Eukaryotic and Prokaryotic Phytochelatin Synthases Differ Less in Functional Terms Than Previously Thought: A Comparative Analysis of Marchantia polymorpha and Geitlerinema sp. PCC 7407
Abstract
1. Introduction
2. Results
2.1. Phytochelatin Synthases from M. polymorpha and Geitlerinema sp. PCC 7407 are Constitutively Expressed and Produce Phytochelatins in vitro
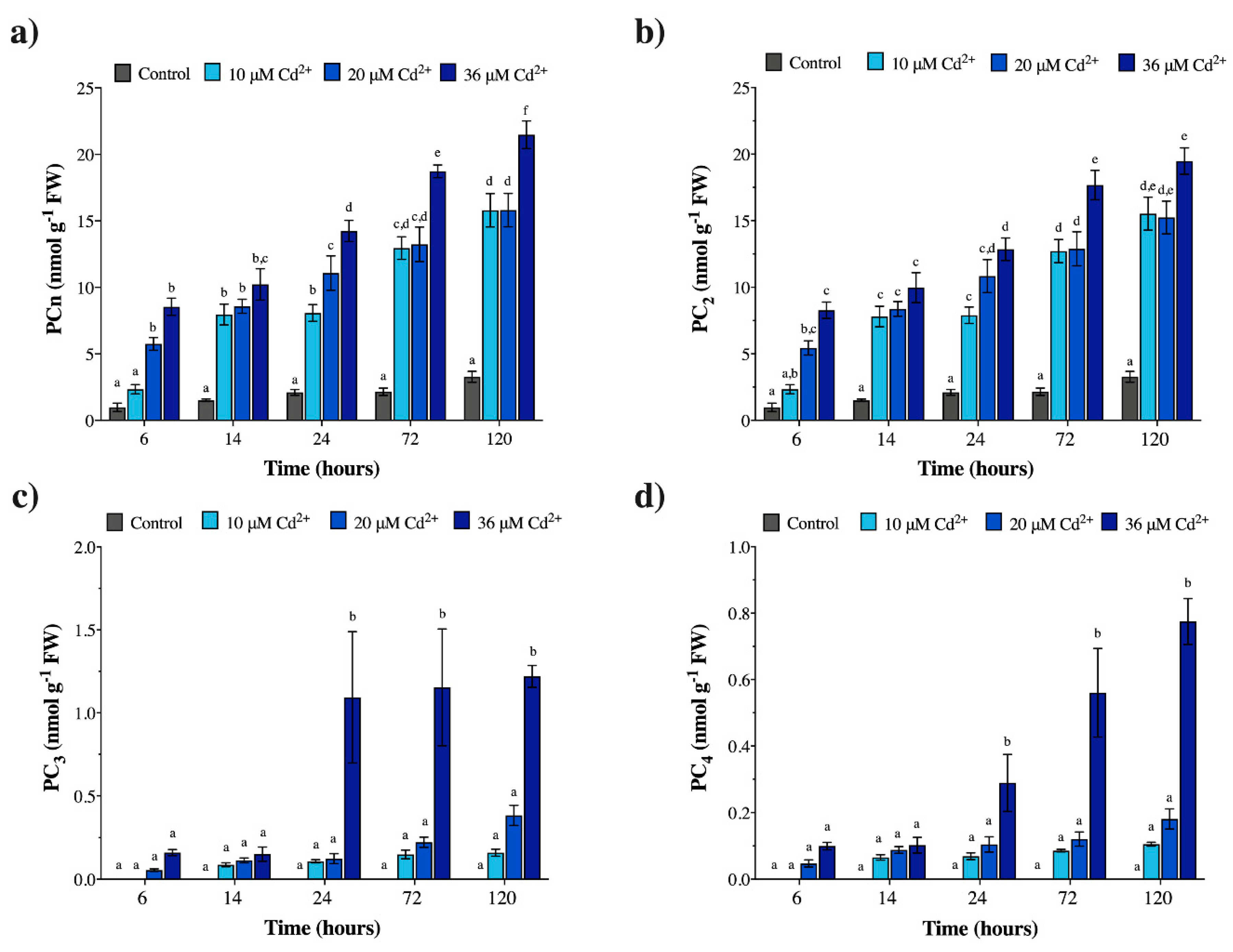
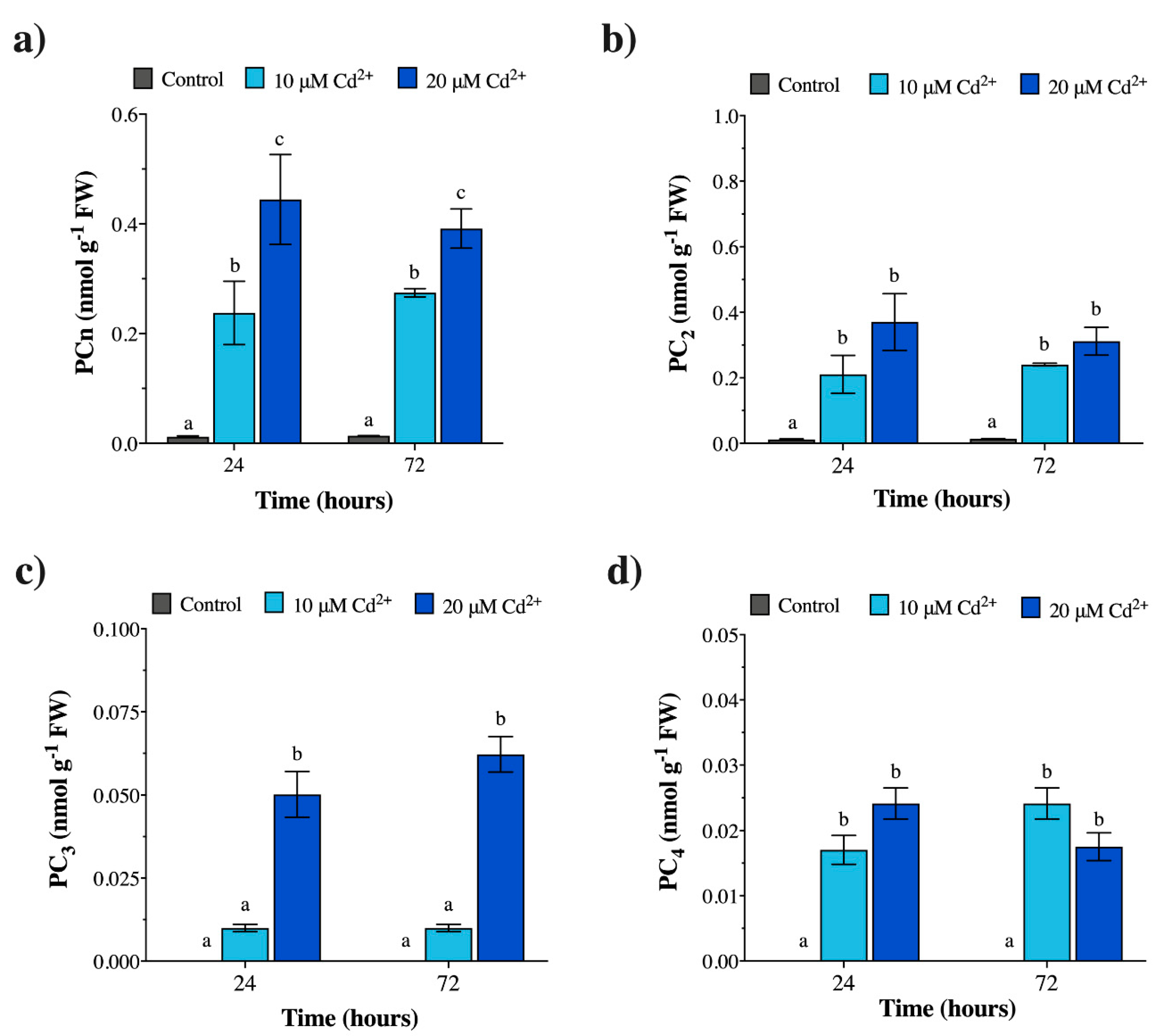
2.2. Cadmium Induces Phytochelatin Synthesis in vivo both in M. polymorpha and Geitlerinema sp. PCC 7407
2.3. Cadmium Exposure Increases GSH Levels in M. polymorpha, but Causes the Opposite Effect in Geitlerinema sp. PCC 7407
3. Discussion
4. Materials and Methods
4.1. Growth Conditions and Experimental Set-Up
4.2. Phytochelatin Synthase Activity Assays
4.3. Extraction, Detection, and Quantification of Thiol-Peptides
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vivares, D.; Arnoux, P.; Pignol, D. A papain-like enzyme at work: Native and acyl-enzyme intermediate structures in phytochelatin synthesis. PNAS 2005, 102, 18848–18853. [Google Scholar] [CrossRef]
- Rea, P.A. Phytochelatin synthase: Of a protease a peptide polymerase made. Physiol. Plant. 2012, 145, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Peršoh, D. Multi-tasking phytochelatin synthases. Plant Sci. 2009, 177, 266–271. [Google Scholar] [CrossRef]
- Petraglia, A.; De Benedictis, M.; Degola, F.; Pastore, G.; Calcagno, M.; Ruotolo, R.; Mengoni, A.; Sanità di Toppi, L. The capability to synthesize phytochelatins and the presence of constitutive and functional phytochelatin synthases are ancestral (plesiomorphic) characters for basal land plants. J. Exp. Bot. 2014, 65, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Perales-Vela, H.V.; Peña-Castro, J.M.; Cañizares-Villanueva, R.O. Heavy metal detoxification in eukaryotic microalgae. Chemosphere 2006, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gupton-Campolongo, T.; Damasceno, L.M.; Hay, A.G.; Ahner, B.A. Characterization of a High Affinity Phytochelatin Synthase From The Cd-Utilizing Marine Diatom Thalassiosira pseudonana. J. Phycol. 2013, 49, 32–40. [Google Scholar] [CrossRef]
- Fontanini, D.; Andreucci, A.; Ruffini Castiglione, M.; Basile, A.; Sorbo, S.; Petraglia, A.; Degola, F.; Bellini, E.; Bruno, L.; Varotto, C.; et al. The phytochelatin synthase from Nitella mucronata (Charophyta) plays a role in the homeostatic control of iron(II)/(III). Plant Physiol. Biochem. 2018, 127, 88–96. [Google Scholar] [CrossRef]
