Acid Rain Increases Impact of Rice Blast on Crop Health via Inhibition of Resistance Enzymes
Abstract
1. Introduction
2. Results
2.1. Effects of Acid Treatment on P. oryzae Colony Traits
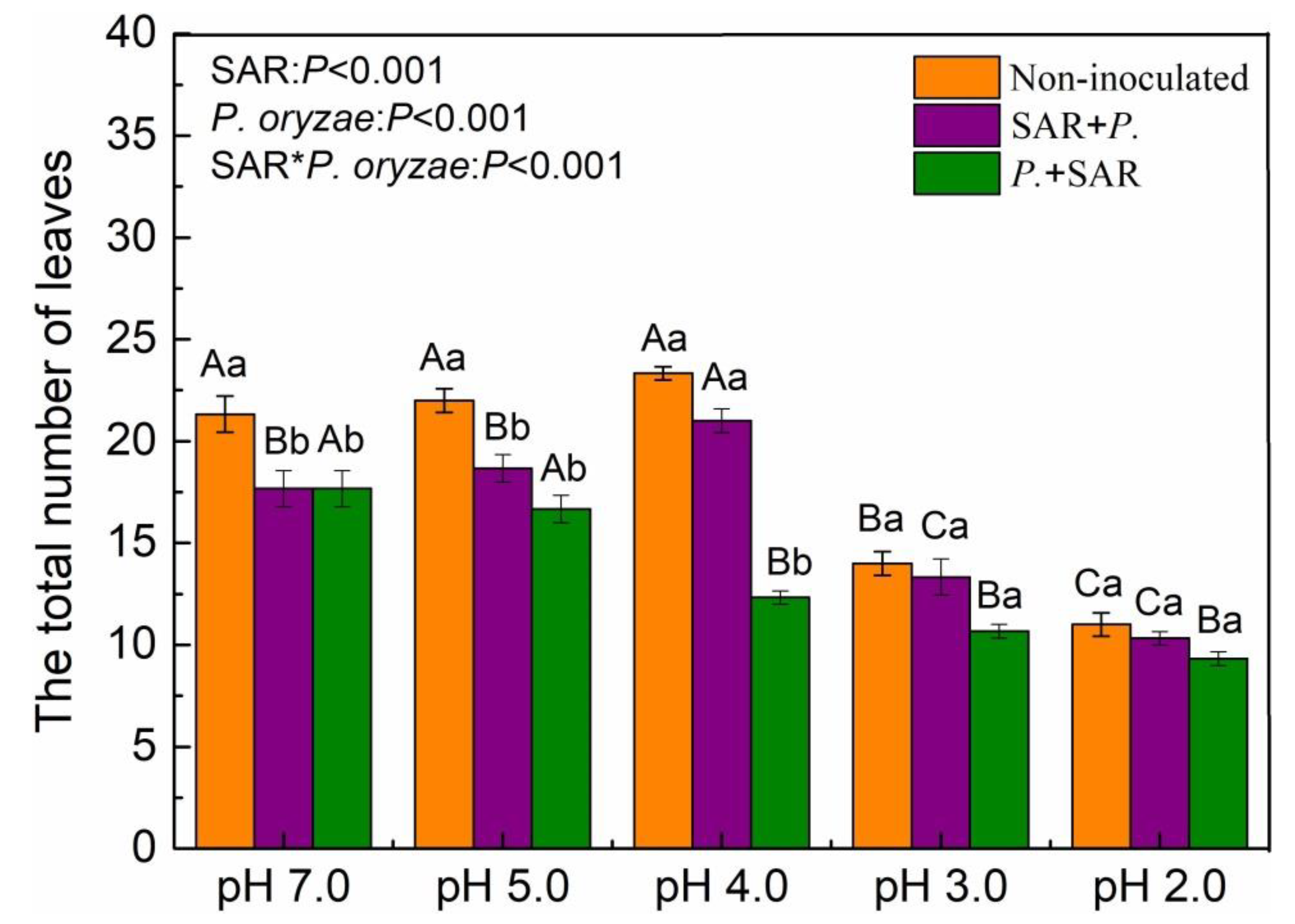
2.2. Effects of SAR and P. oryzae on the Total Number of Leaves
2.3. Disease Resistance-Related Enzyme Activities in Rice Leaves
2.4. Contents of Metabolism Compounds in Rice Leaves
2.5. Effects of SAR and P. oryzae on Rice Blast Disease Index
2.6. Relationships between Disease Index and Total Number of Leaves, Enzyme Activities, and Metabolites
3. Discussion
4. Materials and Methods
4.1. Preparation of Simulated Acid Rain and Cultivation P. oryzae
4.2. Plant Cultivation and Experimental Design
4.3. Determination of P. oryzae Infectivity Related Indicators
4.4. Enzyme Activity Related to Disease Resistance in Rice Leaves Measurement
4.5. Metabolite Related to Disease Resistance Measurement
4.6. Disease Index
4.7. Statistical Aanalysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nalley, L.; Tsiboe, F.; Durand-Morat, A.; Shew, A.; Thoma, G. Economic and environmental impact of rice blast pathogen (Magnaporthe oryzae) alleviation in the United States. PLoS ONE 2016, 11, e167295. [Google Scholar] [CrossRef]
- Marroquin-Guzman, M.; Hartline, D.; Wright, J.D.; Elowsky, C.; Bourret, T.J.; Wilson, R.A. The Magnaporthe oryzae nitrooxidative stress response suppresses rice innate immunity during blast disease. Nat. Microbiol. 2017, 2, 17054. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jia, Y.; Wu, B. Evaluation of Rice Responses to the Blast Fungus Magnaporthe oryzae at Different Growth Stages. Plant Dis. 2019, 103, 132–136. [Google Scholar] [CrossRef]
- Kankanala, P.; Czymmek, K.; Valent, B. Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell 2007, 19, 706–724. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wellings, C.; Chen, X.; Kang, Z.; Liu, T. Wheat stripe (yellow) rust caused by Puccinia striiformis f. sp. tritici. Mol. Plant Pathol. 2014, 15, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.; Grennfelt, P.; Klimont, Z.; Amann, M.; Apsimon, H.; Hettelingh, J.; Holland, M.; Legall, A.; Maas, R.; Posch, M.; et al. From acid rain to climate change. Science 2012, 338, 1153–1154. [Google Scholar] [CrossRef]
- Abbasi, T.; Poornima, P.; Kannadasan, T.; Abbasi, S.A. Acid rain: Past, present, and future. Int. J. Environ. Eng. 2013, 5, 229–272. [Google Scholar] [CrossRef]
- You, S. Improvement of China’s Air Pollution (Sulphur Dioxide and Acid Rain) Control and Countermeasures by Introducing Emissions Trading System. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2010. [Google Scholar] [CrossRef]
- Ramlall, C.; Varghese, B.; Ramdhani, S.; Pammenter, N.; Bhatt, A.; Berjak, P. Effects of simulated acid rain on germination, seedling growth and oxidative metabolism of recalcitrant-seeded Trichilia dregeana grown in its natural seed bank. Physiol. Plant 2015, 153, 149–160. [Google Scholar] [CrossRef]
