Biodiversity of β-Carboline Profile of Banisteriopsis caapi and Ayahuasca, a Plant and a Brew with Neuropharmacological Potential
Abstract
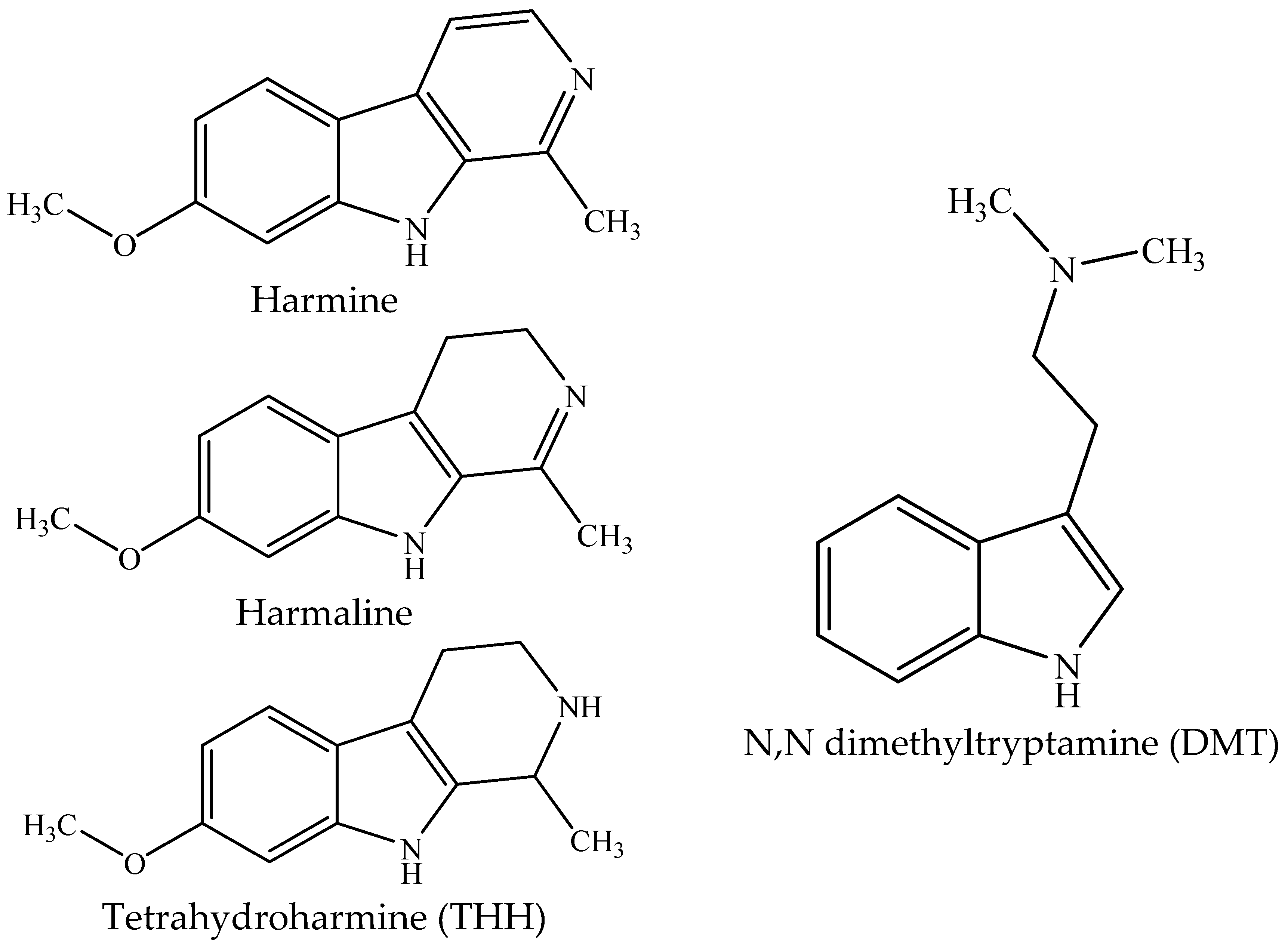
1. Introduction
2. Material and Methods
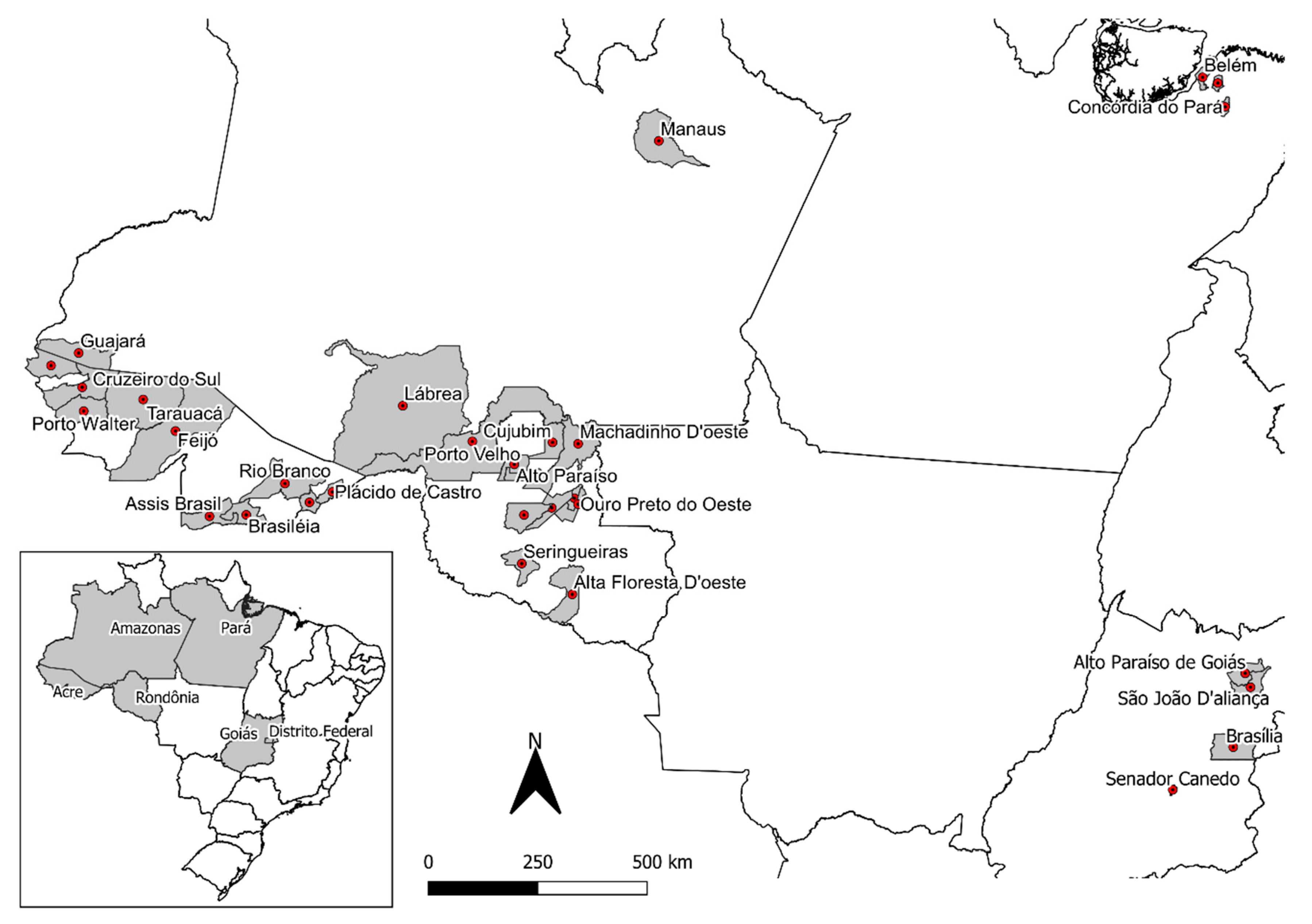
2.1. Banisteriopsis spp., Diplopterys pubipetala, and Ayahuasca Brew Samples
2.2. Chemicals and Reagents
2.3. Synthesis of DMT and THH, Identification and Purity Assessment
2.4. Sample Preparation
2.5. Analysis of the β-carbolines and DMT by Liquid Chromatography–Electrospray Ionization-Tandem Mass Spectrometry (LC-MS/MS)
2.6. Statistical Analysis
3. Results
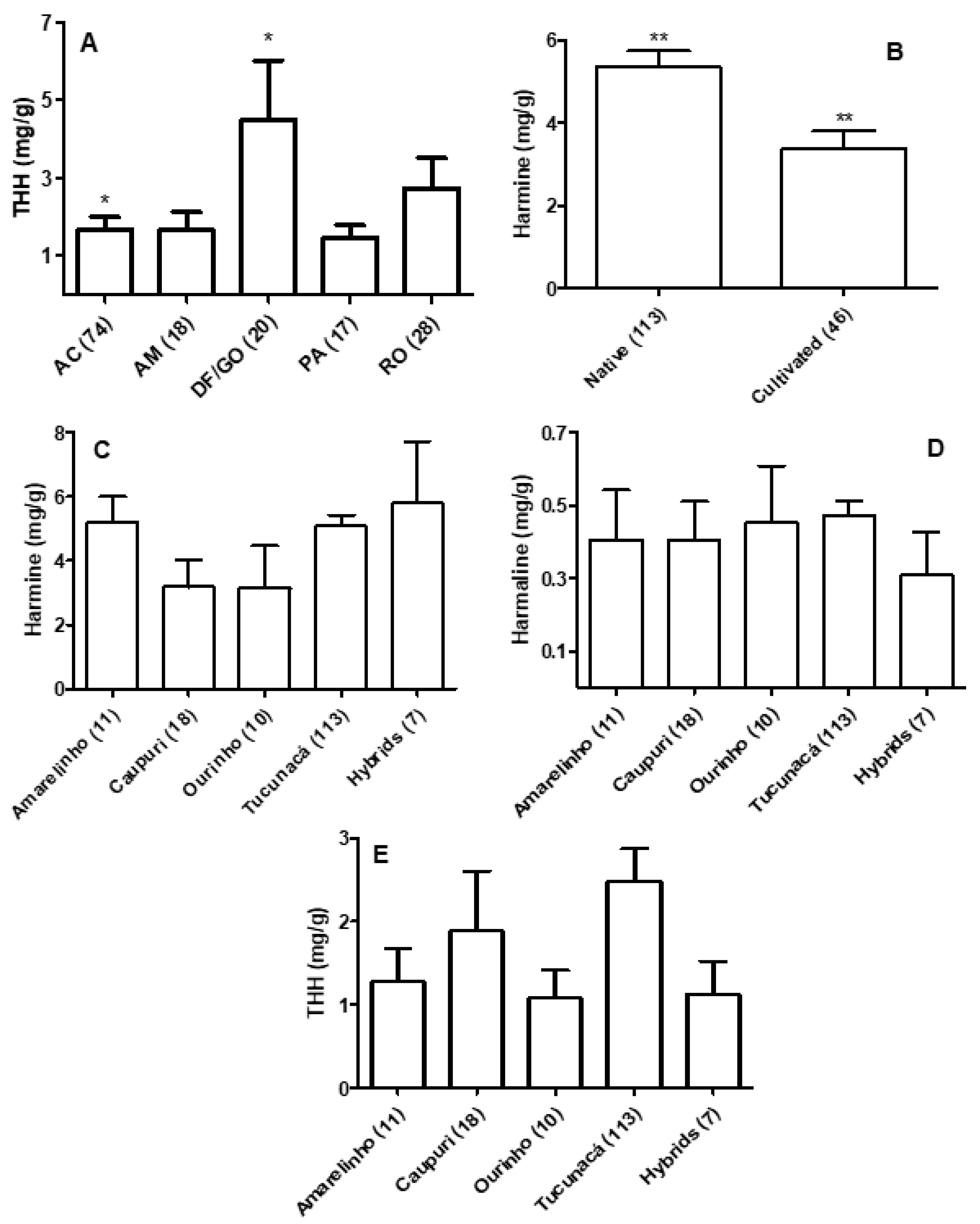
3.1. Quantification of β-carbolines in Banisteriopsis spp. and Diplopterys Pubipetala
3.2. Quantification of β-carbolines and DMT in Ayahuasca Brew Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MacRae, E. The ritual use of ayahuasca by three Brazilian religions. In Drug Use and Cultural Contexts’ Beyond the West’: Tradition, Change and Post-Colonialism; Coomber, R., South, N., Eds.; Free Association Books: London, UK, 2004; pp. 27–45. [Google Scholar]
- Labate, B.C.; Assis, G.L. A critical review of the literature on the diaspora of Brazilian ayahuasca religions. In The Expanding World Ayahuasca Diaspora, 1st ed.; Labate, B., Cavnar, C., Eds.; Routledge: New York, NY, USA, 2018; pp. 1–21. [Google Scholar]
- Luna, L.E. The healing practices of a peruvian shaman. J. Ethnopharmacol. 1984, 11, 123–133. [Google Scholar] [CrossRef]
- McKenna, D.J.; Towers, G.H.N.; Abbott, F. Monoamine oxidase inhibitors in South American hallucinogenic plants: Tryptamine and ß-carboline constituents of Ayahuasca. J. Ethnopharmacol. 1984, 10, 195–223. [Google Scholar] [CrossRef]