- Pawlik-Skowrońska, B.; Toppi, L.S.D.; Favali, M.A.; Fossati, F.; Pirszel, J.; Skowroński, T. Lichens respond to heavy metals by phytochelatin synthesis. New Phytol. 2002, 156, 95–102. [Google Scholar] [CrossRef]
- Bundy, J.G.; Kille, P. Metabolites and metals in Metazoa—What role do phytochelatins play in animals? Metallomics 2014, 6, 1576–1582. [Google Scholar] [CrossRef]
- García-García, J.D.; Girard, L.; Hernández, G.; Saavedra, E.; Pardo, J.P.; Rodríguez-Zavala, J.S.; Encalada, R.; Reyes-Prieto, A.; Mendoza-Cózatl, D.G.; Moreno-Sánchez, R. Zn-bis-glutathionate is the best co-substrate of the monomeric phytochelatin synthase from the photosynthetic heavy metal-hyperaccumulator Euglena gracilis. Metallomics 2014, 6, 604–616. [Google Scholar] [CrossRef]
- Grill, E.; Winnacker, E.-L.; Zenk, M.H. Phytochelatins: The principal heavy-metal complexing peptides of higher plants. Science 1985, 230, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, N.; Nishikori, S.; Iwabe, O.; Shiraki, K.; Miyasaka, H.; Takagi, M.; Hirata, K.; Miyamoto, K. Characterization of phytochelatin synthase-like protein encoded by alr0975 from a prokaryote, Nostoc sp. PCC 7120. Biochem. Biophys. Res. Commun. 2004, 315, 751–755. [Google Scholar] [CrossRef]
- Beck, A.; Lendzian, K.; Oven, M.; Christmann, A.; Grill, E. Phytochelatin synthase catalyzes key step in turnover of glutathione conjugates. Phytochemistry 2003, 62, 423–431. [Google Scholar] [CrossRef]
- Harada, E.; Vonroepenacklahaye, E.; Clemens, S. A cyanobacterial protein with similarity to phytochelatin synthases catalyzes the conversion of glutathione to γ-glutamylcysteine and lacks phytochelatin synthase activity. Phytochemistry 2004, 65, 3179–3185. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, N.; Nishikori, S.; Iwabe, O.; Matsumoto, S.; Shiraki, K.; Miyasaka, H.; Takagi, M.; Miyamoto, K.; Hirata, K. Comparative analysis of the two-step reaction catalyzed by prokaryotic and eukaryotic phytochelatin synthase by an ion-pair liquid chromatography assay. Planta 2005, 222, 181–191. [Google Scholar] [CrossRef]
- Olsson, S.; Penacho, V.; Puente-Sánchez, F.; Díaz, S.; Gonzalez-Pastor, J.E.; Aguilera, A. Horizontal gene transfer of phytochelatin synthases from bacteria to extremophilic green algae. Microb. Ecol. 2017, 73, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Archibald, J.M. Endosymbiosis and eukaryotic cell evolution. Curr. Biol. 2015, 25, R911–R921. [Google Scholar] [CrossRef]
