential for Sporopollenin Synthesis in Pollen of Arabidopsis. Plant Physiol. 2009, 151, 574–589. [Google Scholar] [CrossRef]
- Dobritsa, A.A.; Lei, Z.; Nishikawa, S.-I.; Urbanczyk-Wochniak, E.; Huhman, D.V.; Preuss, D.; Sumner, L.W. LAP5 and LAP6 Encode Anther-Specific Proteins with Similarity to Chalcone Synthase Essential for Pollen Exine Development in Arabidopsis. Plant Physiol. 2010, 153, 937–955. [Google Scholar] [CrossRef]
- Yi, J.; An, S.; An, G. OsMLO12, encoding seven transmembrane proteins, is involved with pollen hydration in rice. Plant Reprod. 2014, 27, 169–180. [Google Scholar] [CrossRef]
- Mayfield, J.A.; Preuss, D. Rapid initiation of Arabidopsis pollination requires the oleosin-domain protein GRP17. Nat. Cell Biol. 2000, 2, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Updegraff, E.P.; Zhao, F.; Preuss, D. The extracellular lipase EXL4 is required for efficient hydration of Arabidopsis pollen. Sex. Plant Reprod. 2009, 22, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Clarke, L.A.; Eason, R.J.; Parker, C.C.; Qi, B.; Scott, R.J.; Doughty, J. PCP-B class pollen coat proteins are key regulators of the hydration checkpoint in Arabidopsis thaliana pollen–stigma interactions. New Phytol. 2016, 213, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Hülskamp, M.; Kopczak, S.D.; Horejsi, T.F.; Kihl, B.K.; Pruitt, R.E. Identification of genes required for pollen-stigma recognition in Arabidopsis thaliana. Plant J. 1995, 8, 703–714. [Google Scholar] [CrossRef]
- Gao, X.-Q.; Liu, C.Z.; Li, D.D.; Zhao, T.T.; Li, F.; Na Jia, X.; Zhao, X.-Y.; Zhang, X.S. The Arabidopsis KINβγ Subunit of the SnRK1 Complex Regulates Pollen Hydration on the Stigma by Mediating the Level of Reactive Oxygen Species in Pollen. PLoS Genet. 2016, 12, e1006228. [Google Scholar] [CrossRef]
- Li, D.; Guan, H.; Liu, C.; Dong, Y.; Zhang, X.S.; Gao, X.-Q. Arabidopsis shaker pollen inward K + channel SPIK functions in SnRK1 complex-regulated pollen hydration on the stigma. J. Integr. Plant Biol. 2017, 59, 604–611. [Google Scholar] [CrossRef]
- Leroux, C.; Bouton, S.; Kiefer-Meyer, M.-C.; Fabrice, T.N.; Mareck, A.; Guénin, S.; Fournet, F.; Ringli, C.; Pelloux, J.; Driouich, A.; et al. PECTIN METHYLESTERASE48 is involved in Arabidopsis pollen grain germination. Plant Physiol. 2014, 167, 367–380. [Google Scholar] [CrossRef]
- Heslop-Harrison, Y.; Shivanna, K.R. The Receptive Surface of the Angiosperm Stigma. Ann. Bot. 1977, 41, 1233–1258. [Google Scholar] [CrossRef]
- Heslop-Harrison, J.; Linskens, H.F. Cellular interaction: A brief conspectus. In Cellular Interactions; Springer Science and Business Media LLC: Berlin, Germany, 1984; pp. 2–17. [Google Scholar]
- Edlund, A.F.; Swanson, R.; Preuss, D. Pollen and Stigma Structure and Function: The Role of Diversity in Pollination. Plant Cell 2004, 16, S84–S97. [Google Scholar] [CrossRef]
- Morant, M.; Jørgensen, K.; Schaller, H.; Pinot, F.; Møller, B.L.; Werck-Reichhart, D.; Bak, S. CYP703 Is an Ancient Cytochrome P450 in Land Plants Catalyzing in-Chain Hydroxylation of Lauric Acid to Provide Building Blocks for Sporopollenin Synthesis in Pollen. Plant Cell 2007, 19, 1473–1487. [Google Scholar] [CrossRef]
- Wheeler, M.J.; Franklin-Tong, V.E.; Franklin, F.C.H. The molecular and genetic basis of pollen-pistil interactions. New Phytol. 2001, 151, 565–584. [Google Scholar] [CrossRef]
- Zinkl, G.M.; Zwiebel, B.I.; Grier, D.G.; Preuss, D. Pollen-stigma adhesion in Arabidopsis: A species-specific interaction mediated by lipophilic molecules in the pollen exine. Development 1999, 126, 5431–5440. [Google Scholar]
- Chapman, L.A.; Goring, D.R. Pollen-pistil interactions regulating successful fertilization in the Brassicaceae. J. Exp. Bot. 2010, 61, 1987–1999. [Google Scholar] [CrossRef] [PubMed]
- Elleman, C.J.; Dickinson, H.G. Identification of pollen components regulating pollination-specific responses in the stigmatic papillae of Brassica oleracea. New Phytol. 1996, 133, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Olsen, K. Ultrastructural Changes During Pollen Wall Development and Germination in Arabidopsis Thalaiana. Theses and Dissertations. 1396. Available online: https://dc.uwm.edu/etd/1396 (accessed on 5 May 2020).