- Riba, J.; McIlhenny, E.H.; Valle, M.; Bouso, J.C.; Barker, S.A. Metabolism and disposition of N,N-dimethyltryptamine and harmala alkaloids after oral administration of ayahuasca. Drug Test. Anal. 2012, 4, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Santillo, M.F.; Liu, Y.; Ferguson, M.; Vohra, S.N.; Wiesenfeld, P.L. Inhibition of monoamine oxidase (MAO) by β-carbolines and their interactions in live neuronal (PC12) and liver (HuH-7 and MH1C1) cells. Toxicol. In Vitro 2014, 28, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Strassman, R.J.; Qualls, C.R. Dose-response study of N,N-dimethyltryptamine in humans, I. Neuroendocrine, autonomic, and cardiovascular effects. Arch. Gen. Psychiatry 1994, 51, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Canton, H.; Barrett, R.J.; Sanders-Bush, E. Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT(2A) and 5-HT(2C) receptors. Pharmacol. Biochem. Behav. 1998, 61, 323–330. [Google Scholar] [CrossRef]
- Ott, J. Pharmahuasca: Human pharmacology of oral DMT plus harmine. J. Psychoact. Drugs 1999, 31, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Brierley, D.I.; Davidson, C. Harmine augments electrically evoked dopamine efflux in the nucleus accumbens shell. J. Psychopharmacol. 2013, 27, 98–108. [Google Scholar] [CrossRef]
- Cao, R.; Peng, W.; Wang, Z.; Xu, A. ß-carboline alkaloids: Biochemical and pharmacological functions. Curr. Med. Chem. 2007, 14, 479–500. [Google Scholar] [CrossRef]
- Glennon, R.A.; Dukat, M.; Grella, B.; Hong, S.; Mattson, M.V. Binding of beta-carbolines and related agents at serotonin (5-HT2 and 5-HT1A), dopamine (D2) and benzodiazepine receptors. Drug Alcohol. Depend. 2000, 60, 121–132. [Google Scholar] [CrossRef]