- Qiu, Y.-L.; Li, L.; Wang, B.; Chen, Z.; Knoop, V.; Groth-Malonek, M.; Dombrovska, O.; Lee, J.; Kent, L.; Rest, J.; et al. The deepest divergences in land plants inferred from phylogenomic evidence. Proc. Natl. Acad. Sci. USA 2006, 103, 15511–15516. [Google Scholar] [CrossRef]
- Ligrone, R.; Duckett, J.G.; Renzaglia, K.S. Major transitions in the evolution of early land plants: A bryological perspective. Ann. Bot. 2012, 109, 851–871. [Google Scholar] [CrossRef]
- Shih, P.M.; Wu, D.; Latifi, A.; Axen, S.D.; Fewer, D.P.; Talla, E.; Calteau, A.; Cai, F.; Tandeau de Marsac, N.; Rippka, R.; et al. Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc. Natl. Acad. Sci. USA 2013, 110, 1053–1058. [Google Scholar] [CrossRef]
- Boyer, S.L.; Johansen, J.R.; Flechtner, V.R.; Howard, G.L. Phylogeny and genetic variance in terrestrial Microcoleus (Cyanophyceae) species based on sequence analysis of the 16S rRNA gene and associated 16S-23S ITS region. J. Phycol. 2002, 38, 1222–1235. [Google Scholar] [CrossRef]
- Meeks, J.C. Cyanobacterial-bryophyte associations. In CRC Handbook of Symbiotic Cyanobacteria; Rai, A.N., Ed.; CRC Press: Boca Raton, FL, USA, 1990; ISBN 978-1-351-08808-4. [Google Scholar]
- Shimamura, M. Marchantia polymorpha: Taxonomy, phylogeny and morphology of a model system. Plant. Cell Physiol. 2016, 57, 230–256. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.L.; Kohchi, T.; Yamato, K.T.; Jenkins, J.; Shu, S.; Ishizaki, K.; Yamaoka, S.; Nishihama, R.; Nakamura, Y.; Berger, F.; et al. Insights into land plant evolution darnered from the Marchantia polymorpha genome. Cell 2017, 171, 287–304.e15. [Google Scholar] [CrossRef] [PubMed]
- Kubota, A.; Ishizaki, K.; Hosaka, M.; Kohchi, T. Efficient Agrobacterium-mediated transformation of the liverwort Marchantia polymorpha using regenerating thalli. Biosci. Biotechnol. Biochem. 2013, 77, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. Marchantia polymorpha L.: A bioaccumulator. Aerobiologia 2007, 23, 181–187. [Google Scholar] [CrossRef]
- Ares, Á.; Itouga, M.; Kato, Y.; Sakakibara, H. Differential metal tolerance and accumulation patterns of Cd, Cu, Pb and Zn in the liverwort Marchantia polymorpha L. Bull. Environ. Contam. Toxicol. 2018, 100, 444–450. [Google Scholar] [CrossRef]
