1. Introduction
Increased human migration, trade, and climate change are significant factors contributing to the spread of plants beyond their natural boundaries [
1,
2,
3]. Some introduced plants survive, spread, and become invasive in new habitats. A variety of factors contribute to the success of invasive plants in their new environment, including biogeographic affinity between their native and invasive range, rapid and high reproductive outputs [
4], rapid growth and high-stress tolerance [
5,
6], lack of specialist natural enemies [
7], high phenotypic plasticity [
8,
9,
10], the ability to release phytotoxic compounds into the environment [
11], and the potential to rob native plants of their mutualists [
12]. The threats posed by exotic invasive plants have gained much attention in recent years, with loss of biodiversity often associated with plant invasion [
13,
14].
Invasive plants change the vegetation structure and composition of their new habitats through direct competition or modification of the environment [
15]. Arthropod communities are vulnerable to these changes due to the impact of microclimatic factors on their development and their close interaction with plants. Several studies report a significant decrease in arthropod diversity and abundance in response to plant invasions, as reviewed by Litt and colleagues [
15], and others suggest that arthropod assemblages could be restored when invasive plants are eradicated [
16,
17,
18]. However, arthropods fill diverse niches and ecological roles, and their responses to plant invasion may vary. For instance, some invasive plants may attract pollinators [
19], provide alternative resources for generalist herbivores, or create favourable conditions for predators and decomposers [
15]. It is therefore important to explore changes in arthropod community composition in different invasion scenarios through the seasons, using a range of sampling techniques to avoid faulty generalisation.
In New Zealand, Tongariro National Park lies within the Central North Island’s Volcanic Plateau, an area originally covered by subalpine shrubland, tussockland, and montane
Nothofagus and
Libocedrus forests. Volcanic activity and forest loss due to burning have created large areas of tussockland, where only a few woody perennials like
Dracophyllum (
Dracophyllum subulatum) and mānuka (
Leptospermum scoparium) persist [
20]. However, this ecosystem is threatened by the spread of exotic invasive weeds including heather (
Calluna vulgaris) and broom (
Cytisus scoparius), both of European origin. Heather was deliberately planted in the Tongariro National Park by European settlers in 1912 [
21] and is now the most widespread weed in the area, while the broom invasion began in the 1960s [
22]. Both the native and invasive species are adapted to these free-draining volcanic ash soils of low fertility. In addition, large temperature extremes and varying rainfall conditions prevail [
23,
24,
25].
Few studies have reported the impact of invasive plants on the surrounding flora and fauna in this ecosystem. A previous study found that plant communities dominated by tussock and other grasses were particularly vulnerable to heather invasion due to the invasive plant’s ability to germinate in inter-tussock spaces, its rapid vegetative growth, and environmental factors such as infertile soils associated with these communities [
20]. Another study [
16] reported displacement of native vegetation in heather-invaded sites on the Central Plateau and the author found that specialised phytophagous arthropods were negatively affected by the invasion, possibly due to a reduction in food availability, habitat loss and increased abundance of predators. The response of arthropod communities to broom invasion on the Central Plateau is not well documented. However, studies in other parts of New Zealand report an increase in generalist phytophages in broom-invaded areas [
26,
27]. This suggests that both heather and broom could have different effects on the composition of arthropod assemblages in this area.