- Chen, S.-Q.; Zhong, W.; Liu, M.-X.; Xie, Z.-W.; Wang, H.-H. Pollen Grain Germination and Pollen Tube Growth in Pistil of Rice. Rice Sci. 2008, 15, 125–130. [Google Scholar] [CrossRef]
- Doucet, J.; Lee, H.K.; Goring, D.R. Pollen Acceptance or Rejection: A Tale of Two Pathways. Trends Plant Sci. 2016, 21, 1058–1067. [Google Scholar] [CrossRef]
- Safavian, D.; Goring, D.R. Secretory Activity Is Rapidly Induced in Stigmatic Papillae by Compatible Pollen, but Inhibited for Self-Incompatible Pollen in the Brassicaceae. PLoS ONE 2013, 8, e84286. [Google Scholar] [CrossRef]
- Moon, S.; Oo, M.M.; Kim, B.; Koh, H.-J.; Oh, S.A.; Yi, G.; An, G.; Park, S.K.; Jung, K.-H. Genome-wide analyses of late pollen-preferred genes conserved in various rice cultivars and functional identification of a gene involved in the key processes of late pollen development. Rice 2018, 11, 28. [Google Scholar] [CrossRef]
- Peng, H.; Chun, J.; Ai, T.-B.; Tong, Y.-A.; Zhang, R.; Zhao, M.-M.; Chen, F.; Wang, S. MicroRNA profiles and their control of male gametophyte development in rice. Plant Mol. Biol. 2012, 80, 85–102. [Google Scholar] [CrossRef]
- Wei, L.Q.; Xu, W.Y.; Deng, Z.Y.; Su, Z.; Xue, Y.; Wang, T. Genome-scale analysis and comparison of gene expression profiles in developing and germinated pollen in Oryza sativa. BMC Genom. 2010, 11, 338. [Google Scholar] [CrossRef]
- Russell, S.D.; Bhalla, P.L.; Singh, M.B. Transcriptome-Based Examination of Putative Pollen Allergens of Rice (Oryza sativa ssp. japonica). Mol. Plant 2008, 1, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.Q.; Yan, L.F.; Wang, T. Deep sequencing on genome-wide scale reveals the unique composition and expression patterns of microRNAs in developing pollen of Oryza sativa. Genome Biol. 2011, 12, R53. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Chen, T.; Chong, K.; Xue, Y.; Liu, S.; Wang, T. Proteomics Identification of Differentially Expressed Proteins Associated with Pollen Germination and Tube Growth Reveals Characteristics of GerminatedOryza sativaPollen. Mol. Cell. Proteom. 2006, 6, 207–230. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Li, L.; Chen, T.; Chong, K.; Xue, Y.; Wang, T. Proteomic analyses ofOryza sativa mature pollen reveal novel proteins associated with pollen germination and tube growth. Proteomics 2006, 6, 2504–2529. [Google Scholar] [CrossRef]
- Yang, N.; Wang, T. Comparative proteomic analysis reveals a dynamic pollen plasma membrane protein map and the membrane landscape of receptor-like kinases and transporters important for pollen tube growth and interaction with pistils in rice. BMC Plant Boil. 2017, 17, 2. [Google Scholar] [CrossRef]
- Loraine, A.E.; McCormick, S.; Estrada, A.; Patel, K.; Qin, P. RNA-Seq of Arabidopsis Pollen Uncovers Novel Transcription and Alternative Splicing. Plant Physiol. 2013, 162, 1092–1109. [Google Scholar] [CrossRef]
- Qin, Y.; Leydon, A.R.; Manziello, A.; Pandey, R.; Mount, D.; Denic, S.; Vasic, B.; Johnson, M.A.; Palanivelu, R. Penetration of the Stigma and Style Elicits a Novel Transcriptome in Pollen Tubes, Pointing to Genes Critical for Growth in a Pistil. PLoS Genet. 2009, 5, e1000621. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.-Z.; Song, L.-F.; Zou, J.-J.; Su, Z.; Wu, W.-H. Transcriptome Analyses Show Changes in Gene Expression to Accompany Pollen Germination and Tube Growth in Arabidopsis. Plant Physiol. 2008, 148, 1201–1211. [Google Scholar] [CrossRef]
- Honys, D.; Twell, D. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Boil. 2004, 5, R85. [Google Scholar] [CrossRef]