- Hendrix, S.; Jozefczak, M.; Wójcik, M.; Deckers, J.; Vangronsveld, J.; Cuypers, A. Glutathione: A key player in metal chelation, nutrient homeostasis, cell cycle regulation and the DNA damage response in cadmium-exposed Arabidopsis thaliana. Plant. Physiol. Biochem. 2020, 154, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Degola, F.; De Benedictis, M.; Petraglia, A.; Massimi, A.; Fattorini, L.; Sorbo, S.; Basile, A.; Sanità di Toppi, L. A Cd/Fe/Zn-responsive phytochelatin synthase is constitutively present in the ancient liverwort Lunularia cruciata (L.) Dumort. Plant Cell Physiol. 2014, 55, 1884–1891. [Google Scholar] [CrossRef]
- Bellini, E.; Borsò, M.; Betti, C.; Bruno, L.; Andreucci, A.; Ruffini Castiglione, M.; Saba, A.; Sanità di Toppi, L. Characterization and quantification of thiol-peptides in Arabidopsis thaliana using combined dilution and high sensitivity HPLC-ESI-MS-MS. Phytochemistry 2019, 164, 215–222. [Google Scholar] [CrossRef]
- Kneer, R.; Zenk, M.H. The formation of Cd-phytochelatin complexes in plant cell cultures. Phytochemistry 1997, 44, 69–74. [Google Scholar] [CrossRef]
- Sanità di Toppi, L.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Grill, E.; Winnacker, E.-L.; Zenk, M.H. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc. Natl. Acad. Sci. USA 1987, 84, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Vögeli-Lange, R.; Wagner, G.J. Relationship between cadmium, glutathione and cadmium-binding peptides (phytochelatins) in leaves of intact tobacco seedlings. Plant. Sci. 1996, 114, 11–18. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2008, 11, 861–905. [Google Scholar] [CrossRef] [PubMed]
- Latifi, A.; Ruiz, M.; Zhang, C.-C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.M.; Hassan, S.H.; Selim, S.; Wadaan, M.A.M.; Mohany, M.; Hozzein, W.N.; AbdElgawad, H. Differential responses of two cyanobacterial species to R-metalaxyl toxicity: Growth, photosynthesis and antioxidant analyses. Environ. Pollut. 2020, 258, 113681. [Google Scholar] [CrossRef]
- Fahey, R.C.; Buschbacher, R.M.; Newton, G.L. The evolution of glutathione metabolism in phototrophic microorganisms. J. Mol. Evol. 1987, 25, 81–88. [Google Scholar] [CrossRef]
- Cameron, J.C.; Pakrasi, H.B. Essential role of glutathione in acclimation to environmental and redox perturbations in the cyanobacterium Synechocystis sp. PCC 6803. Plant. Physiol. 2010, 154, 1672–1685. [Google Scholar] [CrossRef]