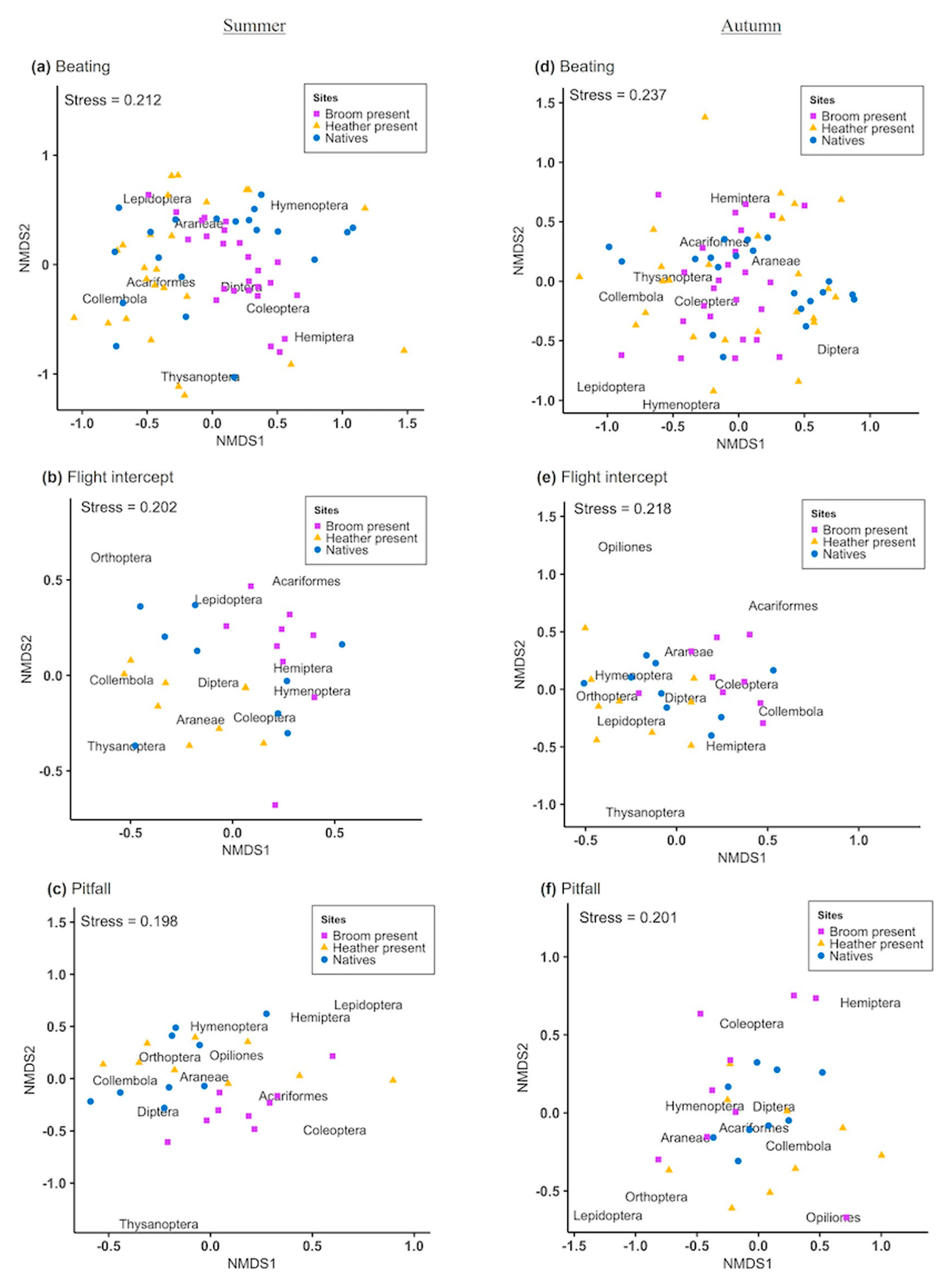
This study aimed to establish the effects of two invasive species (heather and broom) on arthropod assemblages on the Central Plateau, North Island, New Zealand. Three different sampling methods were used; pitfall traps, flight intercept traps, and a beating tray [
28]. Samples were collected from ten sites in summer and autumn, where invasive plants were either present (invaded) or absent (non-invaded) and occurring with different combinations of neighbouring plants. We first explored the differences in diversity (Richness, Simpson’s index, and Shannon’s index) and community composition at the order level for each sampling method for weed-invaded and non-invaded sites. We then used only the samples collected by the beating tray method to explore further the effect of different plant pairings on arthropod diversity and community composition. We predicted that the impact of invasive species on arthropod communities would vary depending on the invader, plant combination, and season. This study provides updated information on arthropod community composition in the region and a better understanding of the impacts of invasive plants on arthropods that will assist conservation efforts.
3. Discussion
Exotic plant invasion modifies vegetation structure and leads to a shift in plant species composition in the new habitat [
29]. This may be detrimental for other community groups like arthropods that rely on surrounding native vegetation for food, shelter, and reproduction sites. Our results demonstrate that during plant invasion, arthropod assemblages are affected differently depending on the invading species. Broom typically increased arthropod abundance, while heather was associated with a reduction in arthropod abundance. Plant-arthropod associations were also affected by the identity of neighbouring plants, and these effects varied in summer and autumn.