- Ren, X.; Zhu, J.; Liu, H.; Xu, X.; Liang, C. Response of antioxidative system in rice (Oryza sativa) leaves to simulated acid rain stress. Ecotoxicol. Environ. Saf. 2018, 148, 851–856. [Google Scholar] [CrossRef]
- Liu, M.; Korpelainen, H.; Dong, L.; Yi, L. Physiological responses of Elaeocarpus glabripetalus seedlings exposed to simulated acid rain and cadmium. Ecotoxicol. Environ. Saf. 2019, 175, 118–127. [Google Scholar] [CrossRef]
- Martin, S.B.; Campbell, C.L.; Bruck, R.I. Influence of acidity level in simulated rain on disease progress and sporangial germination, infection efficiency, lesion expansion, and sporulation in the potato late blight system. Phytopathology 1987, 77, 969–974. [Google Scholar] [CrossRef]
- Andrade, G.C.; Silva, L.C. Responses of tropical legumes from the Brazilian Atlantic Rainforest to simulated acid rain. Protoplasma 2017, 254, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Donatelli, M.; Magarey, R.D.; Bregaglio, S.; Willocquet, L.; Whish, J.P.M.; Savary, S. Modelling the impacts of pests and diseases on agricultural systems. Agric. Syst. 2017, 155, 213–224. [Google Scholar] [CrossRef]
- Debnath, B.; Irshad, M.; Mitra, S.; Li, M.; Rizwan, H.M.; Liu, S.; Pan, T.; Qiu, D. Acid rain deposition modulates photosynthesis, enzymatic and non-enzymatic antioxidant activities in tomato. Int. J. Environ. Res. 2018, 12, 203–214. [Google Scholar] [CrossRef]
- Asai, E.; Futai, K. Effects of inoculum density of pinewood nematode on the development of pine wilt disease in Japanese black pine seedlings pretreated with simulated acid rain. For. Pathol. 2005, 35, 135–144. [Google Scholar] [CrossRef]
- Du, H.M.; Zhou, P.; Huang, B.R. Antioxidant enzymatic activities and gene expression associated with heat tolerance in a cool-season perennial grass species. Environ. Exp. Bot. 2013, 87, 159–166. [Google Scholar] [CrossRef]
- Vaahtera, L.; Brosché, M.; Wrzaczek, M.; Kangasjärvi, J. Specificity in ROS signaling and transcript signatures. Antioxid. Redox Signal. 2014, 21, 1422–1441. [Google Scholar] [CrossRef]
- Fontenele, N.M.B.; Otoch, M.D.L.O.; Gomes-Rochette, N.F.; de Menezes Sobreira, A.C.; Barreto, A.A.G.C.; de Oliveira, F.D.B.; Costa, J.H.; Borges, S.D.S.S.; Do Nascimento, R.F.; de Melo, D.F. Effect of lead on physiological and antioxidant responses in two vigna unguiculata cultivars differing in Pb-accumulation. Chemosphere 2017, 176, 397–404. [Google Scholar] [CrossRef]
- Mahmood, S.; Hussain, I.; Ashraf, A.; Parveen, A.; Javed, S.; Iqbal, M.; Afzal, B. Tyrosine-priming modulates phenylpropanoid pathway in maize grown under different pH regimes. Cereal. Res. Commun. 2017, 45, 214–224. [Google Scholar] [CrossRef][Green Version]