- Rice, C.M.; Ashcroft, W.A.; Batten, D.J.; Boyce, A.J.; Caulfield, J.B.D.; Fallick, A.E.; Hole, M.J.; Jones, E.; Pearson, M.J.; Rogers, G.; et al. A Devonian auriferous hot spring system, Rhynie, Scotland. J. Geol. Soc. 1995, 152, 229–250. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]

| PCS Activity Without Cd2+ (Control) (pmol PCn g−1 FW min−1) | PCS Activity With 100 µM Cd2+ (pmol PCn g−1 FW min−1) | Increase in PCS Activity with 100 µM Cd2+ Compared with Controls (%) | Relative PCS Activity (% of A. thaliana PCS Activity) | |
|---|---|---|---|---|
| M. polymorpha | ||||
| PC2 | 16.94 ± 1.65 a | 70.88 ± 2.32 b | 76.3 ± 1.6 | |
| PC3 | 0.60 ± 0.06 a | 3.08 ± 0.22 b | 80.6 ± 1.2 | |
| PC4 | 0.39 ± 0.05 a | 2.29 ± 0.32 b | 82.5 ± 1.7 | |
| Total PCn | 17.94 ± 1.62 a | 76.26 ± 2.18 b | 76.7 ± 1.5 | 4.91 |
| Geitlerinema sp. PCC 7407 | ||||
| PC2 | 0.156 ± 0.01 a | 1.559 ± 0.01 b | 90.0 ± 0.9 | |
| PC3 | 0.000 ± 0.00 a | 0.064 ± 0.01 b | 100.0 ± 0.0 | |
| PC4 | 0.000 ± 0.00 a | 0.049 ± 0.01 b | 100.0 ± 0.0 | |
| Total PCn | 0.156 ± 0.01 a | 1.672 ± 0.01 b | 90.7 ± 0.9 | 0.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellini, E.; Varotto, C.; Borsò, M.; Rugnini, L.; Bruno, L.; Sanità di Toppi, L. Eukaryotic and Prokaryotic Phytochelatin Synthases Differ Less in Functional Terms Than Previously Thought: A Comparative Analysis of Marchantia polymorpha and Geitlerinema sp. PCC 7407. Plants 2020, 9, 914. https://doi.org/10.3390/plants9070914
Bellini E, Varotto C, Borsò M, Rugnini L, Bruno L, Sanità di Toppi L. Eukaryotic and Prokaryotic Phytochelatin Synthases Differ Less in Functional Terms Than Previously Thought: A Comparative Analysis of Marchantia polymorpha and Geitlerinema sp. PCC 7407. Plants. 2020; 9(7):914. https://doi.org/10.3390/plants9070914
Chicago/Turabian StyleBellini, Erika, Claudio Varotto, Marco Borsò, Lorenza Rugnini, Laura Bruno, and Luigi Sanità di Toppi. 2020. "Eukaryotic and Prokaryotic Phytochelatin Synthases Differ Less in Functional Terms Than Previously Thought: A Comparative Analysis of Marchantia polymorpha and Geitlerinema sp. PCC 7407" Plants 9, no. 7: 914. https://doi.org/10.3390/plants9070914
APA StyleBellini, E., Varotto, C., Borsò, M., Rugnini, L., Bruno, L., & Sanità di Toppi, L. (2020). Eukaryotic and Prokaryotic Phytochelatin Synthases Differ Less in Functional Terms Than Previously Thought: A Comparative Analysis of Marchantia polymorpha and Geitlerinema sp. PCC 7407. Plants, 9(7), 914. https://doi.org/10.3390/plants9070914