- McKenna, D.J. Clinical investigations of the therapeutic potential of ayahuasca: Rationale and regulatory challenges. Pharmacol. Ther. 2004, 102, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Frecska, E.; Bokor, P.; Winkelman, M. The therapeutic potentials of ayahuasca: Possible effects against various diseases of civilization. Front. Pharmacol. 2016, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Morales-García, J.A.; Revenga, M.F.; Alonso-Gil, S.; Rodríguez-Franco, M.I.; Feilding, A.; Perez-Castillo, A.; Riba, J. The alkaloids of Banisteriopsis caapi, the plant source of the Amazonian hallucinogen Ayahuasca, stimulate adult neurogenesis in vitro. Sci. Rep. 2017, 7, 5309. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, J.J.; Réus, G.Z.; Kirsch, T.R.; Stringari, R.B.; Stertz, L.; Kapczinski, F.; Pinto, J.P.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; et al. Acute harmine administration induces antidepressive-like effects and increases BDNF levels in the rat hippocampus. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2009, 33, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, J.J.; Réus, G.Z.; Kirsch, T.R.; Stringari, R.B.; Fries, G.R.; Kapczinski, F.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Quevedo, J. Chronic administration of harmine elicits antidepressant-like effects and increases BDNF levels in rat hippocampus. J. Neural Transm. 2010, 117, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Dueñas, M.; Cardozo-Pelaez, F.; Sánchez-Ramos, J.R. Effects of Banisteriopsis caapi extract on Parkinson’s disease. Sci. Rev. Altern. Med. 2001, 5, 129–134. [Google Scholar]