Our results only partially support the often-reported observation that arthropod abundance and diversity are decreased in habitats dominated by exotic weeds [
15], but rather indicate that the responses of arthropod communities depend on the identity of the invasive plant. While invasive plants may indeed reduce resources for specialist herbivores, arthropod groups occupying other, more generalist, ecological niches may thrive in these environments [
15].
In this study, we found a high number of Acariformes (mostly detritivore oribatid mites) and Coleoptera (mostly silken fungus beetles) associated with broom in pitfall traps, while only small numbers of these groups were associated with heather. This is an example of how different invasive plants can provide different resources. Here, decaying vegetation under broom is creating optimal habitats for detritivores and fungivores [
30,
31].
In the flight intercept traps, Hemiptera, Coleoptera, Araneae, Acariformes, and Diptera were significantly more abundant in broom-infested sites in summer and autumn. Meanwhile, heather had less pronounced effects with only a substantial increase in Thysanoptera (thrips) and Araneae in summer and Orthoptera during autumn. Thrips are well-known generalist florivores and have been suggested to contribute to heather pollination in other ecosystems [
32]. Many invasives have large floral displays or many flowers that can attract generalist pollinators and florivores [
33]. It is, therefore, reasonable to assume that heather attracts native florivores which aid in its reproduction and dispersal, but no hard evidence was found that Hymenoptera (in particular native pollinators) captured by this method were significantly impacted by the presence of heather or broom.
The high numbers of Hemiptera found at the broom-invaded sites were predominantly exotic broom psyllids that were introduced from England to control the spread of broom in New Zealand [
34]. In comparison, Coleoptera and Orthoptera were predominantly native generalist herbivores that would likely use invasive plants as an alternative food source. Araneae and some Diptera are predators or parasitoids of herbivores; thus, their increase may be explained by higher abundance of their prey species [
35]. Certain plant architectures, such as the intricate and dense branching pattern of heather, can also create suitable habitats for spiders and other predators [
16,
36].
The composition of arthropods collected by the beating tray method showed a similar trend as that of flight intercept traps at broom-invaded sites but with higher abundance of Hemiptera, Coleoptera, Thysanoptera, and Acariformes. Contrary to this, a reduction in the abundance of some arthropod groups (Coleoptera, Diptera, Thysanoptera, and Orthoptera) was observed at the heather-invaded sites in samples collected by the beating tray method compared with the flight intercept traps. It is relevant to note that many Coleoptera caught by beating the foliage of mānuka were mānuka beetles, which are endemic insects typically associated with this plant. However, mānuka beetles have been reported to feed on other plants, and they are considered to be pasture pests in some regions [
37], supporting our previous observation that some native insects feed on both native and exotic invasive plants [
38].
An earlier study assessing the impact of invasion by heather on native invertebrates on the Central Plateau also showed some variation in invertebrate assemblages [
16]. Consistent with our findings, the author found that invasion by heather was usually associated with fewer plant-feeders, high abundance of thrips (pollen eaters), and increased predators [
16]. A comparative study of the arthropods associated with broom in two native (France and Scotland) and non-native (New Zealand and Australia) ranges [
27], using the beating tray method, found generalist phytophages to be dominant on broom in exotic habitats and specialists dominant on broom in the native habitats. Thus, the overall abundance of arthropods was high but not significantly different between the two habitats. We also found high arthropod abundance in broom-invaded sites for several groups, suggesting that not only generalist herbivores, but arthropods occupying other niches, benefit from the resources provided by this invasive species. Generally, mechanisms promoting such facilitative interaction between invasive plants and arthropods include habitat modification, diversification of food source, and availability of exploitable hosts [
39].