- Ge, W.; Song, Y.; Zhang, C.; Zhang, Y.; Burlingame, A.L.; Guo, Y. Proteomic analyses of apoplastic proteins from germinating Arabidopsis thaliana pollen. Biochim. Biophys. Acta (BBA) - Proteins Proteom. 2011, 1814, 1964–1973. [Google Scholar] [CrossRef]
- Zou, J.; Song, L.; Zhang, W.; Wang, Y.; Ruan, S.; Wu, W.-H. Comparative Proteomic Analysis ofArabidopsisMature Pollen and Germinated Pollen. J. Integr. Plant Biol. 2009, 51, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Grobei, M.A.; Qeli, E.; Brunner, E.; Rehrauer, H.; Zhang, R.; Roschitzki, B.; Basler, K.; Ahrens, C.H.; Grossniklaus, U. Deterministic protein inference for shotgun proteomics data provides new insights into Arabidopsis pollen development and function. Genome Res. 2009, 19, 1786–1800. [Google Scholar] [CrossRef] [PubMed]
- Clough, E.; Barrett, T. The Gene Expression Omnibus Database. Methods Mol. Biol. 2016, 1418, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, H.; Sarkans, U.; Kolesnikov, N.; Abeygunawardena, N.; Burdett, T.; Dylag, M.; Emam, I.; Farne, A.; Hastings, E.; Holloway, E.; et al. ArrayExpress update—An archive of microarray and high-throughput sequencing-based functional genomics experiments. Nucleic Acids Res. 2011, 39, D1002–D1004. [Google Scholar] [CrossRef]
| Species | Omics Type | Accession No. a | Samples | Reference |
|---|---|---|---|---|
| Rice | Transcriptome (microarray) | Unicellular microspore, bicellular pollen, tricellular pollen, mature pollen and germinated pollen | [71] | |
| Transcriptome (microarray) | GSE29080 | Unicellular microspore, bicellular pollen and tricellular pollen | [72] | |
| Transcriptome (microarray) | GSE27988 | Unicellular microspore, bicellular pollen, tricellular pollen, mature pollen and germinated pollen | [73] | |
| Transcriptome (microarray) | GSE17002 | Mature pollen | [74] | |
| Transcriptome (RNA-Seq) | Unicellular microspore, bicellular pollen and tricellular pollen | [75] | ||
| Proteome | Mature pollen and germinated pollen | [76,77] | ||
| Proteome | Germinated pollen | [78] | ||
| Transcriptome (RNA-Seq) | SRP022162 | Mature pollen | [79] | |
| Arabidopsis | Transcriptome (microarray) | GSE17343 | Pollen grains (MP), germinated pollen and pollen tubes from cut pistil explants | [80] |
| Transcriptome (microarray) | GSE6696 | Pollen grains (MP), hydrated pollen grains and growing pollen tubes (PT) | [81] | |
| Transcriptome (microarray) | Mature pollen | [82] | ||
| Proteome | Mature pollen and germinated pollen | [83] | ||
| Proteome | Mature pollen and pollen tube | [84] | ||
| Proteome | Mature pollen | [85] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.; Jung, K.-H. First Steps in the Successful Fertilization of Rice and Arabidopsis: Pollen Longevity, Adhesion and Hydration. Plants 2020, 9, 956. https://doi.org/10.3390/plants9080956
Moon S, Jung K-H. First Steps in the Successful Fertilization of Rice and Arabidopsis: Pollen Longevity, Adhesion and Hydration. Plants. 2020; 9(8):956. https://doi.org/10.3390/plants9080956
Chicago/Turabian StyleMoon, Sunok, and Ki-Hong Jung. 2020. "First Steps in the Successful Fertilization of Rice and Arabidopsis: Pollen Longevity, Adhesion and Hydration" Plants 9, no. 8: 956. https://doi.org/10.3390/plants9080956
APA StyleMoon, S., & Jung, K.-H. (2020). First Steps in the Successful Fertilization of Rice and Arabidopsis: Pollen Longevity, Adhesion and Hydration. Plants, 9(8), 956. https://doi.org/10.3390/plants9080956