- Schwarz, M.J.; Houghton, P.J.; Rose, S.; Jenner, P.; Lees, A.D. Activities of extract and constituents of Banisteriopsis caapi relevant to parkinsonism. Pharmacol. Biochem. Behav. 2003, 75, 627–633. [Google Scholar] [CrossRef]
- Samoylenko, V.; Rahman, M.M.; Tekwani, B.L.; Tripathi, L.M.; Wang, Y.H.; Khan, S.I.; Khan, I.A.; Miller, L.S.; Joshi, V.C.; Muhammad, I. Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson’s disease. J. Ethnopharmacol. 2010, 127, 357–367. [Google Scholar] [CrossRef]
- Wang, Y.H.; Samoylenko, V.; Tekwani, B.L.; Khan, I.A.; Miller, L.S.; Chaurasiya, N.D.; Rahman, M.M.; Tripathi, L.M.; Khan, S.I.; Joshi, V.C.; et al. Composition, standardization and chemical profiling of Banisteriopsis caapi, a plant for the treatment of neurodegenerative disorders relevant to Parkinson’s disease. J. Ethnopharmacol. 2010, 128, 662–671. [Google Scholar] [CrossRef]
- Pic-Taylor, A.; Da Motta, L.G.; De Morais, J.A.; Junior, W.M.; Santos, A.F.A.; Campos, L.A.; Mortari, M.R.; Von Zuben, M.; Caldas, E.D. Behavioural and neurotoxic effects of ayahuasca infusion (Banisteriopsis caapi and Psychotria viridis) in female Wistar rat. Behav. Process. 2015, 118, 102–110. [Google Scholar] [CrossRef]
- Da Silva, F.S.; Silva, E.A.S.; Sousa, G.M., Jr.; Maia-de-Oliveira, J.P.; Soares-Rachetti, V.P.; De Araujo, D.B.; Sousa, M.B.C.; Lobão-Soares, B.; Hallak, J.; Galvão-Coelho, N.L. Acute effects of ayahuasca in a juvenile non-human primate model of depression. Rev. Bras. Psychiatry 2019, 41, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Colaço, C.S.; Alves, S.S.; Nolli, L.M.; Pinheiro, W.O.; Oliveira, D.G.R.; Santos, B.W.L.; Pic-taylor, A.; Mortari, M.R.; Caldas, E.D. Toxicity of ayahuasca after 28 days daily exposure and effects on monoamines and brain-derived neurotrophic factor (BDNF) in brain of Wistar rats. Metab. Brain Dis. 2020, 35, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Nolli, L.M.; Gustavo, D.; Oliveira, R.; Alves, S.S.; Von Zuben, M.V.; Pic-taylor, A.; Mortari, M.R.; Caldas, E.D. Effects of the hallucinogenic beverage ayahuasca on voluntary ethanol intake by rats and on cFos expression in brain areas relevant to drug addiction. Alcohol 2020, 84, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Lawn, W.; Hallak, J.E.; Crippa, J.A.; Dos Santos, R.; Porffy, L.; Barratt, M.J.; Ferris, J.A.; Winstock, A.R.; Morgan, C. Well-being, problematic alcohol consumption and acute subjective drug effects in past-year ayahuasca users: A large, international, self-selecting online survey. Sci. Rep. 2017, 7, 15201. [Google Scholar] [CrossRef]
- Palhano-Fontes, F.; Barreto, D.; Onias, H.; Andrade, K.C.; Novaes, M.M.; Pessoa, J.A.; Mota-Rolim, S.A.; Osório, F.L.; Sanches, R.; Dos Santos, R.G.; et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: A randomized placebo-controlled trial. Psychol. Med. 2019, 49, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Garrido, D.F.; Gómez-Sousa, M.; Ona, G.; Dos Santos, R.G.; Hallak, J.; Alcázar-Córcoles, M.A.; Bouso, J.C. Effects of ayahuasca on mental health and quality of life in naïve users: A longitudinal and cross-sectional study combination. Sci. Rep. 2020, 10, 4075. [Google Scholar] [CrossRef]
- Schultes, R.E. Hallucinogens of plant origin. Science 1969, 163, 245–254. [Google Scholar] [CrossRef]