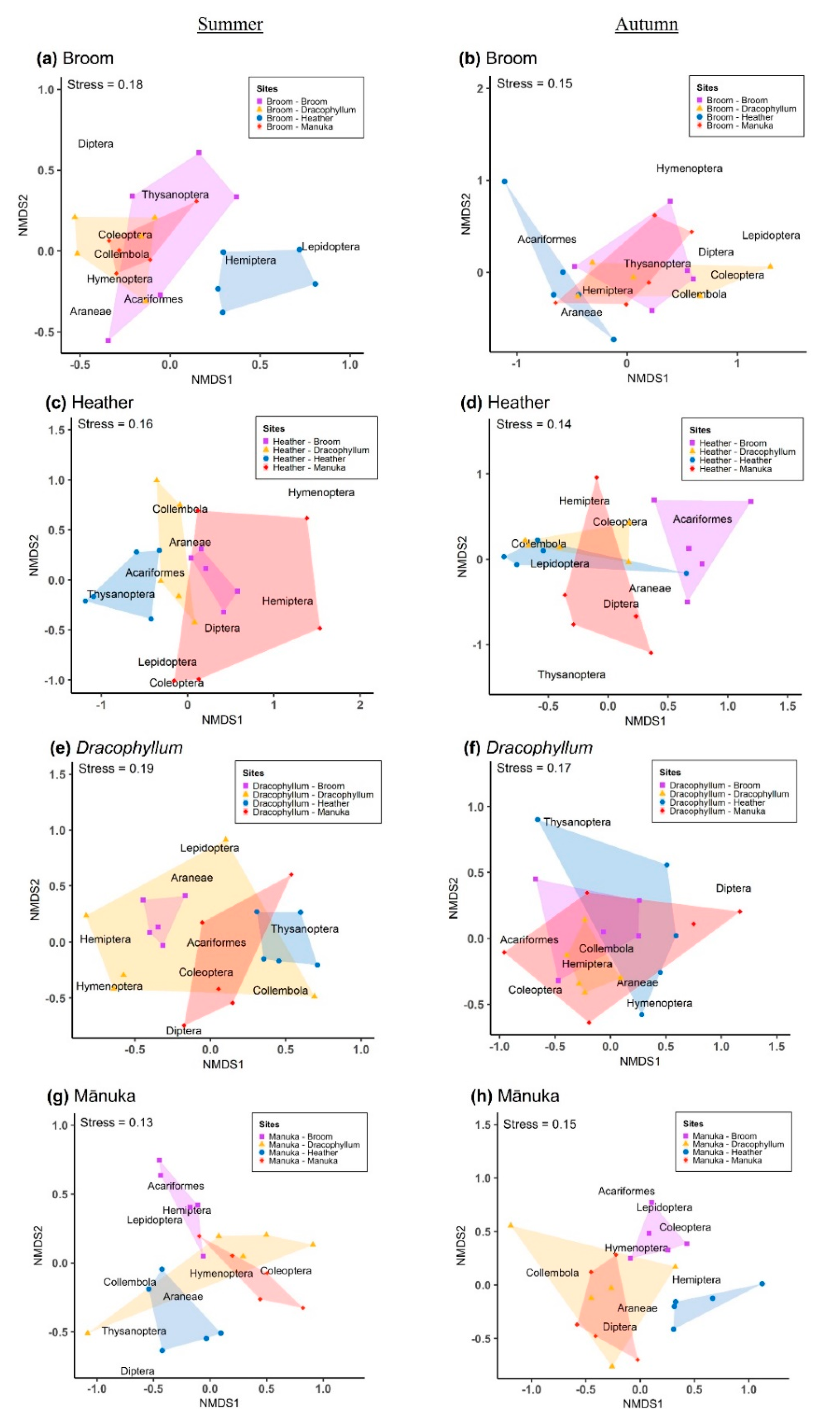
Plant species composition can be used as a predictor of arthropod assemblages, as revealed by a study using multiple sites with different levels of vegetation cover and a range of sampling methods [
40]. However, that study revealed that this is not necessarily due to the direct use of particular plants as a resource, but their correlation with some other factors (i.e., microclimate, habitat structure, changes in trophic webs). Our results strongly support the hypothesis that it is plant community composition, rather than the presence of invaders only, that is a strong driver of change in arthropod assemblages. We found significant differences in arthropod community composition between sites where the same invasive was present but in combination with other species, and the same was true for native plants.
Overall, our results highlight that it is difficult to generalise when considering the impacts of invasive plants on arthropod communities, and that sampling multiple sites with different plant assemblages, using a variety of different trapping methods over multiple seasons, is needed to elucidate the complex effects of invasive plants on arthropod communities and their associated ecosystem services. Further studies investigating lower taxonomic levels, focusing on native and endemic arthropods and with a multitrophic approach [
41], will be of great assistance to expand these findings and support conservation efforts.