- Wan, J.; Zhang, X.C.; Stacey, G. Chitin signaling and plant disease resistance. Plant Signaling Behav. 2008, 3, 831–833. [Google Scholar] [CrossRef]
- Ju, S.M.; Yin, N.N.; Wang, L.P. Effects of silicon on Oryza sativa L. seedling roots under simulated acid rain stress. PLoS ONE 2017, 12, e0173378. [Google Scholar] [CrossRef]
- Wang, S.H.; Sun, P.L.; Zhou, W.N.; Liu, T.; Ma, Z.H. Effect of simulated acid rain on disease progress of wheat yellow rust. J. Plant Prot. 2018, 45, 173–180. [Google Scholar] [CrossRef]
- Shriner, D.S. Effects of simulated acidic rain on host-parasite interactions in plant diseases. Phytopathology 1978, 68, 3–218. [Google Scholar] [CrossRef]
- Jacobson, E.S. Pathogenic roles for fungal melanins. Clin. Microbiol. Rev. 2000, 13, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Effects and the Underlying Mechanisms of Tomato-Pathogen Interaction under Different Environmental Factors and Its Regulation; Zhejiang University: Hangzhou, China, 2016; Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?FileName=1016124874.nh&DbName=CMFD2018 (accessed on 8 June 2020).
- Stroo, H.F.; Alexander, M. Effect of simulated acid rain on mycorrhizal infection of Pinus strobus L. Water Air Soil Pollut. 1985, 25, 107–114. [Google Scholar] [CrossRef]
- Shu, X.; Yin, L.; Zhang, Q.; Wang, W. Effect of Pb toxicity on leaf growth, antioxidant enzyme activities, and photosynthesis in cuttings and seedlings of Jatropha curcas L. Environ. Sci. Pollut. Res. 2012, 19, 893–902. [Google Scholar] [CrossRef]
- Zhang, X.B.; Du, Y.P.; Wang, L.H.; Zhou, Q.; Huang, X.H.; Sun, Z.G. Combined effects of lanthanum (iii) and acid rain on antioxidant enzyme system in soybean roots. PLoS ONE 2015, 10, e0134546. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Xie, C.; Zu, Y.; Zhan, F.; Mei, X.; Xia, Y.; Li, Y. Effects of UV-B radiation on the infectivity of Magnaporthe oryzae and rice disease-resistant physiology in Yuanyang terraces. Photochem. Photobiol. Sci. 2018, 17, 8–17. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Effects of calcium on antioxidant activities and water relations associated with heat tolerance in two cool-season grasses. J. Exp. Bot. 2001, 52, 341–349. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Yoon, J.; Choi, H.; An, G. Roles of lignin biosynthesis and regulatory genes in plant development. J. Int. Plant Biol. 2015, 57, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Miron, A.; Aprotosoaie, A.C.; Trifan, A.; Xiao, J. Flavonoids as modulators of metabolic enzymes and drug transporters. Ann. N. Y. Acad. Sci. 2017, 1398, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Long, Y. Effect of Enhanced UV-B Radiation and Simulated Acid Rain on Growth and Metabolism of C4 Plant Maize and Amaranth; Southwest University: Chongqing, China, 2011; Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?FileName=1012280356.nh&DbName=CDFD2012 (accessed on 8 June 2020).