- Schultes, R.E.; Hofmann, A. The Botany and Chemistry of Hallucinogens; Charles C. Thomas Pub: Springfield, IL, USA, 1980; 437p. [Google Scholar]
- Langdon, E.J. Las clasificaciones del yagé Dentro del grupo Siona: Etnobotánica, etnoquímica e historia. Am. Indíg. 1986, 46, 101–116. [Google Scholar]
- Schultes, R.E.; Hofmann, A.; Rätsch, C. Plants of the Gods: Their Sacred, Healing and Hallucinogenic Powers; Healing Arts Press: Rochester, NY, USA, 2001; 208p, Available online: https://archive.org/details/SchultesHofmannPlantsOfTheGodsHealingArts2001/page/n15/mode/2up (accessed on 22 June 2020).
- Rivier, L.; Lindgren, J.E. ‘Ayahuasca,’ the South American hallucinogenic drink: An ethnobotanical and chemical investigation. Econ. Bot. 1972, 26, 101–129. [Google Scholar] [CrossRef]
- Callaway, J.C.; Brito, G.S.; Neves, E.S. Phytochemical analyses of Banisteriopsis caapi and Psychotria viridis. J. Psychoact. Drugs 2005, 37, 145–150. [Google Scholar] [CrossRef]
- Qu, S.J.; Wang, G.F.; Duan, W.H.; Yao, S.Y.; Zuo, J.P.; Tan, C.H.; Zhu, D.Y. Tryptamine derivatives as novel non-nucleosidic inhibitors against hepatitis B virus. Bioorg. Med. Chem. 2011, 19, 3120–3127. [Google Scholar] [CrossRef] [PubMed]
- Callaway, J.C.; Raymon, L.P.; Hearn, W.L.; McKenna, D.J.; Grob, C.S.; Brito, G.S.; Mash, D.C. Quantitation of N,N-dimethyltryptamine and harmala alkaloids in human plasma after oral dosing with ayahuasca. J. Anal. Toxicol. 1996, 20, 492–497. [Google Scholar] [CrossRef] [PubMed]
- INMETRO. Orientações Sobre Validação de Métodos Analíticos (DOQ-CGCRE-008); Revisão 5; INMETRO: Rio de Janeiro, Brazil, 2016; 31p.
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- De Castro-Neto, E.F.; Da Cunha, R.H.; Da Silveira, D.X.; Yonamine, M.; Gouveia, T.L.F.; Cavalheiro, E.A.; Amado, D.; Naffah-Mazzacoratti, M.D.G. Changes in aminoacidergic and monoaminergic neurotransmission in the hippocampus and amygdala of rats after ayahuasca ingestion. World J. Biol. Chem. 2013, 4, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Schultes, R.E.; Holmstedt, B.; Lindgren, J. Phytochemical examination of Spruce’s original collection of Banisteriopsis caapi. Bot. Mus. Leafl. Harv. Univ. 1969, 22, 121–132. [Google Scholar]
- Franco, A.C.; Bustamante, M.; Caldas, L.S.; Goldstein, G.; Meinzer, F.C.; Kozovits, A.R.; Rundel, P.; Coradin, V.T.R. Leaf functional traits of Neotropical savanna trees in relation to seasonal water deficit. Trees 2005, 19, 326–335. [Google Scholar] [CrossRef]
- Teixeira, D.C.; Quinteiro, M.D.C.; Baptista, A.A.; Da Silva, J.G. Uso e manejo de plantas ritualísticas na Comunidade do Santo Daime em Galdinópolis, Nova Friburgo/Rj, Brasil. Rev. Ciên. Vida 2008, 28, 63–74. [Google Scholar]
- Callaway, J.C. Various alkaloid profiles in decoctions of Banisteriopsis caapi. J. Psychoact. Drugs 2005, 37, 151–155. [Google Scholar] [CrossRef]
- Boerngen-Lacerda, R.; Jamal, Y.; Correia, D.; Goeldner, F.O. Contribution of serotonin 5-HT2 receptor to ethanol-induced sensitization and ethanol consumption in mice. In Serotonin: Biosynthesis, Regulation and Health Implications; Hall, F.S., Ed.; Nova Science Publishers Inc: Happauge, NY, USA, 2013; pp. 224–255. [Google Scholar]
- Souza, R.C.Z.; Zandonadi, F.S.; Freitas, D.P.; Tófoli, L.F.F. Validation of an analytical method for the determination of the main ayahuasca active compounds and application to real ayahuasca samples from Brazil. J. Chromatogr. B 2019, 1124, 197–203. [Google Scholar] [CrossRef]
- Pires, A.P.S.; Oliveira, C.D.R.; Moura, S.; Dörr, F.A.; Silva, W.A.E.; Yonamine, M. Gas chromatographic analysis of dimethyltryptamine and ß-carboline alkaloids in ayahuasca, an Amazonian psychoactive beverage. Phitochem. Anal. 2009, 20, 149–153. [Google Scholar] [CrossRef]
- McIlhenny, E.H.; Pipkin, K.E.; Standish, L.J.; Wechkin, H.A.; Strassman, R.; Barker, S.A. Direct analysis of psychoactive tryptamine and harmala alkaloids in the Amazonian botanical medicine ayahuasca by liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 8960–8968. [Google Scholar] [CrossRef] [PubMed]
- Da Motta, L.G.; Morais, J.A.; Tavares, A.C.A.M.; Vianna, L.M.S.; Mortari, M.R.; Amorim, R.F.B.; Carvalho, R.R.; Paumgartten, F.J.R.; PicTaylor, A.; Caldas, E.D. Maternal and developmental toxicity of the hallucinogenic plant-based beverage ayahuasca in rats. Reprod. Toxicol. 2018, 77, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.D.R.; Moreira, C.Q.; De Sá, L.R.M.; Spinosa, H.D.S.; Yonamine, M. Maternal and developmental toxicity of ayahuasca in Wistar rats. Birth Defects Res. Part B 2010, 89, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Assis, G.L. A Religião of the Floresta: Apontamentos Sociológicos em Direção a uma Genealogia do Santo Daime e seu Processo de Diáspora. Ph.D. Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2017; 484p. Available online: http://hdl.handle.net/1843/BUBD-ANYQSW (accessed on 22 June 2020).