- Moura, J.C.; Bonine, C.A.; de Oliveira, F.V.J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef]
- Lee, Y.; Rubio, M.C.; Alassimone, J.; Geldner, N. A mechanism for localized lignin deposition in the endodermis. Cell 2013, 153, 402–412. [Google Scholar] [CrossRef]
- Voxeur, A.; Wang, Y.; Sibout, R. Lignification: Different mechanisms for a versatile polymer. Curr. Opin. Plant Biol. 2015, 23, 83–90. [Google Scholar] [CrossRef]
- De Ascensao, A.R.; Dubery, I.A. Panama Disease: Cell wall reinforcement in banana roots in response to elicitors from Fusarium oxysporum f. sp. cubense race four. Phytopathology 2000, 90, 1173–1180. [Google Scholar] [CrossRef]
- Albert, N.W.; Lewis, D.H.; Zhang, H.; Irving, L.J.; Jameson, P.E.; Davies, K.M. Light-induced vegetative anthocyanin pigmentation in Petunia. J. Exp. Bot. 2009, 60, 2191–2202. [Google Scholar] [CrossRef]
- Pawlak-Sprada, S.; Stobiecki, M.; Deckert, J. Activation of phenylpropanoid pathway in legume plants exposed to heavy metals. Part II. Profiling of isoflavonoids and their glycoconjugates induced in roots of lupine (Lupinus luteus) seedlings treated with cadmium and lead. Acta. Biochim. Pol. 2011, 58, 217–223. [Google Scholar] [CrossRef]
- Asai, E.; Futai, K. The effects of long-term exposure to simulated acid rain on the development of pine wilt disease caused by Bursaphelenchus xylophilus. For. Pathol. 2001, 31, 241–253. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, B. Effect of exogenous calcium on growth, nutrients uptake and plasma membrane H+-ATPase and Ca2+-ATPase activities in soybean (Glycine max) seedlings under simulated acid rain stress. Ecotoxicol. Environ. Saf. 2018, 165, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, X.; He, L.; Feng, N.; Hu, M.; Niu, Y.; Zeng, L. 5-Year study of rainwater chemistry in a coastal mega-city in South China. Atmos. Res. 2010, 97, 185–193. [Google Scholar] [CrossRef]
- Samalova, M.; Meyer, A.J.; Gurr, S.J.; Fricker, M.D. Robust anti-oxidant defences in the rice blast fungus magnaporthe oryzae confer tolerance to the host oxidative burst. New Phytol. 2013, 201, 556–573. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.J.; Ferrari, M.A. The role of melanin in appressorium function. Exp. Mycol. 1989, 13, 403–418. [Google Scholar] [CrossRef]
- Lu, X. Report on the State of Guangdong Provincial Ecology and Environment; Guangdong Provincial Department of Ecology and Environment: Guangzhou, China, 2018. [Google Scholar]
- Ballini, E.; Nguyen, T.T.; Morel, J. Diversity and genetics of nitrogen-induced susceptibility to the blast fungus in rice and wheat. Rice 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Bassam, S.E.; Benhamou, N.; Carisse, O. The role of melanin in the antagonistic interaction between the apple scab pathogen Venturia inaequalis and Microsphaeropsis ochracea. Can. J. Microbiol. 2002, 48, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, F.; Azenha, M.; Silva, A.F.; De Sousa, A.; Santiago, A.; Ferraz, P.; Teixeira, J. Copper-induced stress in Solanum nigrum L. and antioxidant defense system responses. Food Energy Secur. 2013, 2, 70–80. [Google Scholar] [CrossRef]
- Mirecki, R.M.; Teramura, A.H. The dependence of plant sensitivity on the photosynthetic photon flux density during and after leaf expansion. Plant Physiol. 1984, 74, 475–480. [Google Scholar] [CrossRef]