- De Oliveira Silveira, G.; Guimarães Dos Santos, R.; Rebello Lourenço, F.; Novak Rossi, G.; Hallak, J.E.C.; Yonamine, M. Stability evaluation of DMT and harmala alkaloids in ayahuasca tea samples. Molecules 2020, 25, 2072. [Google Scholar] [CrossRef] [PubMed]

| Banisteriopsis caapi (N = 159) | Ayahuasca (N = 33) | |||
|---|---|---|---|---|
| Compound | Range (Mean) | RSD, % | Range (Mean) | RSD, % |
| Harmine | <LOD-18.27 mg/g (4.79) a | 78.9 | 0.109–7.11 mg/mL (1.28) | 99.1 |
| Harmaline | <LOD-2.08 mg/g (0.451) a | 92.4 | 0.012–0.945 mg/mL (0.195) | 102 |
| THH | <LOD-29.04 mg/g (2.18) a | 170 | 0.09–3.05 mg/mL (1.16) | 60.3 |
| DMT | - | - | 0.10 b–3.12 mg/mL (1.08) | 63.8 |
| Total β-carbolines | <LOD-32.1 mg/g (7.4) a | 40.4 | 0.21–11.1 mg/mL (2.65) | 76.2 |
| Harmaline/harmine | 0.006–3.0 (0.15) c | 187 | 0.04–0.27 (0.155) | 39.4 |
| THH/harmaline | 0.55–109 (5.6) c | 198 | 2.0–17.0 (8.4) | 41.1 |
| THH/harmine | 0.01–38 (1.2) c | 331 | 0.35–3.4 (1.2) | 54.2 |
| Ratio a | |||
|---|---|---|---|
| Sample | Harmaline/Harmine | THH/Harmaline | THH/Harmine |
| Ayahuasca 1 | 0.255 | 2.09 | 8.19 |
| B. caapi 1 | 0.174 | 0.518 | 2.99 |
| Ayahuasca 2 | 0.201 | 1.63 | 8.08 |
| B. caapi 2 | 0.153 | 0.505 | 3.30 |
| Ayahuasca 3 | 0.068 | 0.414 | 6.12 |
| B. caapi 3 | 0.041 | 0.038 | 0.912 |
| Ayahuasca 4 | 0.188 | 1.97 | 10.4 |
| B. caapi 4 | 0.087 | 0.187 | 2.14 |
| Ayahuasca 5 | 0.125 | 1.64 | 13.0 |
| B. caapi 5 | 0.094 | 5.34 | 56.9 |
| Ayahuasca 6 | 0.227 | 1.43 | 6.27 |
| B. caapi 6 | 0.129 | 4.52 | 35.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, B.W.L.; Oliveira, R.C.d.; Sonsin-Oliveira, J.; Fagg, C.W.; Barbosa, J.B.F.; Caldas, E.D. Biodiversity of β-Carboline Profile of Banisteriopsis caapi and Ayahuasca, a Plant and a Brew with Neuropharmacological Potential. Plants 2020, 9, 870. https://doi.org/10.3390/plants9070870
Santos BWL, Oliveira RCd, Sonsin-Oliveira J, Fagg CW, Barbosa JBF, Caldas ED. Biodiversity of β-Carboline Profile of Banisteriopsis caapi and Ayahuasca, a Plant and a Brew with Neuropharmacological Potential. Plants. 2020; 9(7):870. https://doi.org/10.3390/plants9070870
Chicago/Turabian StyleSantos, Beatriz Werneck Lopes, Regina Célia de Oliveira, Julia Sonsin-Oliveira, Christopher William Fagg, José Beethoven Figueiredo Barbosa, and Eloisa Dutra Caldas. 2020. "Biodiversity of β-Carboline Profile of Banisteriopsis caapi and Ayahuasca, a Plant and a Brew with Neuropharmacological Potential" Plants 9, no. 7: 870. https://doi.org/10.3390/plants9070870
APA StyleSantos, B. W. L., Oliveira, R. C. d., Sonsin-Oliveira, J., Fagg, C. W., Barbosa, J. B. F., & Caldas, E. D. (2020). Biodiversity of β-Carboline Profile of Banisteriopsis caapi and Ayahuasca, a Plant and a Brew with Neuropharmacological Potential. Plants, 9(7), 870. https://doi.org/10.3390/plants9070870