- Kakani, V.G.; Reddy, K.R.; Zhao, D.; Gao, W. Senescence and hyperspectral reflectance of cotton leaves exposed to ultraviolet-B radiation and carbon dioxide. Physiol. Plant. 2004, 121, 250–257. [Google Scholar] [CrossRef]
- Cai, Y.; Li, G.; Nie, J.; Lin, Y.; Nie, F.; Zhang, J.; Xu, Y. Study of the structure and biosynthetic pathway of lignin in stone cells of pear. Sci. Hortic. 2010, 125, 374–379. [Google Scholar] [CrossRef]
- Yu, L.; Liu, H.; Shao, X.; Yu, F.; Wei, Y.; Ni, Z.; Xu, F.; Wang, H. Effects of hot air and methyl jasmonate treatment on the metabolism of soluble sugars in peach fruit during cold storage. Postharvest Biol. Technol. 2016, 113, 8–16. [Google Scholar] [CrossRef]



| SAR (pH) | Inoculation (I) | Flavonoids (A305/g FW) | Total Phenol (μg/cm−2 FW) | Lignin (% DW) | Soluble Sugar (mg·g−1 FW) |
|---|---|---|---|---|---|
| CK | Non-inoculated | 1.90 ± 0.05Cb | 23.41 ± 0.36Db | 18.41 ± 0.38Aa | 3.00 ± 0.02Db |
| SAR + P. | 3.13 ± 0.07Ba | 27.60 ± 0.52Ca | 13.61 ± 0.07Ab | 5.27 ± 0.10Ba | |
| P. + SAR | 3.13 ± 0.07Aa | 27.60 ± 0.52Ba | 13.61 ± 0.07Ab | 5.27 ± 0.10Ba | |
| 5.0 | Non-inoculated | 2.87 ± 0.04Ab | 29.74 ± 0.45Bb | 17.55 ± 0.45Aa | 5.38 ± 0.02Ba |
| SAR + P. | 3.24 ± 0.07Ba | 31.54 ± 0.54Ba | 14.41 ± 0.19Ab | 5.14 ± 0.12Ca | |
| P. + SAR | 2.83 ± 0.06Bb | 25.69 ± 0.66Cc | 13.46 ± 0.13Ab | 4.88 ± 0.09Bb | |
| 4.0 | Non-inoculated | 3.01 ± 0.06Ab | 32.29 ± 0.44Ab | 17.03 ± 0.20Aa | 6.22 ± 0.50Ab |
| SAR + P. | 3.57 ± 0.08Aa | 34.39 ± 0.59Aa | 14.18 ± 0.15Ab | 7.89 ± 0.07Aa | |
| P. + SAR | 2.61 ± 0.06Cb | 31.53 ± 0.80Ab | 10.18 ± 0.16Bc | 5.97 ± 0.10Ab | |
| 3.0 | Non-inoculated | 2.49 ± 0.08Ba | 28.01 ± 0.27Ca | 10.35 ± 0.36Ba | 6.45 ± 0.07Aa |
| SAR + P. | 2.51 ± 0.02Ca | 26.01 ± 0.34Ca | 8.32 ± 0.11Bb | 5.45 ± 0.07Bb | |
| P. + SAR | 2.16 ± 0.10Cb | 23.17 ± 0.36Ca | 6.60 ± 0.19Cc | 4.52 ± 0.07Cc | |
| 2.0 | Non-inoculated | 2.32 ± 0.05Ba | 24.55 ± 0.52Da | 8.10 ± 0.12Ca | 4.65 ± 0.09Ca |
| SAR + P. | 2.20 ± 0.09Ca | 22.75 ± 0.84Da | 6.44 ± 0.23Cb | 4.29 ± 0.06Cb | |
| P. + SAR | 2.14 ± 0.05Ca | 20.41 ± 0.67Db | 4.19 ± 0.08Dc | 3.60 ± 0.09Dc |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-R.; Xiang, H.-M.; Zhong, J.-W.; Ren, X.-Q.; Wei, H.; Zhang, J.-E.; Xu, Q.-Y.; Zhao, B.-L. Acid Rain Increases Impact of Rice Blast on Crop Health via Inhibition of Resistance Enzymes. Plants 2020, 9, 881. https://doi.org/10.3390/plants9070881
Li H-R, Xiang H-M, Zhong J-W, Ren X-Q, Wei H, Zhang J-E, Xu Q-Y, Zhao B-L. Acid Rain Increases Impact of Rice Blast on Crop Health via Inhibition of Resistance Enzymes. Plants. 2020; 9(7):881. https://doi.org/10.3390/plants9070881
Chicago/Turabian StyleLi, Hong-Ru, Hui-Min Xiang, Jia-Wen Zhong, Xiao-Qiao Ren, Hui Wei, Jia-En Zhang, Qiu-Yuan Xu, and Ben-Liang Zhao. 2020. "Acid Rain Increases Impact of Rice Blast on Crop Health via Inhibition of Resistance Enzymes" Plants 9, no. 7: 881. https://doi.org/10.3390/plants9070881
APA StyleLi, H.-R., Xiang, H.-M., Zhong, J.-W., Ren, X.-Q., Wei, H., Zhang, J.-E., Xu, Q.-Y., & Zhao, B.-L. (2020). Acid Rain Increases Impact of Rice Blast on Crop Health via Inhibition of Resistance Enzymes. Plants, 9(7), 881. https://doi.org/10.3390/plants9070881






